Abstract
The human pathogen enterohemorrhagic Escherichia coli (EHEC) O157:H7 colonizes human and animal gut via formation of attaching and effacing lesions. EHEC strains use a type III secretion system to translocate a battery of effector proteins into the mammalian host cell, which subvert diverse signal transduction pathways implicated in actin dynamics, phagocytosis, and innate immunity. The genomes of sequenced EHEC O157:H7 strains contain two copies of the effector protein gene nleH, which share 49% sequence similarity with the gene for the Shigella effector OspG, recently implicated in inhibition of migration of the transcriptional regulator NF-κB to the nucleus. In this study we investigated the role of NleH during EHEC O157:H7 infection of calves and lambs. We found that while EHEC ΔnleH colonized the bovine gut more efficiently than the wild-type strain, in lambs the wild-type strain exhibited a competitive advantage over the mutant during mixed infection. Using the mouse pathogen Citrobacter rodentium, which shares many virulence factors with EHEC O157:H7, including NleH, we observed that the wild-type strain exhibited a competitive advantage over the mutant during mixed infection. We found no measurable differences in T-cell infiltration or hyperplasia in colons of mice inoculated with the wild-type or the nleH mutant strain. Using NF-κB reporter mice carrying a transgene containing a luciferase reporter driven by three NF-κB response elements, we found that NleH causes an increase in NF-κB activity in the colonic mucosa. Consistent with this, we found that the nleH mutant triggered a significantly lower tumor necrosis factor alpha response than the wild-type strain.
The human diarrheal pathogens enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic E. coli (EHEC) (31) and the murine pathogen Citrobacter rodentium (reviewed in reference 28) colonize the host gastrointestinal tract via attaching and effacing (A/E) lesions, which are characterized by intimate adhesion to intestinal epithelia and localized effacement of brush border microvilli (21). EPEC was the first type of E. coli to be associated with human disease and is a major cause of infantile diarrhea in developing countries (reviewed in reference 2), while EHEC (O157 and non-O157 strains) is prevalent in developed countries and causes a wide spectrum of illnesses ranging from mild diarrhea to severe diseases such as hemorrhagic colitis and hemolytic uremic syndrome mediated by Shiga toxins (31).
EPEC, EHEC, and C. rodentium harbor the locus of enterocyte effacement (LEE) pathogenicity island, which is necessary for A/E lesion formation in vivo (26). The LEE encodes several gene regulators, the outer membrane adhesin intimin (18), the structural components of a type III secretion system (T3SS) (17), chaperones, and translocator and several effector proteins, including Tir (19), Map, EspG, EspF, and EspH (reviewed in reference 12), that are injected into enterocytes via the T3SS and differentially modulate cellular actin dynamics in vitro.
In addition, EPEC, EHEC, and C. rodentium use the LEE-encoded T3SS to inject a large number of effectors which are encoded by genes that are scattered around the bacterial genome, carried mainly on prophages and genomic islands (8). Among these effectors are Cif (which causes irreversible cell cycle arrest at the G2/M transition) (24), EspJ (which is involved in inhibition of receptor-mediated phagocytosis) (23), TccP/EspFU and TccP2 (which can activate N-WASP) (1, 13, 39), EspI/NleA (which affects exocytosis) (16, 29), and NleH of unknown function (34).
EHEC infection in humans frequently results from direct or indirect contact with ruminant feces; however, the molecular mechanisms underlying colonization of reservoir hosts are ill defined. The LEE-carried genes required for A/E lesion formation (e.g., those for Tir and intimin) play pivotal roles in colonization of such hosts by EHEC O157:H7 (4, 7, 37), and structural components of the T3SS were found by signature-tagged mutagenesis to be required for colonization of calves by EHEC O157:H7 and O26:H− (9, 36). In addition to Tir, EspK has been shown to influence colonization of the bovine intestine (38), while Map (9), TccP/EspFU (37), and NleD (25) had no measurable effect. The roles of other T3SS effectors in colonization of ruminants are not known.
C. rodentium is a natural mouse pathogen that, while causing colonic hyperplasia, shares many virulence factors with EPEC and EHEC (reviewed in reference 28). Following inoculation via the oral route, bacteria colonize the colon, typically peaking at day 9 before clearance at around day 17 (27). Recently we refined the C. rodentium mouse model by developing noninvasive real-time bioluminescence imaging (BLI) to monitor infection dynamics and tissue tropism in vivo (41). Using this method, we have shown that C. rodentium first targets the murine cecal patch and rectum before the infection spreads to the large intestine.
The genome sequences of EHEC O157:H7 strains EDL933 and Sakai, EPEC strain E2348/69, and C. rodentium strain ICC168 revealed that EHEC and EPEC contain two nleH alleles (34; A. Iguchi et al., unpublished data), while C. rodentium harbors only one nleH gene. NleH shares 49% sequence similarity with the Shigella flexneri T3SS serine/threonine kinase effector protein OspG, which prevents ubiquitination and subsequent degradation of phospho-IκBα and downstream activation of the transcriptional factor NF-κB, possibly via the phosphorylation of the E3 ubiquitin ligase SCFβ-TrCP (Skp-Cullin-F box protein) complex (20). NF-κB proteins are transcriptional factors that, when activated, control the transcription of a large number of genes, many of which are involved in the immune (inflammatory) response. The similarities between NleH and OspG prompted us to investigate its role in colonization and activation of the NF-κB pathway in vivo.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
The bacterial strains, plasmids, and primers used in this study are described in Tables 1, 2, and 3, respectively. Bacteria were grown at 37°C in Luria-Bertani (LB) broth or agar supplemented with ampicillin (100 μg ml−1), chloramphenicol (25 μg ml−1), kanamycin (50 μg ml−1), and nalidixic acid (Nal) (15 to 25 μg ml−1) as appropriate. C. rodentium ICC169 ΔnleH, luminescent C. rodentium ICC180 ΔnleH, and EHEC O157:H7 85-170 Nalr ΔnleH1 ΔnleH2 mutant strains (strains ICC229, ICC285, and ICC232, respectively) were generated using the one-step PCR λ Red-mediated mutation protocol (6) (Table 1). Primers 1 and 2 (Table 3) were used to amplify the kanamycin cassette in pKD4 for deletion of nleH in ICC169. Primers 3 and 4 were used to amplify the chloramphenicol cassette in pKD3 for deletion of nleH in ICC180. For construction of the EHEC O157:H7 85-170 Nalr ΔnleH1 ΔnleH2 mutant, primers 5 and 6 for nleH1 and 7 and 8 for nleH2 were used for amplification of the kanamycin cassette from pKD4. Prior to deletion of nleH1 from 85-170 ΔnleH2, the kanamycin cassette was deleted as described previously (6) using the pCP20 vector (3).
TABLE 1.
Strains
| Species and strain | Characteristicsa | Source or reference |
|---|---|---|
| C. rodentium | ||
| ICC169 | Spontaneous Nalr mutant of wild-type C. rodentium | 40 |
| ICC229 | ICC169 ΔNleH1 (Kanr) | This study |
| ICC180 | Bioluminescent strain harboring the Photorhabdus luxCDABE operon (Kanr) | 40 |
| ICC285 | ICC180 ΔNleH1 (Cmr) | This study |
| E. coli | ||
| 85-170 | Spontaneous Stx1− and Stx2− EHEC O157:H7 strain | 35 |
| 85-170 Nalr | Spontaneous Nalr derivative of 85-170 | 33 |
| ICC232 | 85-170 Nalr ΔNleH1ΔNleH2 (Nalr Kanr Cmr) | This study |
| ICC299 | Commensal strain isolated from the cecum of a C57BL/6 mouse | This study |
Kanr, Cmr, and Nalr, kanamycin, chloramphenicol, and nalidixic acid resistant, respectively.
TABLE 2.
Plasmids
| Plasmid | Characteristics | Source or reference |
|---|---|---|
| pKD3 | oriRg blaM; Cmr cassette flanked by FRT sites | 6 |
| pKD4 | oriRg blaM; Kanr cassette flanked by FRT sites | 6 |
| pKD46 | ori101 repA101(ts) araBp-gam-bet-exo blaM | 6 |
| pCP20 | FLP synthesis under thermal control | 3 |
| pET28-a | His6 tag expression vector | Novagen |
| pET:nleH1 | Derivative of pET28-a expressing NleH1-His6 | This study |
| pET:nleH1K159A | Derivative of pET28-a expressing NleH1K159A-His6 | This study |
TABLE 3.
Primers
| Primer no. | Primer name | Sequence (5′→3′) |
|---|---|---|
| 1 | NleH CRICC169 Fw | ATGTTATCACCAGCTCCTGTAAATTTGGGATGTTCATGGAATTCTTTAACTGTGTAGGCTGGAGCTGCTTCG |
| 2 | NleH CRICC169 Rv | AATTCTACTTAATACCACTCTGATAAGATCTTGCTTTCCTCCATGATAAGCATATGAATATCCTCCTTAG |
| 3 | NleH CRICC180 Fw | ATGTTATCACCAGCTCCTGTAAATTTGGGATGTTCATGGAATTCTTTAACGTATACTTATAGGAGGAATC |
| 4 | NleH CRICC180 Rv | TTAAATTCTACTTAATACCACTCTGATAAGATCTTGCTTTCCTCCATGATACACATCCGACCTCGACGAAGC |
| 5 | NleH1 EHEC Fw | TGAAGGTTGAAATGTATGTTATCGCCATATTCTGTAAATTTGGGATGTTCTGTGTAGGCTGGAGCTGCTTCG |
| 6 | NleH1 EHEC Rv | CACTACACTGGATAAAATTACTAAATTTTACTTAATACCACACTAATAAGCATATGAATATCCTCCTTAG |
| 7 | NleH2 EHEC Fw | ATGTTATCGCCCTCTTCTATAAATTTGGGATGTTCATGGAATTCTTTAACGTGTAGGCTGGAGCTGCTTC |
| 8 | NleH1 EHEC Rv | TATCTTACTTAATACTACACTAATAAGATCCAGCTTTCCTCCGTGATAAGCATATGAATATCCTCCTTA |
| 9 | TNF-α-Fw | ATGAGCACAGAAAGCATGATC |
| 10 | TNF-α-Rv | TACAGGCTTGTCACTCGAATT |
| 11 | B-actin-Fw | AGAGGGAAATCGTGCGTGAC |
| 12 | B-actin-Rv | CAATAGTGATGACCTGGCCGT |
| 13 | IFN-γ-Fw | TGAACGCTACACACTGCATCTTGG |
| 14 | IFN-γ-Rv | CGACTCCTTTTCCGCCTTCCTGAG |
The PCR product of the resistance cassette, flanked by approximately 50 bp of nleH, was digested with DpnI and the cassette electroporated into the recipient strains carrying pKD46, encoding the λ Red recombinase. Mutants were selected on selective LB plates with kanamycin or chloramphenicol. Recombinant clones were cured of pKD46 and the mutation confirmed by PCR using primers flanking nleH and primers within the antibiotic resistance gene. Growth curves have confirmed that the mutant and wild-type stains have identical growth rates in LB and minimal media. Mutations of the nleH1 and nleH2 genes created using the same method in EPEC strains were successfully complemented in trans during in vitro studies.
Oral inoculation of calves.
All animal experiments were performed in accordance with the Animals (Scientific Procedures) Act 1986 and were approved by the local Ethical Review Committee. Groups of four 12-day-old Friesian bull calves were separately inoculated with approximately 1010 CFU of wild-type 85-170 Nalr or 85-170 Nalr ΔnleH1 ΔnleH2 as described previously by Stevens et al. (33). The magnitude and duration of fecal excretion of the bacteria were followed daily for 14 days. Wild-type and mutant bacteria were enumerated by plating of triplicate serial dilutions of fresh feces collected by rectal palpation onto sorbitol-MacConkey agar supplemented with potassium tellurite (2.5 mg ml−1) (T-SMAC) containing 25 μg ml−1 Nal (T-SMAC-Nal) and onto T-SMAC-Nal containing 50 μg ml−1 kanamycin, respectively. Recovery of wild-type and mutant bacteria was confirmed by PCR from selected colonies using nleH-flanking primers. The sensitivity of detection was 102 CFU/g. Fecal excretion data were statistically analyzed for the effect of mutation by means of an F test, with the data taken as repeated measurements and the animal as a covariant (Proc Mixed, Statistical Analysis System 1995; SAS Institute, Cary, NC). P values of <0.05 were considered significant.
Oral inoculation of lambs.
Fifteen 6-week-old crossbred lambs were randomly divided into three equal groups, supplied with food and water ad libitum, and confirmed to be free of E. coli O157:H7 by enrichment and O157 immunomagnetic separation. All lambs were housed in biosecure containment level 2 accommodations. Each group was housed in a separate room with its own air handling. The animals were visited by experienced staff, who changed protective clothing between groups. Five lambs were each dosed orally with 109 CFU of either wild-type 85-170 Nalr or the isogenic ΔnleH1 ΔnleH2 mutant separately, or with wild-type 85-170 Nalr and the ΔnleH1 ΔnleH2 mutant together, suspended in 10 ml of 0.1 M phosphate-buffered saline (PBS) (pH 7.4). Approximately 24 h after oral inoculation and as required thereafter for up to 28 days, rectal fecal samples from each lamb were collected for direct plating onto SMAC supplemented with either 15 μg of Nal/ml or 25 μg of kanamycin/ml. Samples that were O157 negative on direct plating were enriched in buffered peptone water for 6 h at 37°C and then plated onto SMAC plates supplemented with the appropriate antibiotic. Representative colonies were confirmed to be E. coli O157 by latex agglutination (Oxoid). For coinfection studies, the competitive index (CI) was calculated; it is defined as the ratio between the mutant and wild-type strains within the output (bacteria recovered from the host after infection) divided by their ratio within the input (initial inoculum). For this experiment, the input ratio was 1:1. The null hypothesis that CI = 1 was tested by a two-sided t test.
Murine models.
Specific-pathogen-free female 6- to 8-week-old mice were used in this study. Wild-type inbred C57BL/6 and DBA-1 mice were purchased from Harlan UK Ltd. (Bicester, United Kingdom), while the transgenic light-producing animal model DBA-1 NF-κB-luc (Oslo) (P/N 119335) was purchased from Xenogen-Caliper Corp. (Alameda, CA). The transgenic light-producing animal model NF-κB-RE-luc (Oslo)-Xen, commonly called NF-κB-RE-luc (Oslo), carries a transgene containing three NF-κB responsive-element (RE) sites from the immunoglobulin κ light-chain promoter and modified firefly luciferase cDNA. All animals were housed in individually HEPA-filtered cages with sterile bedding and free access to sterilized food and water. Independent experiments were performed at least twice (but only once for histology) using at least four mice per group.
Oral infection of mice, harvesting and collection of tissues, and bacterial stool counts.
Mice were orally inoculated using a gavage needle with 200 μl of overnight LB-grown bacterial suspension in PBS (∼5 × 109 CFU). The number of viable bacteria used as the inoculum was determined by retrospective plating onto LB agar containing antibiotics. Stool samples were recovered aseptically at various time points after inoculation and the number of viable bacteria per gram of stool determined after homogenization at 0.1g ml−1 in PBS and plating onto LB agar containing the appropriate antibiotics. At selected time intervals postinfection, blood was collected by cardiac puncture and mice were sacrificed by cervical dislocation. Sections of distal colon were collected and snap frozen in liquid nitrogen before storage at −70°C prior to analysis. For coinfection studies, the CI was calculated as described previously by Mundy et al. (30). For this experiment, the input ratio of wild-type strain to mutant strain was approximately 1:2. The null hypothesis that CI = 1 was tested by a nonparametric Wilcoxon two-tailed test using the GraphPad InStat software (GraphPad Software Inc., San Diego, CA).
In vivo BLI.
Prior to imaging, the abdominal region of each mouse was depilated to minimize any potential signal impedance by melanin within pigmented skin and fur. Bioluminescence (photons s−1 cm−2 sr−1) from living infected animals was measured after gaseous anesthesia with isofluorane using the IVIS50 camera system (Xenogen-Caliper Corp., Alameda, CA). The sample shelf was set to position D (field of view, 15 cm). A photograph (reference image) was taken under low illumination prior to quantification of photons emitted from strain ICC180 at a binning of 4 over 1 to 10 min using the software program Living Image (Xenogen-Caliper Corp.) as an overlay on Igor (Wavemetrics, Seattle, WA). For anatomical localization, a pseudocolor image representing light intensity (from blue [least intense] to red [most intense]) was generated using the Living Image software and superimposed over the gray-scale reference image. Bioluminescence within specific regions of individual mice was also quantified using the region-of-interest tool in the Living Image software program (given as photons s−1). For expression of light from the NF-κB reporter mice, 180 mg kg−1 d-luciferin (Gold Biotechnology, St. Louis, MO) dissolved in 250 μl PBS (pH 7.8) was administered by intraperitoneal injection 5 min prior to imaging. As a positive control for luciferase gene expression from the NF-κB-RE-luc (Oslo) mice, tumor necrosis factor alpha (TNF-α) (2 μg per mouse) was administered by intraperitoneal injection.
Histopathology.
Segments of the cecum and the terminal colon of each mouse were collected at 9 and 14 days postinoculation, rinsed of their content, and fixed in 10% buffered formalin for microscopic examination. Formalin-fixed tissues were then processed, paraffin embedded, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E) according to standard techniques. Sections were examined by light microscopy for the presence of intimately adhering bacteria on intestinal cells, as previously described (15). Crypt length was also evaluated, and the lengths of at least four well-oriented crypts were measured for each section. A nonparametric analysis of variance (ANOVA) with a posteriori comparisons was performed using commercially available GraphPad InStat v3.06 software (GraphPad Software Inc., San Diego, CA). P values of ≤0.05 were considered significant.
Immunohistochemistry.
Snap-frozen colonic tissues, embedded in OCT mounting medium (VWR BDH, Lutterworth, United Kingdom), were sectioned using a cryostat to a thickness of 5 μm. Sections were mounted on polysine slides (VWR BDH) and air dried overnight before fixing in acetone at room temperature for 20 min. After air drying for 1 h, sections were rehydrated in Tris-buffered saline (TBS) for 5 min and then incubated with antibodies against CD3, CD4, and CD8 (Serotec, Oxford, United Kingdom) at a dilution of 1:50 to 1:100 for 1 h. Sections were gently washed with TBS three times before addition of biotinylated anti-rat immunoglobulin G (Serotec) at a dilution of 1:200 with 4% (vol/vol) normal murine serum for blocking (Sera Laboratories International, Horsted Keynes, United Kingdom) for 30 min. After washing, a 1:200 dilution of 0.1% avidin-peroxidase (Sigma-Aldrich, Dorset, United Kingdom) was added and left for 30 min before further washing and treatment with diaminobenzadine substrate (Sigma-Aldrich) for 5 min. The reaction was stopped with excess TBS, and sections were counterstained with Mayer's hematoxylin (Sigma-Aldrich) for 30 s, dipped in acid alcohol, and washed in tap water for 5 min. Sections were dehydrated through an ethanol gradient of 70%, 90%, and 100% solutions (2 min each), followed by clearing in Histoclear (VWR BDH) and mounting in DPX (VWR BDH). A control slide using no primary antibody was also made to show endogenous peroxidase-containing cells. Stained cell populations were counted in five randomly selected fields per section, and data were expressed as the number of T cells per 250 μm2 of lamina propria.
SEM.
Intestinal segments were fixed in 2.5% glutaraldehyde and processed for scanning electron microscopy (SEM) as previously described (14). SEM samples were examined blindly at 25 kV using a JEOL JSM-5300 SEM [JEOL (UK) Ltd., Herts, United Kingdom].
IFA.
An indirect immunofluorescence assay (IFA) was used for the detection of C. rodentium (serotype O152) in formalin-fixed, paraffin-embedded sections as previously described (14). Tetramethyl rhodamine isothiocyanate-conjugated donkey anti-rabbit (Jackson ImmunoResearch Europe Ltd., Soham, Cambridgeshire, United Kingdom) secondary antibody was used to visualize O152-positive bacteria, while DNA of both bacteria and epithelial cells was counterstained with Hoechst 33342 (Sigma-Aldrich Co., United Kingdom). Sections were examined with an Axio Imager M1 microscope (Carl Zeiss MicroImaging GmbH, Germany). Images were acquired using an AxioCam MRm monochrome camera and computer processed using AxioVision (Carl Zeiss MicroImaging GmbH, Germany) and Adobe Photoshop 5.0 and Adobe Illustrator 8.0 software (Adobe Systems Incorporated, CA).
RNA extraction and quantitative RT-PCR.
Total RNA was isolated from frozen colonic tissue using the RNeasy Plus minikit (Qiagen). All tissues used were harvested from mice 14 days after oral gavage. Total RNA was measured using a Nanodrop. TNF-α or gamma interferon (IFN-γ) and β-actin mRNAs were measured by semiquantitative reverse transcription-PCR (RT-PCR) using primers 9 to 14 listed in Table 3 and the one-step Reverse-iT hot-start kit (Thermo). The PCR amplification cycle was 20 s at 94°C, 30 s at 60°C, and 60 s at 72°C for 35 cycles. One microgram of RNA was used for measurement of TNF-α and IFN-γ transcript levels, and 100 ng of RNA was used for detection of β-actin mRNA. The PCR products were run on a 1% agarose gel alongside the 100-bp ladder from NEB in Tris-borate-EDTA buffer. GeneTools (Syngene) was used to conduct densitometric analysis. All TNF-α/β-actin and IFN-γ/β-actin ratios were compared between mice infected with wild-type and nleH mutant bacteria. Statistical analyses were conducted with the one-way ANOVA Bonferroni multiple-comparison test using the GraphPad InStat software (GraphPad Software Inc., San Diego, CA), as all groups displayed normal distributions.
RESULTS
The NleH T3SS effector homologues.
NleH belongs to a family of T3SS effectors found in diverse enteric pathogens. EPEC O127:H6 E2348/69 and EHEC O157:H7 EDL933 and Sakai contain two NleH paralogues, which share 83 to 84% protein identity. C. rodentium contains only one nleH gene, which shares 83 and 81% amino acid sequence identity with EHEC O157:H7 NleH1 and NleH2, respectively. NleH shares 49% amino acid sequence similarity with the serine/threonine kinases OspG and YspK (Yersinia secreted protein kinase) of Yersinia enterocolitica.
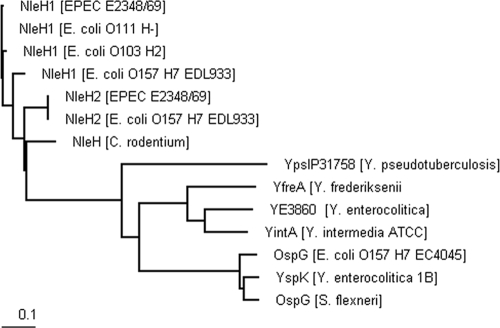
We employed BLASTp searches of the NCBI protein database using EPEC E2348/69 NleH1 as the query sequence. The Clustal W2 software (EMBL-EBI) was then used to create a phylogram of the different homologues (Fig. 1). Two major clusters were formed, one containing all the NleH effectors and the other containing closer homologues of OspG, including an OspG-like effector (90.8% identity to OspG of Shigella) from EHEC O157:H7 strain EC4045. To determine the role of NleH proteins in vivo, we generated EHEC O157:H7 (strain 85-170) ΔnleH1 ΔnleH2 and C. rodentium (ICC169 and bioluminescent strain ICC180) ΔnleH mutants.
FIG. 1.
Distance tree of the NleH family. Homologues were identified using BLASTp searches of NCBI nr protein database with EPEC 2348/69 NleH1 as the query sequence. Protein sequences selected and analyzed included E. coli O111:H− NleH1 (GI:164457622), E. coli O103:H2 NleH1 (GI:164457632), E. coli O157:H7 EDL933 NleH2 (GI:15801938), C. rodentium NleH (GI:44888794), phage cdtI gp23 (GI:148609405), Y. pseudotuberculosis IP 31758 (GI:153930640), Y. frederiksenii ATCC 33641 YfreA (GI:77972806), Y. enterocolitica subsp. enterocolitica 8081 (GI:123442682), Y. intermedia ATCC 29909 YintA (GI:77977119), Y. enterocolitica subsp enterocolitica 8081 YspK (GI:13442682), S. flexneri OspG (GI:13449175), and E. coli O157:H7 str EC4045 OspG (GI:168711271), as well as EPEC 2348/69 NleH2 (unpublished). The BioEdit software was used to create a ClustalW alignment. Clustal W2 (EMBL-EBI ebi.ac.uk) was used to create a phylogram, and TreeView (Bioedit) was used for visualization.
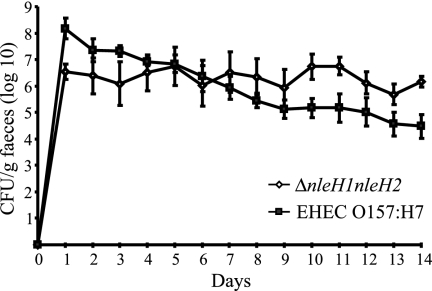
The EHEC O157:H7 ΔnleH1 ΔnleH2 double mutant is shed in greater numbers than the parental strain from orally challenged calves.
To assess the role of NleH in intestinal colonization of calves by EHEC O157:H7, wild-type and mutant bacteria were separately inoculated into four 12-day-old Friesian bull calves. Both wild-type and mutant strains colonized the calves efficiently, and the mutant was excreted at lower levels than the wild type during the first 4 days. From day 7 the mutant was shed at higher levels than the wild-type strain, and the difference became statistically significant (P < 0.05) from day 10 onwards (Fig. 2). The course of the fecal excretion of the wild-type strain was consistent with previously observed patterns (10, 33, 37).
FIG. 2.
Course of fecal excretion of EHEC O157:H7 following oral challenge of 12-day-old calves with wild-type strain 85-170 Nalr or the 85-170 Nalr ΔnleH1 ΔnleH2 mutant. Data represent the mean daily fecal count (n = 4 per strain) ± standard error of the mean.
Contribution of NleH1 and NleH2 to colonization of conventional 6-week-old-lambs.
A 6-week-old lamb model was next used to compare the persistence of an E. coli O157:H7 ΔnleH1 ΔnleH2 double mutant and the isogenic wild-type strain. The ability of the mutant to establish itself and persist in lambs was investigated by monitoring the viable counts recovered in fecal pellets collected per animal. When given as a single inoculum, the wild-type E. coli O157:H7 isolate produced the classical shedding pattern in lambs, as described previously (5, 42), persisting in relatively high numbers during the early stages of infection and then declining by day 11 postinoculation (Fig. 3A). The ΔnleH1 ΔnleH2 mutant demonstrated a similar shedding pattern, except that positive fecal samples were noted only until day 12 postinoculation (Fig. 3A). When both isolates were administered in the same inoculum, the wild type persisted for 4 days longer than the ΔnleH1 ΔnleH2 mutant (data not shown). The mean CI was significantly less than 1 for all time points where it could be calculated except day 1 (Fig. 3B), demonstrating that the wild-type strain outcompeted the ΔnleH1 ΔnleH2 mutant in the ovine model.
FIG. 3.
(A) Course of fecal excretion of EHEC O157:H7 following oral challenge of 6-week-old lambs with wild-type strain 85-170 Nalr or the 85-170 Nalr ΔnleH1 ΔnleH2 mutant. Data represent the mean daily fecal count (n = 5 per strain) ± standard error of the mean. (B) CI of EHEC O157: H7 85-170 Nalr ΔnleH1 ΔnleH2 in lambs infected with an inoculum containing a 1:1 ratio of wild-type EHEC and the ΔnleH1 ΔnleH2 double mutant. The null hypothesis that CI = 1 was tested by a two-sided t test and shows that the mean CI postchallenge is significantly less than 1 for all time points where it could be calculated except day 1.
Contribution of NleH to colonization in the C. rodentium murine model.
We followed the anatomical localization and pathogenic burden using BLI and viable counts in stools of mice infected with either wild-type or ΔnleH C. rodentium. Using BLI, we observed that both the wild-type and mutant strains colonized the cecal patch and rectum by day 3 to 4 postinfection. After adaptation to the in vivo environment (day 6 to 7), the entire distal colon was then heavily infected. Clearance began by day 10, at which point light intensity decreased. By day 14 the gastrointestinal tract had mostly cleared the infection (Fig. 4). Viable counts of bacteria in stool mirrored the results obtained by BLI; no significant differences in viable bacterial counts of the wild-type and ΔnleH mutant strains were seen (Fig. 5A). However, the ΔnleH C. rodentium mutant was significantly out competed by the wild-type strain in a mixed infection (Fig. 5B).
FIG. 4.
Anatomical localization of luminescent wild-type C. rodentium ICC180 and ΔnleH strains during in vivo infection of mice. Images were acquired using an IVIS50 system and displayed as pseudocolor images of bioluminescence. The variations in color represent light intensity at a given location, with red being the most intense light emission and blue being the weakest signal. The colored scale to the right indicates relative signal intensity in photons s−1cm−2 sr−1. At each time point postgavage, mice were imaged with an integration time of 1 min.
FIG. 5.
(A) Course of fecal excretion following oral challenge of groups of six 6- to 8-week-old C57BL/6 mice with either wild-type C. rodentium ICC180 or the ΔnleH mutant. (B) Mean CI of C. rodentium ΔnleH in mice infected with an inoculum containing a 1:2 ratio of wild-type C. rodentium and the ΔnleH mutant. The null hypothesis that CI = 1 was tested by a two-tailed nonparametric Wilcoxon test and showed that the mean CI postchallenge is significantly less than 1 for all time points where it could be calculated.
NleH does not affect A/E lesion formation and T-cell infiltration in the C. rodentium murine model.
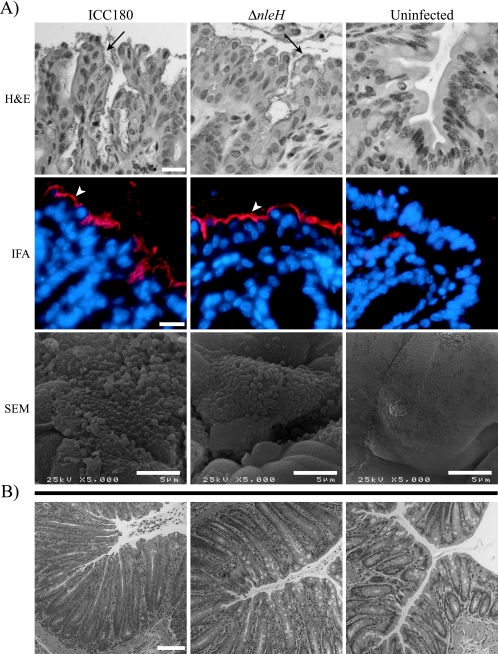
The hallmarks of C. rodentium infection include induction of extensive colonic hyperplasia and influx of T cells into the colonic lamina propria. Histological examination and measurement of crypt length did not reveal any differences between the parent and mutant strains (data not shown). Immunohistochemistry, performed to investigate the influx of CD3+, CD4+, and CD8+ T cell subsets into the colonic lamina propria at 14 days postinoculation, revealed that although the ΔnleH mutant-infected animals showed slightly fewer CD3- and CD4-positive T cells in the lamina propria, no significant differences were observed between animals infected with the mutant or wild-type C. rodentium strains (data not shown). Immunofluorescent staining with anti-O152 and SEM analysis at day 9 or day 14 postinoculation did not reveal any differences between the wild-type and ΔnleH C. rodentium strains (Fig. 6).
FIG. 6.
Interaction of wild-type C. rodentium strain ICC180 and its ΔnleH mutant with the colonic epithelium in vivo. (A) At day 9 postchallenge, typical foci of intimately adherent bacteria (arrows) were observed on H&E-stained sections, accompanied by a highly disorganized epithelium. IFA staining revealed these bacteria to be of the O152 serotype (arrowheads), corresponding to C. rodentium serotype. These foci of intimately adherent bacteria were confirmed to be A/E lesions by SEM. Neither O152-positive bacteria nor A/E lesions were observed on sections or samples derived from uninfected mice. (B) No adherent bacteria were observed in the colons of mice infected with ICC180 and the ICC180 ΔnleH mutant at day 14 postchallenge, although crypt hyperplasia was still present. For the IFA panel: Hoechst 33342 (blue, false color), DNA; tetramethyl rhodamine isothiocyanate (intense blue, false color), O152-positive bacteria. Representative micrographs are shown. Bar, 20 μm (H&E and IFA) or 100 μm (B).
NleH influences NF-κB levels and expression of TNF-α in vivo.
A recent study has shown that OspG inhibits degradation of IκB and hence activation of NF-κB and that a Shigella ospG mutant induces a stronger inflammatory response than the wild-type strain after inoculation of rabbit ileal loops (20). In order to determine if NleH influences activation of NF-κB in vivo, we infected NF-κB-RE-luc reporter mice with C. rodentium wild-type strain ICC169 or ICC169 ΔnleH; mice inoculated with the commensal E. coli strain ICC299 (Table 1) or PBS were used as controls. The number of nleH mutant bacteria shed from this mouse strain was similar to that of the wild type (data not shown). No significant differences in whole-body (including chest, neck, abdomen, and rectum) luminescence counts were recorded by live imaging, at any time point, between PBS-gavaged animals and those gavaged with wild-type C. rodentium, the nleH mutant, or the commensal E. coli. On day 14 postchallenge, we recorded luminescence counts in different organs. While no significant difference in the luminescence counts was seen in the mesenteric lymph nodes, spleens, and ceca of the different mouse groups (Table 4), there was a significant (P = 0.0024) 2.5-fold increase in the signal from the colon in mice infected with the wild-type C. rodentium compared to those inoculated with C. rodentium ΔnleH, E. coli ICC299, or PBS (Fig. 7A and B). This difference was similar to the three- to fourfold-increased luminescent signal seen after mice were inoculated with TNF-α as a positive control (data not shown).
TABLE 4.
Specific bioluminescence from each organ at harvest
| Inoculum | Specific bioluminescence (mean ± SD) from:
|
||||
|---|---|---|---|---|---|
| Cecum | Mesenteric lymph nodes | Spleen | Colon | Intestines | |
| ICC169 | 2.59 × 105 ± 8.00 × 104 | 2.35 × 106 ± 9.47 × 105 | 9.25 × 104 ± 2.29 × 104 | 1.41 × 106 ± 2.92 × 105 | 1.72 × 106 ± 4.69 × 105 |
| ΔnleH mutant | 1.80 × 105 ± 9.89 × 104 | 2.75 × 106 ± 1.26 × 106 | 2.12 × 105 ± 2.38 × 105 | 5.69 × 105 ± 2.53 × 105 | 2.08 × 106 ± 1.04 × 106 |
| PBS | 3.61 × 105 ± 8.78 × 104 | 3.02 × 106 ± 8.61 × 105 | 8.33 × 104 ± 1.71 × 104 | 6.34 × 105 ± 2.37 × 105 | 1.53 × 106 ± 6.35 × 105 |
| Commensal strain | 2.69 × 105 ± 4.42 × 104 | 2.58 × 106 ± 8.11 × 105 | 1.39 × 105 ± 2.81 × 104 | 5.02 × 105 ± 1.03 × 105 | 2.38 × 106 ± 4.27 × 105 |
FIG. 7.
NF-κB activation in organs of mice at 14 days postchallenge. Luminescence was measured in extracted organs. NF-κB-RE-luc reporter mice were inoculated with wild-type (strain ICC169) or ΔnleH C. rodentium. (A) Representative bioluminescence images captured using the IVIS50 camera system are shown (cecum [1], mesenteric lymph nodes [2], colon [3], spleen [4], and intestine [5]. Luminescence was quantified using the region-of-interest tool in the Living Image software (given as photons s−1). (B) Graph of luminescence in extracted colons shows a significant loss of NF-κB activation in the absence of nleH. ***, P < 0.005.
As NF-κB induces the transcription of many proinflammatory cytokines such as TNF-α and IFN-γ, we compared the levels of their transcripts in uninfected mice and mice infected with either wild-type or ΔnleH mutant C. rodentium. RNA extracted from distal colons of mice was subjected to semiquantitative RT-PCR. Transcript levels were quantified as a ratio of TNF-α or IFN-γ to β-actin. This revealed that the levels of TNF-α mRNA were significantly higher in tissues extracted from infected mice than in those from uninfected control mice. Mice infected with the wild-type C. rodentium had significantly higher TNF-α transcript levels than those infected with the ΔnleH mutant (Fig. 8). The levels of IFN-γ transcript were comparable in all mouse groups (data not shown).
FIG. 8.
Semiquantitative TNF-α and β-actin RT-PCRs were performed on RNA isolated from colons of uninfected mice or mice orally challenged with wild-type or ΔnleH C. rodentium. Median TNF-α levels (measured as a ratio of TNF-α to β-actin) in C. rodentium-infected mice were considered the basal level of activation by wild-type bacteria in each experiment and taken as 100%. All TNF-α/β-actin ratios were compared to this and expressed as a percentage of the median TNF-α level during wild-type infection. GeneTools (Syngene) was used for densitometric analysis. Two repetitions, each using four or five mice per group, were conducted and graphed. The one-way ANOVA Bonferroni multiple-comparison test was used to determine significance, as the three groups had normal distributions. ***, P < 0.001.
DISCUSSION
Many gram-negative bacterial pathogens use T3SS effectors to modulate host cell signaling pathways. Effector proteins can trigger local (e.g., alteration in the cytoskeleton) or systemic (e.g., immune response) changes. Colonization of the mucosal surface requires temporal modulation of the host immune status. While downregulation of innate immunity might aid the pathogen to launch an infection and to reach a critical mass, activation of the immune system might assist the pathogen in its competition with the resident gut microflora. It is therefore not surprising that pathogenic bacteria are equipped with virulence factors which have antagonistic immune modulation activities. Indeed, in a recent report Lupp et al. demonstrated that C. rodentium population expansion in vivo is mediated by an inflammatory response that disrupts the endogenous intestinal microbiota (22). In contrast, using polarized culture models, Ruchaud-Sparagano et al. have shown that EPEC inactivates innate immune responses in vitro (32).
In this study we investigated the role of NleH in colonization, competitiveness, and activation of the NF-κB pathway in vivo. EPEC O127:H6 E2348/69 and EHEC O157:H7 EDL933 and Sakai contain two, almost identical, copies of nleH, while C. rodentium harbors only a single nleH gene. The functional consequences of this gene duplication are not known. Interestingly, a recent shotgun sequencing of a number of EHEC O157:H7 isolates has shown that they contain, in addition to nleH, a gene whose product shares 90.8% sequence identity with OspG (NCBI BLASTp).
Investigation of the contribution of NleH toward colonization and competitiveness of EHEC O157:H7 in bovine and ovine hosts, which are important animal reservoirs of the pathogen, showed that the EHEC O157 ΔnleH1 ΔnleH2 double mutant was shed in greater numbers than the parental strain from orally challenged calves, significantly from day 10 postinoculation. The precise effect of deleting nleH on factors or regulatory mechanisms involved in EHEC O157:H7 colonization in bovine intestines remains to be investigated. In single-infection studies with lambs, there was no statistical difference in shedding after oral inoculation with the same strains. However, CIs measured following oral inoculation of lambs with a mixture of wild-type EHEC O157:H7 and the ΔnleH1 ΔnleH2 double mutant revealed that the mutant was significantly outcompeted. The reasons for the different phenotypes observed in the bovine and ovine models are currently not known. However, these results show that effector proteins might have dissimilar or even opposite functions in different hosts.
In order to determine the contribution of NleH to infection with C. rodentium, we mutated nleH in the wild-type ICC169 and luminescent ICC180 strains. Deletion of nleH from C. rodentium had no significant effect on in vivo tissue tropism, bacterial burden, colonic hyperplasia, or infiltration of CD3+, CD4+, and CD8+ cells to the lamina propria. A subtle phenotype was recently reported for C. rodentium ΔnleH in single infection, as expansion of the mutant population in vivo lagged behind that of the wild-type population during early stages (6 days postchallenge), although at later stages (10 days) the mutant and wild type colonized at comparable levels (11). The reasons for the different results might be due to the mouse status (i.e., composition of the normal gut flora), preparation of the inocula, or bacterial strains. Importantly, we found that in a mixed infection the nleH mutant was significantly outcompeted by the wild-type strain, suggesting that expression of NleH increases the bacterial fitness in vivo. This could explain the need for conservation and multiplicity of the gene in EHEC and EPEC strains.
As we did not observe any difference in colonization and clearance dynamics, hyperplasia, and T-cell infiltration between the wild-type and ΔnleH mutant strains, NleH appears to have a local rather than systemic role, possibly in displacing the normal gut flora. Considering that both NleH (C. Hemrajani, unpublished data) and OspG (20) are protein kinases and share a high level of sequence identity, we investigated, using reporter mice, if NleH plays a role in activation of the NF-κB pathway. Unexpectedly, we found lower activation of NF-κB in the colon in mice infected with the C. rodentium ΔnleH mutant than in those infected with the parental wild-type strain. Consistent with these results, we found lower colonic levels of TNF-α at 14 days postchallenge in mice infected with the ΔnleH C. rodentium mutant than in those infected with the wild-type strain, while no difference was recorded for IFN-γ. These data suggest that NleH triggers local activation of NF-κB, which in turn leads to a differential increase in the levels of proinflammatory cytokines.
Collectively these results demonstrate that the role of NleH during infection is host specific. NleH, one of the core and conserved T3SS effectors in A/E pathogens, is likely to work with other effectors in optimizing the level of local gut inflammatory responses and the relationship with the endogenous gut flora for the benefit of the pathogen.
Acknowledgments
We thank Robin Sayers (VLA) for the statistical analysis of the ovine data.
This work was supported by grants from the MRC and the Wellcome Trust.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 25 August 2008.
REFERENCES
- 1.Campellone, K. G., D. Robbins, and J. M. Leong. 2004. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev. Cell 7217-228. [DOI] [PubMed] [Google Scholar]
- 2.Chen, H. D., and G. Frankel. 2005. Enteropathogenic Escherichia coli: unravelling pathogenesis. FEMS Microbiol. Rev. 2983-98. [DOI] [PubMed] [Google Scholar]
- 3.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 1589-14. [DOI] [PubMed] [Google Scholar]
- 4.Cornick, N. A., S. L. Booher, and H. W. Moon. 2002. Intimin facilitates colonization by Escherichia coli O157:H7 in adult ruminants. Infect. Immun. 702704-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahan, S., S. Wiles, R. M. La Ragione, A. Best, M. J. Woodward, M. P. Stevens, R. K. Shaw, Y. Chong, S. Knutton, A. Phillips, and G. Frankel. 2005. EspJ is a prophage-carried type III effector protein of attaching and effacing pathogens that modulates infection dynamics. Infect. Immun. 73679-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 664560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng, W., J. L. Puente, S. Gruenheid, Y. Li, B. A. Vallance, A. Vazquez, J. Barba, J. A. Ibarra, P. O'Donnell, P. Metalnikov, K. Ashman, S. Lee, D. Goode, T. Pawson, and B. B. Finlay. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. USA 1013597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dziva, F., P. M. van Diemen, M. P. Stevens, A. J. Smith, and T. S. Wallis. 2004. Identification of Escherichia coli O157:H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology 1503631-3645. [DOI] [PubMed] [Google Scholar]
- 10.Dziva, F., I. Vlisidou, V. F. Crepin, T. S. Wallis, G. Frankel, and M. P. Stevens. 2007. Vaccination of calves with EspA, a key colonisation factor of Escherichia coli O157:H7, induces antigen-specific humoral responses but does not confer protection against intestinal colonisation. Vet. Microbiol. 123254-261. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Angulo, V. A., W. Deng, N. A. Thomas, B. B. Finlay, and J. L. Puente. 2008. Regulation of expression and secretion of NleH, a new non-locus of enterocyte effacement-encoded effector in Citrobacter rodentium. J. Bacteriol. 1902388-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garmendia, J., G. Frankel, and V. F. Crepin. 2005. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect. Immun. 732573-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garmendia, J., A. D. Phillips, M. F. Carlier, Y. Chong, S. Schuller, O. Marches, S. Dahan, E. Oswald, R. K. Shaw, S. Knutton, and G. Frankel. 2004. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell. Microbiol. 61167-1183. [DOI] [PubMed] [Google Scholar]
- 14.Girard, F., F. Dziva, P. van Diemen, A. D. Phillips, M. P. Stevens, and G. Frankel. 2007. Adherence of enterohemorrhagic Escherichia coli O157, O26, and O111 strains to bovine intestinal explants ex vivo. Appl. Environ. Microbiol. 733084-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard, F., I. P. Oswald, I. Taranu, P. Helie, G. D. Appleyard, J. Harel, and J. M. Fairbrother. 2005. Host immune status influences the development of attaching and effacing lesions in weaned pigs. Infect. Immun. 735514-5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruenheid, S., I. Sekirov, N. A. Thomas, W. Deng, P. O'Donnell, D. Goode, Y. Li, E. A. Frey, N. F. Brown, P. Metalnikov, T. Pawson, K. Ashman, and B. B. Finlay. 2004. Identification and characterization of NleA, a non-LEE-encoded type III translocated virulence factor of enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 511233-1249. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis, K. G., J. A. Giron, A. E. Jerse, T. K. McDaniel, M. S. Donnenberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 927996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 877839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91511-520. [DOI] [PubMed] [Google Scholar]
- 20.Kim, D. W., G. Lenzen, A. L. Page, P. Legrain, P. J. Sansonetti, and C. Parsot. 2005. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 10214046-14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knutton, S., D. R. Lloyd, and A. S. McNeish. 1987. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect. Immun. 5569-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lupp, C., M. L. Robertson, M. E. Wickham, I. Sekirov, O. L. Champion, E. C. Gaynor, and B. B. Finlay. 2007. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2204. [DOI] [PubMed] [Google Scholar]
- 23.Marches, O., V. Covarelli, S. Dahan, C. Cougoule, P. Bhatta, G. Frankel, and E. Caron. 2008. EspJ of enteropathogenic and enterohaemorrhagic Escherichia coli inhibits opsono-phagocytosis. Cell. Microbiol. 101104-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marches, O., T. N. Ledger, M. Boury, M. Ohara, X. Tu, F. Goffaux, J. Mainil, I. Rosenshine, M. Sugai, J. De Rycke, and E. Oswald. 2003. Enteropathogenic and enterohaemorrhagic Escherichia coli deliver a novel effector called Cif, which blocks cell cycle G2/M transition. Mol. Microbiol. 501553-1567. [DOI] [PubMed] [Google Scholar]
- 25.Marches, O., S. Wiles, F. Dziva, R. M. La Ragione, S. Schuller, A. Best, A. D. Phillips, E. L. Hartland, M. J. Woodward, M. P. Stevens, and G. Frankel. 2005. Characterization of two non-locus of enterocyte effacement-encoded type III-translocated effectors, NleC and NleD, in attaching and effacing pathogens. Infect. Immun. 738411-8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 921664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mundy, R., F. Girard, A. J. FitzGerald, and G. Frankel. 2006. Comparison of colonization dynamics and pathology of mice infected with enteropathogenic Escherichia coli, enterohaemorrhagic E. coli and Citrobacter rodentium. FEMS Microbiol. Lett. 265126-132. [DOI] [PubMed] [Google Scholar]
- 28.Mundy, R., T. T. MacDonald, G. Dougan, G. Frankel, and S. Wiles. 2005. Citrobacter rodentium of mice and man. Cell. Microbiol. 71697-1706. [DOI] [PubMed] [Google Scholar]
- 29.Mundy, R., L. Petrovska, K. Smollett, N. Simpson, R. K. Wilson, J. Yu, X. Tu, I. Rosenshine, S. Clare, G. Dougan, and G. Frankel. 2004. Identification of a novel Citrobacter rodentium type III secreted protein, EspI, and roles of this and other secreted proteins in infection. Infect. Immun. 722288-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mundy, R., D. Pickard, R. K. Wilson, C. P. Simmons, G. Dougan, and G. Frankel. 2003. Identification of a novel type IV pilus gene cluster required for gastrointestinal colonization of Citrobacter rodentium. Mol. Microbiol. 48795-809. [DOI] [PubMed] [Google Scholar]
- 31.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruchaud-Sparagano, M. H., M. Maresca, and B. Kenny. 2007. Enteropathogenic Escherichia coli (EPEC) inactivate innate immune responses prior to compromising epithelial barrier function. Cell. Microbiol. 91909-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens, M. P., A. J. Roe, I. Vlisidou, P. M. van Diemen, R. M. La Ragione, A. Best, M. J. Woodward, D. L. Gally, and T. S. Wallis. 2004. Mutation of toxB and a truncated version of the efa-1 gene in Escherichia coli O157:H7 influences the expression and secretion of locus of enterocyte effacement-encoded proteins but not intestinal colonization in calves or sheep. Infect. Immun. 725402-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobe, T., S. A. Beatson, H. Taniguchi, H. Abe, C. M. Bailey, A. Fivian, R. Younis, S. Matthews, O. Marches, G. Frankel, T. Hayashi, and M. J. Pallen. 2006. An extensive repertoire of type III secretion effectors in Escherichia coli O157 and the role of lambdoid phages in their dissemination. Proc. Natl. Acad. Sci. USA 10314941-14946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzipori, S., H. Karch, K. I. Wachsmuth, R. M. Robins-Browne, A. D. O'Brien, H. Lior, M. L. Cohen, J. Smithers, and M. M. Levine. 1987. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect. Immun. 553117-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Diemen, P. M., F. Dziva, M. P. Stevens, and T. S. Wallis. 2005. Identification of enterohemorrhagic Escherichia coli O26:H− genes required for intestinal colonization in calves. Infect. Immun. 731735-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vlisidou, I., F. Dziva, R. M. La Ragione, A. Best, J. Garmendia, P. Hawes, P. Monaghan, S. A. Cawthraw, G. Frankel, M. J. Woodward, and M. P. Stevens. 2006. Role of intimin-Tir interactions and the Tir-cytoskeleton coupling protein in the colonization of calves and lambs by Escherichia coli O157:H7. Infect. Immun. 74758-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlisidou, I., O. Marches, F. Dziva, R. Mundy, G. Frankel, and M. P. Stevens. 2006. Identification and characterization of EspK, a type III secreted effector protein of enterohaemorrhagic Escherichia coli O157:H7. FEMS Microbiol. Lett. 26332-40. [DOI] [PubMed] [Google Scholar]
- 39.Whale, A. D., R. T. Hernandes, T. Ooka, L. Beutin, S. Schuller, J. Garmendia, L. Crowther, M. A. Vieira, Y. Ogura, G. Krause, A. D. Phillips, T. A. Gomes, T. Hayashi, and G. Frankel. 2007. TccP2-mediated subversion of actin dynamics by EPEC 2—a distinct evolutionary lineage of enteropathogenic Escherichia coli. Microbiology 1531743-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiles, S., S. Clare, J. Harker, A. Huett, D. Young, G. Dougan, and G. Frankel. 2004. Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium. Cell. Microbiol. 6963-972. [DOI] [PubMed] [Google Scholar]
- 41.Wiles, S., K. M. Pickard, K. Peng, T. T. MacDonald, and G. Frankel. 2006. In vivo bioluminescence imaging of the murine pathogen Citrobacter rodentium. Infect. Immun. 745391-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodward, M. J., A. Best, K. A. Sprigings, G. R. Pearson, A. M. Skuse, A. Wales, C. M. Hayes, J. M. Roe, J. C. Low, and R. M. La Ragione. 2003. Non-toxigenic Escherichia coli O157:H7 strain NCTC12900 causes attaching-effacing lesions and eae-dependent persistence in weaned sheep. Int. J. Med. Microbiol. 293299-308. [DOI] [PubMed] [Google Scholar]