Abstract
The precise mechanisms by which Streptococcus pneumoniae overcomes epithelial and endothelial barriers to access underlying human tissues remain to be determined. The plasminogen system is highly important for the tissue barrier degradation which allows cell migration. Plasminogen is known to interact with pneumococci via enolase, glyceraldehyde-3-phosphate dehydrogenase, and choline-binding protein E. These observations prompted us to evaluate the role of this proteolytic system in the pneumococcal invasion process. We observed that coating of S. pneumoniae R6 strain with plasminogen or inactivated plasmin increased adherence to pulmonary epithelial A549 and vascular endothelial EaHy cells in vitro. This indicates that plasminogen-mediated adherence is independent of the protease activity and involves plasminogen binding to receptors on eukaryotic cell surfaces. Conversely, decreased adherence of bacterial cells coated with active plasmin was observed, indicating that the protease activity limits bacterial attachment on the cell surface. We were then interested in investigating the role of the proteolytic plasmin activity in the traversal of tissue barriers. We observed that adherence of plasmin-coated D39 (encapsulated) or R6 (unencapsulated) pneumococci induced sporadic disruptions of EaHy and A549 monolayer cell junctions. This was not observed when plasmin was inhibited by aprotinin. Endothelial junction disorganization may proceed by proteolysis of the cell junction components. This is supported by our observation of the in vitro cleavage by plasmin bound to pneumococci of recombinant vascular endothelial cadherin, the main component of endothelial adherens junctions. Finally, junction damage induced by plasmin may be related to tissue barrier traversal, as we measured an increase of S. pneumoniae transmigration across epithelial A549 and endothelial EaHy layers when active plasmin was present on the bacterial surface. Our results highlight a novel function for the plasminogen recruitment at the bacterial surface in facilitating adherence of pneumococci to endothelial and epithelial cells, while active plasmin degrades intercellular junctions. This process promotes migration of pneumococci through cell barriers by a pericellular route, a prerequisite for dissemination of S. pneumoniae in the host organism.
Streptococcus pneumoniae, a major human pathogen, claims about 1.6 million lives per year worldwide according to the WHO, involving mostly young children, the elderly, and immunocompromised patients. S. pneumoniae is the leading cause of noninvasive diseases like otitis, sinusitis, and pneumonia and invasive diseases such as sepsis and meningitis. Pneumococci colonize asymptomatically the nasopharynx but disseminate into the lungs, where they cause pneumonia, which can degenerate into sepsis. This implies that S. pneumoniae gains access to the systemic blood circulation from the alveolar space, suggesting interactions with the pulmonary epithelial cells and the vascular endothelial cells of the alveolar capillaries (10). However, the precise mechanisms by which S. pneumoniae overcomes these cellular boundaries to access the underlying tissues remain to be determined.
Intracellular uptake of S. pneumoniae by pulmonary epithelial A549 cells was observed by transmission electron microscopy: pneumococci were either enclosed within vacuoles of intact cells or free in the cytoplasm of damaged cells (26). Endocytosis of S. pneumoniae seems to be dependent on the platelet-activating factor receptor, and uptake of the vacuole containing pneumococci involves clathrin (25). Recently, another mechanism allowing S. pneumoniae migration through the epithelial barrier was described: Beisswenger et al. proposed that inflammatory responses to the pneumococcus contribute to cell barrier disruption and promote invasion (4).
Pathogens are known to penetrate the epithelial or endothelial barriers, due to the proteolytic cleavage of cell junctions following the recruitment of host proteases. Group A streptococci (GAS), displaying active human plasmin, penetrate through transwell-grown pharyngeal epithelial cells in a significantly higher number than untreated GAS, highlighting a new function of plasminogen-converted plasmin, i.e., the ability to pericellularly invade cells as a result of proteolytic activity (23). Similarly, plasmin at the surface of Borrelia burgdorferi was found to significantly enhance the microorganism's penetration of umbilical vein endothelial cell monolayers and of the underlying connective tissues, in contrast to untreated or plasminogen-bound spirochetes (9). Moreover, in the presence of plasmin, B. burgdorferi binds to human brain microvascular endothelial cells by the tips near or at cell borders, suggesting again a pericellular route of transmigration (13).
The mammalian plasminogen-plasmin proteolytic system plays a crucial role in fibrinolysis and in extracellular matrix degradation. The latter is required for cell migration under physiological conditions but is also exploited by metastatic cancer cells and by several invasive bacterial pathogens as discussed above. The single-chain proenzyme plasminogen circulates in plasma at high concentration (2 μM). It is composed of five kringle domains, which are loop structures of about 80 amino acids, and by the catalytic domain. Activation of plasminogen into plasmin, a broad-specificity serine protease, is triggered by mammalian tissue-type plasminogen activator and urokinase and by the streptococcal streptokinase (SK). The activation of plasminogen to the two-chain active plasmin is due to the cleavage of the peptide bond Arg561-Val562.
The plasminogen-plasmin receptors at the surface of S. pneumoniae include the enolase and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) enzymes (5, 6). Recently, we have shown that the pneumococcal surface-exposed choline-binding protein E (CBPE) is also a receptor for plasminogen, the activation of which into plasmin facilitates traversal of S. pneumoniae through a reconstituted basement membrane (2). Results presented herein support a pericellular traversal of epithelial and endothelial cell barriers by pneumococci, due to the presence of plasmin complexed on the surface of the bacteria.
MATERIALS AND METHODS
Pneumococcal strains.
Strain D39 is an encapsulated strain from serotype 2, and R6 is a nonencapsulated derivative of D39 (Rockefeller University). Pneumococcal strains were cultured in Todd-Hewitt broth (BD Sciences). Whenever required, pneumococcal bacteria were labeled with fluorescein isothiocyanate (FITC). Bacteria from mid-exponentially grown cultures were washed with phosphate-buffered saline (PBS, pH 7.4) before incubating for 20 min at room temperature (RT) with FITC at 1 mg/ml for adherence and transmigration assays or at 0.2 mg/ml for immunofluorescence microscopy observations. Bacteria were washed five times with PBS before use.
Cell culture.
The human lung alveolar carcinoma cell line A549 (type II pneumocytes) and the human vascular endothelial cell line EaHy were grown and maintained at 37°C in a 5% CO2 atmosphere in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% bovine growth serum (BGS; HyClone). Cells were grown to confluent monolayers in 24-well plates for adherence assays or on glass coverslips for immunofluorescence microscopy. For transmigration assays, 5 × 104 cells were layered on a 3-μm transparent membrane insert (Greiner Bio One) and allowed to grow to confluent monolayers for 3 days at 37°C with 5% CO2 in DMEM supplemented with 10% BGS. The tightness of the cell layers was verified by addition of 200 μl of DMEM with 10% BGS in the upper compartment followed by incubation at 37°C in 5% CO2 for 16 h. No medium was found in the lower compartment when 5 × 104 cells were layered, while this was not the case when 5 × 102 cells were used.
Proteins and antibodies.
cDNA encoding a fragment of human VE-cadherin (amino acid residues 1 to 431 of mature VE-cadherin) was inserted into the pET30b+ vector (Novagen) to generate the expression vector pET-VE-cadherin. After transformation of the Escherichia coli host strain BL21(DE3) with pET-VE-cadherin, the C-terminal His-tagged protein was expressed in inclusion bodies. Affinity chromatography purification was performed under denaturing conditions in the presence of 6 M urea. The recombinant VE-cadherin was refolded by fast dilution and repurified by size exclusion chromatography. The fragment Cad3 (amino acids 259 to 439 of human VE-cadherin) was used as an antigen to raise the anti-VE-cadherin antibody (17). Phosphorylcholine esterase (PCE) and CBPE expression and purification were done as previously described (11, 2).
In vitro VE-cadherin degradation by plasmin.
Plasminogen at 1 μM (Sigma) was activated by 100 nM SK (Sigma) in a final volume of 100 μl in PBS and incubated for 30 min at RT in order to generate plasmin. A volume of 1 μl containing 0.5 μg of recombinant purified VE-cadherin or PCE was mixed with 9 μl of freshly prepared plasmin and incubated for 1 h at 37°C with or without 100 μg/ml of aprotinin (Roche). The reaction was stopped by addition of 3 μl of 4× Laemmli buffer and heating at 100°C for 10 min. The reaction mixtures were then electrophoresed on 12.5% sodium dodecyl sulfate (SDS)-polyacrylamide gels and stained with Coomassie blue.
VE-cadherin degradation catalyzed by plasmin bound on S. pneumoniae.
Bacteria (1 × 108 CFU) were incubated with or without 1 μM plasminogen in 40 μl of PBS for 30 min at 37°C. Bacteria were washed with PBS to remove unbound plasminogen, resuspended in 40 μl of 100 nM SK in PBS, and incubated for 30 min at RT to allow conversion of plasminogen into plasmin. Bacteria were washed once with PBS to remove unbound plasmin and resuspended in 40 μl of PBS with or without 100 μg/ml of aprotinin. A quantity of 0.5 μg of recombinant VE-cadherin was added to the plasmin-bacteria suspension and subjected to degradation for 1 h at 37°C. The reaction was stopped by addition of 4× denaturing buffer (Bio-Rad) and heated for 10 min at 100°C. Samples were electrophoresed on 4 to 12% bis-Tris Criterion XT precast gels (Bio-Rad) in XT morpholinepropanesulfonic acid running buffer (Bio-Rad), transferred onto nitrocellulose membranes, and subsequently saturated with PBS containing 0.3% Tween 20, 5% milk (blocking buffer). Successive 1-h incubations were conducted with anti-Cad3 antibody (dilution, 1/1,000) and horseradish peroxidase-conjugated anti-rabbit antibody (dilution, 1/10,000; Sigma), both diluted in the blocking buffer before chemiluminescence detection with the ECL system (GE Healthcare). Immunodetected bands of VE-cadherin were quantified (Molecular Imager Gel Doc XR system; Bio-Rad). The total bacteria extracts were electrophoresed on 12.5% SDS-polyacrylamide gels and stained with Coomassie blue; the total amount of proteins was quantified in order to normalize the quantity of bacteria used under each condition.
Coating of bacteria with plasminogen or plasmin.
Plasminogen at 1 μM was added to 5 × 108 CFU/ml of FITC-labeled bacteria in DMEM for 30 min at 37°C. Bacteria were washed with DMEM to remove unbound plasminogen, resuspended in DMEM with or without 100 nM SK for plasminogen conversion into plasmin, and incubated for 30 min at RT. Bacteria were washed with DMEM. In transmigration assays (see below), FITC-labeled bacteria were diluted to 5 × 109 CFU/ml in DMEM, 2 μM plasminogen was used, and conversion into plasmin was performed with 200 nM SK in DMEM. Whenever required, 100 μg/ml of aprotinin was added to inhibit plasmin activity.
Adherence assay and immunofluorescence microscopy on endothelial EaHy and epithelial A549 cells.
Confluent cell monolayers were washed once with PBS and incubated with 200 μl of bacteria (1 × 108 CFU) previously treated with plasminogen/plasmin or with 200 μl of DMEM (used as the blank control). Efficient bacterium-cell contact was ensured by low-speed centrifugation (2 min at 300 × g) before incubation at 37°C with 5% CO2 for 30 min. The cells were then washed three times with PBS.
In the adherence assay, cells were treated with 200 μl of trypsin (0.5% in PBS) for 10 min at 37°C in 5% CO2 and then transferred to black 96-well microtiter plates (Greiner Bio One) for FITC fluorescence (excitation, 490 nm; emission, 520 nm) measurement on a multiwell fluorescence reader (Fluostar Optima; BMG). The ratio of binding of plasminogen-plasmin-treated bacteria to that of binding of untreated bacteria was calculated in order to normalize the data. Each condition tested was repeated three times, and the adhesion assays were carried out in three independent experiments.
For the immunofluorescence microscopy analysis, cells were fixed with 3% paraformaldehyde in PBS for 20 min at RT and then permeabilized by treatment with 0.5% Triton-3% paraformaldehyde in PBS for 3 min at RT. This step was followed by two washes with PBS for 10 min. VE-cadherin was detected in EaHy cells by using anti-Cad3 antibody diluted 1/200 in PBS and revealed with the Cy3-conjugated anti-rabbit antibody (dilution, 1/300; Jackson ImmunoResearch). Each incubation lasted for 1 h. Epithelial A549 cells were labeled with phalloidin coupled to a far-red fluorescent dye (Alexa Fluor 647 phalloidin; Invitrogen) diluted 1/40 in PBS. Glass coverslips were mounted in Mowiol-4′6-diamidino-2-phenylindole (DAPI) and incubated overnight at RT before observation (fluorescence microscope BX61; Olympus). The assays were done at least twice.
Quantification of plasminogen activators secreted by endothelial EaHy and epithelial A549 cells.
Cells were grown to confluent monolayers in 24-well plates, washed once with PBS, and incubated with 1 μM plasminogen in 200 μl of DMEM for 30 min at 37°C in 5% CO2. Positive and negative controls were performed using 200 μl of 1 μM plasminogen in DMEM in the absence of cells; 0.9 μl of 22.6 μM SK or DMEM was added and incubated for 30 min at RT with gentle agitation. Finally, 100 μl of the plasmin chromogenic substrate N-(p-tosyl)-Gly-Pro-Lys 4-nitroanilide acetate salt (500 μM; Sigma) was added and incubated for 30 min at 37°C. Absorbance at 406 nm was measured using the multiwell spectrophotometer Fluostar Optima after transfer of 150 μl of the reaction mixture supernatant to transparent 96-well microtiter plates (Greiner Bio One). The assays were performed three times independently, and each condition was repeated four times.
Pneumococcal transmigration through epithelial A549 and endothelial EaHy cell monolayers.
FITC-labeled R6 (5 × 109 CFU/ml in DMEM) was prepared as described above. Plasmin-coated bacteria, 1 × 109 CFU in 200 μl of DMEM, were added to the upper compartment of the transwell, where cells were washed once with PBS before the beginning of the assay. A control was performed using inserts deprived of the cell layer. The lower compartment contained 700 μl of DMEM with 10% BGS. The culture chambers were incubated at 37°C in 5% CO2. FITC fluorescence present in the lower compartment was read using the Fluorimeter Fluostar Optima at 1-hour time intervals for 10 h. The assay was done twice, and each experiment was carried out using two or three inserts for each condition.
Statistical analyses.
Error bars correspond to the standard errors of the means, or to the standard deviations when only two assays were performed. The statistical significance of between-group comparisons was determined with Student's t test. A probability value P of <0.05 or 0.1 was considered statistically significant.
RESULTS
Plasminogen bound to the surface of pneumococci enhances adhesion to EaHy and A549 cells.
Levels of adherence to human cell monolayers of FITC-labeled R6 coated with plasminogen, active plasmin, or aprotinin-inhibited plasmin were compared relative to values obtained with R6 alone (Fig. 1A and B). The patterns of adherence obtained with the endothelial EaHy and epithelial A549 cells were very similar, indicating that these two cell types have the same ability to interact with plasminogen- or plasmin-coated bacteria. Therefore, the detailed analysis below focuses mainly on the EaHy cells.
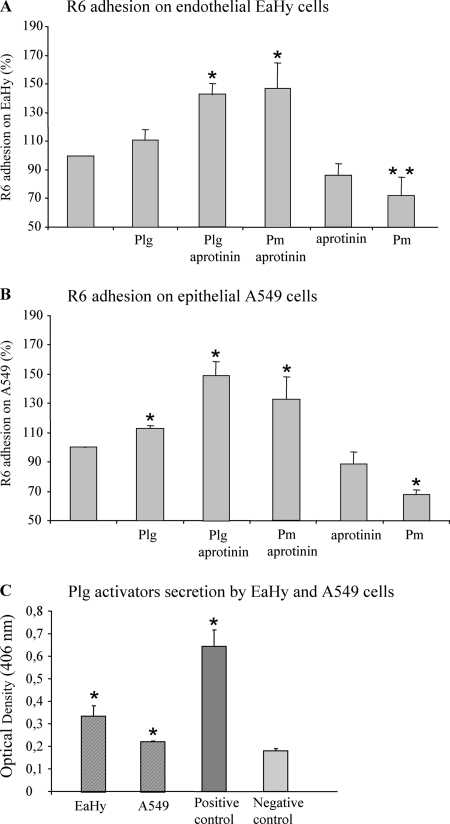
FIG. 1.
In vitro adherence of R6 to EaHy and A549 cells. (A and B) A total of 1 × 108 CFU of FITC-labeled R6, treated with plasminogen or plasmin with or without aprotinin, were incubated with cell monolayers for 30 min at 37°C in 5% CO2, and cell-associated bacterial fluorescence was measured. The standard error was derived from three independent experiments. Values were significantly different, as indicated by a single asterisk (P < 0.05) or a double asterisk (P < 0.1). (C) Secretion of plasminogen activators by EaHy and A549. Cells were incubated with 1 μM plasminogen for 30 min at 37°C in 5% CO2. Controls were performed in the absence of cells and using 1 μM plasminogen and 100 nM SK (positive control) or DMEM buffer (negative control). Plasmin activity was measured by addition of substrate and by reading absorbance at 406 nm. The standard error was derived from three independent experiments. Absorbance values at 406 nm were significantly different from the negative control, as indicated (*, P < 0.05).
Bacterial adherence on EaHy cells was not changed over R6 when the bacteria were precoated with plasminogen (Fig. 1A), while a slightly significant increase was observed when the assay was performed on A549 cells (Fig. 1B). This effect was even more pronounced when R6 was preincubated with either aprotinin-inhibited plasminogen or plasmin, as the bacterial adherence increased by 143% and 147%, respectively (Fig. 1A). This property was not due to aprotinin, because R6 incubation with aprotinin did not modify the adherence level compared to R6 alone. We then tested the effect of preincubating the R6 strain with active plasmin, prior to adherence to cell monolayers. This treatment led to a decrease of about 30% in bacterial adherence for both cell lines (Fig. 1A and B).
The lack of effect of plasminogen, in the absence of aprotinin, might be due to an activation of plasminogen by secretion by EaHy and A549 cells of plasminogen activators. Amounts of plasminogen activators secreted in the cell culture media were measured and corresponded approximately to 87 fmol and 58 fmol per EaHy and A549 cells, respectively (Fig. 1C). This observation has also been described for human brain microvascular endothelial cells and epithelial pharyngeal cells (13, 23). Therefore, it is likely that a fraction of surface-bound plasminogen was activated into plasmin, generating a mixed population, leading to an adhesion level intermediate between the increasing effect of plasminogen and the decreasing one of plasmin.
In summary, both plasminogen and inactivated plasmin, bound at the surface of pneumococci, enhance bacterial adherence to endothelial and epithelial cells. Conversely, enzymatically active plasmin lowers the level of R6 adhesion on both cell lines.
Plasmin at the surface of pneumococci disrupts intercellular junctions of endothelial EaHy and epithelial A549 monolayers.
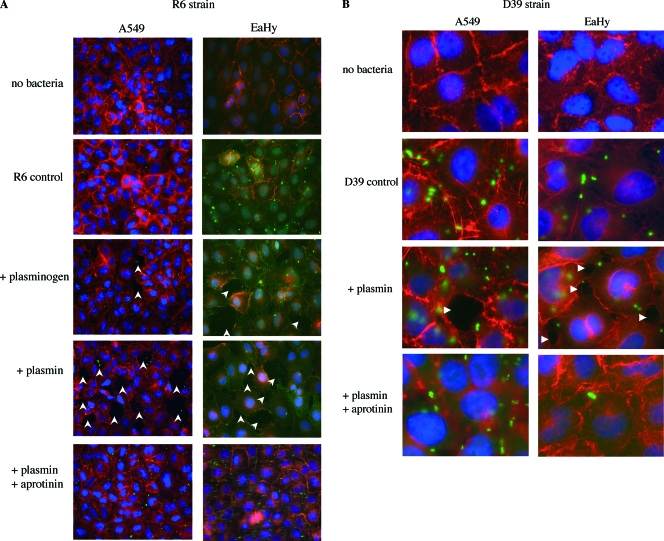
Experimental conditions used in the adherence assays were reproduced on cells cultured on coverslips. The integrity of cell EaHy and A549 monolayers was monitored using fluorescence microscopy, after infection with R6 and D39 pneumococcal strains preincubated with plasminogen, plasmin, in the presence or absence of the aprotinin inhibitor (Fig. 2). Monolayers of both cell types remained confluent after the incubation period with untreated pneumococci (Fig. 2A, compare “no bacteria” to R6 control). When the R6 strain was precoated with plasminogen or plasmin, EaHy and A549 monolayers showed disruption. The effect of plasminogen is likely the result of its activation into plasmin by plasminogen activators produced by the cells, as described above. On the contrary, EaHy and A549 monolayers remained intact when the enzymatic activity of the plasmin bound at the bacterial surface was inhibited by aprotinin. Similar experiments were performed in the absence of pneumococci, and plasmin alone at a concentration of 2 μM did not trigger cell monolayer disruption. When plasmin was added to the cell culture medium simultaneously with R6 instead of being preincubated with the bacteria, no significant difference in intercellular junction disorganization was observed compared to the R6-alone culture. These results indicate that bacteria-bound plasmin, harboring protease activity, is required for the disorganization of the monolayer cell junctions. The holes in the monolayers were counted, and the results indicated that, in the presence of plasmin, these defects were about seven times more frequent than in the absence of plasmin.
FIG. 2.
Immunofluorescence of EaHy and A549 monolayers infected with R6 and D39 strains. (A and B) A total of 1 × 108 CFU of FITC-labeled R6 (A) or D39 (B) were coated with 1 μM plasminogen activated into plasmin, with or without aprotinin, and incubated for 30 min at 37°C in 5% CO2 with confluent EaHy or A549 monolayers. EaHy cells were labeled by incubation with anti-VE-cadherin antibody and subsequently with Cy3-conjugated anti-rabbit antibody. A549 cells were labeled with Alexa Fluor 647 phalloidin. Glass coverslips were mounted on Mowiol-DAPI. Magnification, ×40 (with an Olympus microscope). FITC-labeled D39 were incubated on A549 and EaHy cells labeled with Alexa Fluor 647 phalloidin. Magnification, ×100 (with an Olympus microscope). White arrows indicate holes in the monolayers. These assays were performed at least twice for each cell line, and a representative experiment is shown.
The D39 pneumococcal strain was used to assess the influence of the capsule on plasmin-mediated disruption of the cell monolayers. Active plasmin disrupted intercellular junctions of EaHy and A549 monolayers when present at the surface of the encapsulated D39 strain as was observed for the unencapsulated R6 strain (Fig. 2B). This effect required active plasmin, as treatment with aprotinin prevented it.
Plasmin bound to pneumococci degrades human VE-cadherin in vitro.
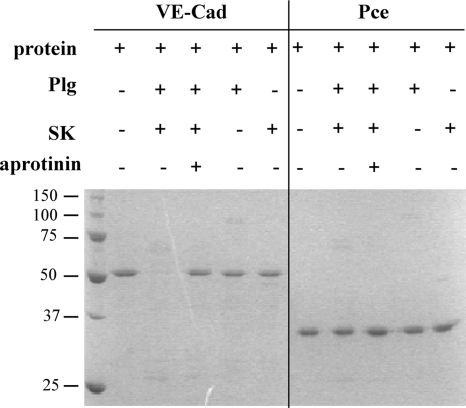
We investigated the ability of free plasmin to specifically cleave human VE-cadherin, the main protein of the vascular endothelium adherens junctions. A recombinant 50-kDa fragment of human VE-cadherin was purified and incubated with plasmin, generated from plasminogen activation. PCE, the recombinant catalytic domain of the pneumococcal CBPE, was used as a negative control protein in the proteolytic assay. Samples were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining as shown in Fig. 3. Plasmin specifically degraded recombinant VE-cadherin but not PCE, and this proteolytic activity was inhibited by aprotinin (Fig. 3). Neither plasminogen nor SK cleaved human VE-cadherin recombinant protein (Fig. 3).
FIG. 3.
Proteolysis of VE-cadherin by plasmin. Plasmin was obtained by incubation of 1 μM plasminogen (Plg) with 100 nM SK. VE-cadherin (VE-cad; 0.5 μg) and PCE were subjected to plasmin degradation for 1 h at 37°C with or without aprotinin. Samples were analyzed by SDS-PAGE and stained with Coomassie blue.
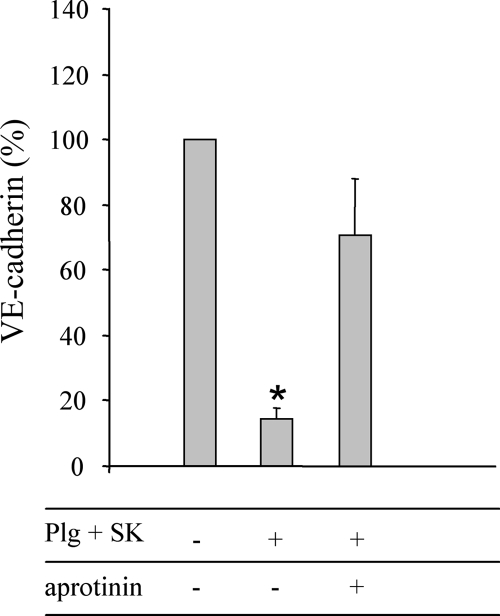
Does plasmin bound at the surface of R6 strains degrade the recombinant human VE-cadherin fragment? To answer this question, plasmin was generated at the surface of the pneumococcal strains by SK-mediated plasminogen activation. The bacteria were then incubated with recombinant VE-cadherin for 1 hour at 37°C prior to analysis by Western blotting using an anti-VE-cadherin polyclonal antibody (Fig. 4). No bands were detected with the anti-VE-cadherin antibody on R6 extracts (data not shown). In parallel to Western blot analysis results, bacterial extracts were analyzed by Coomassie blue-stained SDS-PAGE gels, and the levels of total protein were quantified to allow relative qualification of residual VE-cadherin detected by Western blotting (Fig. 4). When plasmin was bound at the surface of the R6 strain, the residual amount of intact VE-cadherin was 15% (Fig. 4). Inhibition of protease activity by aprotinin protected VE-cadherin from degradation. In summary, plasmin bound at the surface of pneumococci is able to cleave an extracellular fragment of human VE-cadherin, a major protein of the vascular adherens junctions.
FIG. 4.
Proteolysis of VE-cadherin by plasmin bound at the surface of pneumococci. A total of 1 × 108 CFU of R6 treated with 1 μM plasminogen-converted plasmin by SK, with or without 100 μg/ml of aprotinin, were incubated with 0.5 μg of VE-cadherin (VE-cad) for 1 h at 37°C. VE-cadherin was immunodetected and quantified. The amount of total protein of bacteria loaded in each well was quantified from a Coomassie blue-stained SDS-PAGE gel. A ratio was calculated between the amount of detected VE-cadherin and the total quantity of bacterial protein. The standard deviation was derived from two independent experiments. Values were significantly different from the reference R6 (100%) as indicated (*, P < 0.05).
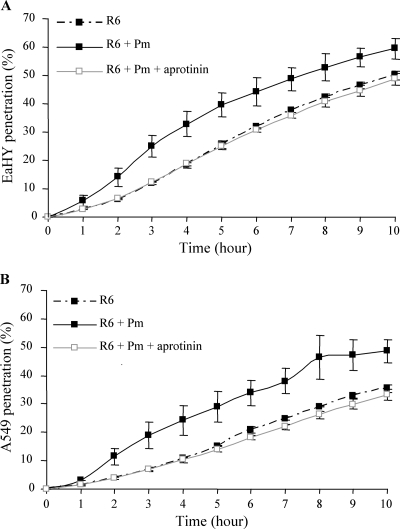
S. pneumoniae traversal of endothelial EaHy or epithelial A549 cell monolayers is facilitated by bacteria-bound plasmin.
Based on the results presented above, we have tested the effect of plasmin on the ability of pneumococci to migrate through epithelial and endothelial barriers. EaHy and A549 cells were grown on semipermeable transwell membranes, and FITC-labeled pneumococcal bacteria, coated or not with plasmin, were added in the upper compartment. The migration kinetics of the bacteria across EaHy or A549 monolayers was measured by monitoring the bacterial fluorescence present in the lower compartment. The fraction of bacteria migrating across the cell layers was calculated as the ratio of the fluorescence level in the lower compartment, at each time point, versus the fluorescence in the upper compartment at the beginning of the experiment (Fig. 5). In both EaHy and A549 cell layers, traversal was facilitated when active plasmin was bound at the surface of the R6 bacteria (Fig. 5). When the plasmin proteolytic activity was inhibited by aprotinin, no difference was observed compared to the bacteria free of plasmin (Fig. 5). In conclusion, active plasmin harbored at the surface of R6 facilitates transmigration through endothelial and epithelial cell layers.
FIG. 5.
Pneumococcal transmigration across endothelial EaHy and epithelial A549 cell monolayers. FITC-labeled R6 (1 × 109 CFU in 200 μl of DMEM) precoated with plasmin (Pm), in the presence or absence of 100 μg/ml aprotinin were added to the upper well of the inserts. FITC fluorescence in the lower well was read at 1-h time intervals to follow the penetration of bacteria through monolayer cells. The percentage of bacteria able to penetrate the monolayers was calculated as the ratio between the fluorescence measured in the lower compartment versus total bacterial fluorescence. These assays were performed twice, in duplicate or triplicate wells. In both panels, the standard deviations were derived from two independent experiments. Values for R6 + Pm were significantly different from the reference R6, as indicated (*, P < 0.05).
DISCUSSION
The data presented in this report show that acquisition of plasminogen, but not active plasmin, by S. pneumoniae promotes epithelial and endothelial cell adherence. This observation prompted us to investigate in more detail the effect of plasmin on pneumococcal invasion. We showed that active plasmin, bound at the bacterial surface, induces intercellular junction disorganization and favors cell barrier traversal. The pneumococcal pericellular transmigration promoted by surface-bound plasmin may be attributed to enzymatic cleavage of cell junction proteins, in accordance with our experimental data concerning the cleavage of human VE-cadherin, a major component of the adherens junction. Taken together, these results highlight a new function of the plasminogen/plasmin system in pneumococcal adherence to epithelial and vascular endothelial cell barriers and its involvement in the pericellular invasion process.
Even though encapsulated strains of S. pneumoniae are more virulent than nonencapsulated ones, several reports have supported the regulation of capsule expression in hosts (21). Pneumococci in intimate contact with cells are devoid of capsule, which is not the case in the absence of cellular contact (16). Recently, Hermans and colleagues compared the levels of adherence of various encapsulated strains of S. pneumoniae to epithelial cells in vitro with those of their isogenic nonencapsulated mutated forms; it was observed that mutant strains displayed significantly higher adherence, confirming that the capsule reduces the adherence and colonization ability of S. pneumoniae (8). In addition, Magee and Yother proposed that a reduction in the amount of capsule might be required to ensure efficient colonization, as the surface molecules involved in adherence would be exposed (19). In this work, we showed that both encapsulated strain D39 and unencapsulated R6 strain recruit plasmin, triggering disorganization of the intercellular junctions. We have also verified that the D39 strain transmigrates to the same degree as the R6 strain (data not shown). These results indicate that plasmin, independently of the presence and/or the quantity of capsule expressed, can reach its surface bacterial receptors. Therefore, the use of unencapsulated strain R6 is fully relevant in virulence studies when investigating the interactions of surface-exposed proteins with human cells and molecular components.
The apical surface of the pulmonary epithelium faces the external environment and forms the first barrier to bacterial entry into the human organism. The fibrinolytic system is present in bronchoalveolar fluid, from which bacteria can recruit plasminogen (20). In the presence of plasminogen or inhibited plasmin, adherence of pneumococci to epithelial and endothelial cell cultures increases. Similar findings have been observed with GAS on pharyngeal epithelial cells (23). Therefore, plasmin(ogen) molecules bind to their receptor(s) at the eukaryotic cell surface and hence favor bacterial attachment. Different human plasminogen receptors have been described (24): enolase at the surface of human epithelial pharyngeal cells (23), the voltage-dependent anion channel, and the glucose-regulated protein 78 at the surface of human umbilical vascular endothelial cells (12).
Our data show that disruption of cell monolayers by pneumococci coated with plasmin happened within 30 min of contact, but this period was too short to allow full transmigration in an in vitro system. This observation underlines the fact that bacterial transmigration is a complex multistep process that includes endocytosis and basolateral cell extravasion. In addition, basal extracellular matrix degradation by metalloproteinases is most probably also required, as shown with B. burgdorferi (13). Our data are reminiscent of recent observations showing that epithelial monolayer disorganization by S. pneumoniae occurred prior to detection of bacteria in the basolateral compartment (4).
Tight junctions seal the intercellular space between adjacent endothelial or epithelial cells to create a primary barrier to underlying tissues. Assembly and disassembly of the adherens junctions between cells are in part controlled by cadherins (14, 15, 22). It has been shown that pathogens like Porphyromonas gingivalis and Bacteroides fragilis produce endogenous proteases degrading E-cadherin, triggering migration through epithelial barriers in a pericellular manner (3, 18, 27). In this report, we showed that plasmin bound at the surface of R6 is able to degrade an extracellular fragment of the human VE-cadherin. This result exemplified the ability of plasmin-bound pneumococci to cleave proteins involved in cell junctions, inducing intercellular junction disorganization, as observed with the R6 and D39 pneumococcal strains. However, more investigations are now required to detail this process: adherens junctions are located below the tight junctions, which are not functional in the cell lines used in this study. It would be interesting to verify if components of the tight junctions, like the claudins, are degraded by pneumococci during the transmigration and if other host proteases, or endogenous pneumococcal proteases, could be involved in the cleavage of cell junction proteins.
The plasminogen-plasmin receptors identified in S. pneumoniae are the enolase and the GAPDH proteins, and recently we showed that the surface-exposed CBPE is also a receptor for plasminogen (2, 5, 6). All the experiments described in this work have been performed in parallel with an R6 strain with the cbpE gene deleted, and reduced effects were observed with the mutant strain compared with the parental strain (data not shown). We verified that the expression of enolase was not affected in the cbpE mutant strain. Therefore, our data indicate that CBPE, functioning as a plasminogen-plasmin receptor, is to some extent involved in the pericellular transmigration of S. pneumoniae. Nevertheless, more studies are now needed to analyze the respective contributions of each plasminogen receptor, enolase, GAPDH, and CBPE, in pneumococcal adherence and pericellular invasion.
Interactions of pneumococci with proteins of the cadherin family were previously described: the pneumococcal lipoprotein PsaA recognizes E-cadherin of nasopharyngeal epithelial cells (1), and the moonlighting protein fructose biphosphate aldolase was described as a putative flamingo cadherin receptor (7). Furthermore, S. pneumoniae has been shown to colocalize with E-cadherin at intercellular junctions (4). In summary, we propose that S. pneumoniae localizes to adherens junctions by targeting cadherins through surface-exposed receptors and recruits host proteases (like plasmin) to degrade cell-cell junctions, allowing pericellular tissue invasion.
Acknowledgments
We thank Marjolaine Noirclerc-Savoye (Fondation Rhône-Alpes Futur) and Benoît Gallet (IBS/LIM) of the RoBioMol platform.
This work was funded in part by an ANR Jeunes Chercheurs 2005 (ANR-05-JCJC-0049-01) grant to A.M.D.G. and by the FPG EUR-INTAFAR LSHM-CT-2004-512138 project.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 25 August 2008.
REFERENCES
- 1.Anderton, J. M., G. Rajam, S. Romero-Steiner, S. Summer, A. P. Kowalczyk, G. M. Carlone, J. S. Sampson, and E. W. Ades. 2007. E-cadherin is a receptor for the common protein pneumococcal surface adhesin A (PsaA) of Streptococcus pneumoniae. Microb. Pathog. 42225-236. [DOI] [PubMed] [Google Scholar]
- 2.Attali, C., C. Frolet, C. Durmort, J. Offant, T. Vernet, and A. M. Di Guilmi. 2008. Streptococcus pneumoniae choline-binding protein E interaction with plasminogen/plasmin stimulates migration across the extracellular matrix. Infect. Immun. 76466-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkovetz, D. F., and J. Katz. 2003. Bacterial invasion by a paracellular route: divide and conquer. Microbes Infect. 5613-619. [DOI] [PubMed] [Google Scholar]
- 4.Beisswenger, C., C. B. Coyne, M. Shchepetov, and J. N. Weiser. 2007. Role of p38 MAP kinase and transforming growth factor-signaling in transepithelial migration of invasive bacterial pathogens. J. Biol. Chem. 28228700-28708. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann, S., M. Rohde, G. S. Chhatwal, and S. Hammerschmidt. 2001. α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 401273-1287. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann, S., M. Rohde, and S. Hammerschmidt. 2004. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus pneumoniae is a surface-displayed plasminogen-binding protein. Infect. Immun. 722416-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blau, K., M. Portnoi, M. Shagan, A. Kaganovich, S. Rom, D. Kafka, V. Chalifa Caspi, A. Porgador, N. Givon-Lavi, J. M. Gershoni, R. Dagan, and Y. Mizrachi Nebenzahl. 2007. Flamingo cadherin: a putative host receptor for Streptococcus pneumoniae. J. Infect. Dis. 1951828-1837. [DOI] [PubMed] [Google Scholar]
- 8.Bootsma, H. J., M. Egmont-Petersen, and P. W. Hermans. 2007. Analysis of the in vitro transcriptional response of human pharyngeal epithelial cells to adherent Streptococcus pneumoniae: evidence for a distinct response to encapsulated strains. Infect. Immun. 755489-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman, J. L., T. J. Sellati, J. E. Testa, R. R. Kew, M. B. Furie, and J. L. Benach. 1995. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect. Immun. 632478-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cundell, D. R., J. N. Weiser, J. Shen, A. Young, and E. I. Tuomanen. 1995. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect. Immun. 63757-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garau, G., D. Lemaire, T. Vernet, O. Dideberg, and A. M. Di Guilmi. 2005. Crystal structure of phosphorylcholine esterase domain of the virulence factor choline-binding protein E from Streptococcus pneumoniae: new structural features among the metallo-beta-lactamase superfamily. J. Biol. Chem. 28028591-28600. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Gronow, M., S. J. Kaczowka, S. Payne, F. Wang, G. Gawdi, and S. V. Pizzo. 2007. Plasminogen structural domains exhibit different functions when associated with cell surface GRP78 or the voltage-dependent anion channel. J. Biol. Chem. 28232811-32820. [DOI] [PubMed] [Google Scholar]
- 13.Grab, D. J., G. Perides, J. S. Dumler, K. J. Kim, J. Park, Y. V. Kim, O. Nikolskaia, K. S. Choi, M. F. Stins, and K. S. Kim. 2005. Borrelia burgdorferi, host-derived proteases, and the blood-brain barrier. Infect. Immun. 731014-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gumbiner, B., and K. Simons. 1986. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J. Cell Biol. 102457-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gumbiner, B. M. 2005. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6622-634. [DOI] [PubMed] [Google Scholar]
- 16.Hammerschmidt, S., S. Wolff, A. Hocke, S. Rosseau, E. Muller, and M. Rohde. 2005. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect. Immun. 734653-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermant, B., S. Bibert, E. Concord, B. Dublet, M. Weidenhaupt, T. Vernet, and D. Gulino-Debrac. 2003. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J. Biol. Chem. 27814002-14012. [DOI] [PubMed] [Google Scholar]
- 18.Katz, J., Q. B. Yang, P. Zhang, J. Potempa, J. Travis, S. M. Michalek, and D. F. Balkovetz. 2002. Hydrolysis of epithelial junctional proteins by Porphyromonas gingivalis gingipains. Infect. Immun. 702512-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magee, A. D., and J. Yother. 2001. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect. Immun. 693755-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuoka, H., T. H. Sisson, T. Nishiuma, and R. H. Simon. 2006. Plasminogen-mediated activation and release of hepatocyte growth factor from extracellular matrix. Am. J. Respir. Cell Mol. Biol. 35705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson, A. L., A. M. Roche, J. M. Gould, K. Chim, A. J. Ratner, and J. N. Weiser. 2007. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect. Immun. 7583-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niessen, C. M. 2007. Tight junctions/adherens junctions: basic structure and function. J. Investig. Dermatol. 1272525-2532. [DOI] [PubMed] [Google Scholar]
- 23.Pancholi, V., P. Fontan, and H. Jin. 2003. Plasminogen-mediated group A streptococcal adherence to and pericellular invasion of human pharyngeal cells. Microb. Pathog. 35293-303. [DOI] [PubMed] [Google Scholar]
- 24.Plow, E. F., T. Herren, A. Redlitz, L. A. Miles, and J. L. Hoover-Plow. 1995. The cell biology of the plasminogen system. FASEB J. 9939-945. [DOI] [PubMed] [Google Scholar]
- 25.Radin, J. N., C. J. Orihuela, G. Murti, C. Guglielmo, P. J. Murray, and E. I. Tuomanen. 2005. β-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect. Immun. 737827-7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talbot, U. M., A. W. Paton, and J. C. Paton. 1996. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect. Immun. 643772-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu, S., K. C. Lim, J. Huang, R. F. Saidi, and C. L. Sears. 1998. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc. Natl. Acad. Sci. USA 9514979-14984. [DOI] [PMC free article] [PubMed] [Google Scholar]