Abstract
Chikungunya virus (CHIKV) has caused recent, large epidemics on islands in the Indian Ocean, raising the possibility of more widespread CHIKV epidemics. Historically, CHIKV has been vectored by Aedes aegypti, but these outbreaks likely also involved Ae. albopictus. To examine the potential for an outbreak of CHIKV in Florida, we determined the susceptibility to CHIKV of F1 Ae. aegypti and Ae. albopictus from Florida. In addition, we also evaluated two well-characterized laboratory strains (Rockefeller and Lake Charles) of these species. We determined infection and dissemination rates as well as total body titer of mosquitoes 7 days post-exposure (pe) (Ae. albopictus) and 3, 7, and 10 days pe (Ae. aegypti). All mosquito strains were susceptible to both infection and dissemination, with some variation between strains. Our results suggest Florida would be vulnerable to transmission of CHIKV in urban and rural areas where the two vector species occur.
INTRODUCTION
The 2004–2007 outbreak of chikungunya virus (CHIKV) started in Kenya and spanned the countries bordering the Indian Ocean and caused illness in more than 250,000 persons on La Réunion island, and possibly more than a million in India.1–4 Although isolated in 1952,5 CHIKV has received little attention because of the low mortality associated with it and the limited extent of previous epidemics relative to the current outbreak. This virus has a sylvatic cycle in Africa between wild primates and forest Aedes mosquitoes, while in Asia and the Indian Ocean it cycles exclusively between humans and Aedes spp. mosquitoes.6 The large scale of the Indian Ocean outbreak, and increased frequency of epidemics throughout south Asia suggests the possibility CHIKV could emerge in non-endemic areas, such as the United States.7,8 Numerous imported cases of CHIKV to the United States and Europe have been recently reported.9–12
The two principal vectors of CHIKV in the Indian Ocean basin are Aedes aegypti and Ae. albopictus.13–15 Aedes aegypti has been established in North America for probably more than 300 years,16 and since 1985 the southeastern United States has been invaded by Ae. albopictus, whose distribution now ranges from Illinois to Florida.17–19 Aedes aegypti decreased in range and abundance concomitant with this invasion and is currently limited to urban settings in Florida and other southern states, where it may be locally abundant.17,20 Determining the ability of local populations of these two mosquito species to acquire and transmit the outbreak strain of CHIKV is an important step for considering public health responses in the event of an outbreak.
Previous studies have found variation in the susceptibility of mosquito species and strains to CHIKV. An early study found Ae. aegypti the most refractory of five mosquito species to infection, half as susceptible as Ae. albopictus.21 Tesh and others,22 working with two strains of CHIKV, reported large vector competence variation between several strains of Ae. albopictus. In a more recent study, Ae. albopictus was also more likely than Ae. aegypti to become infected and develop a disseminated infection with a strain of CHIKV isolated from an outbreak in Bangkok, Thailand in 1962.23 This study also found significant variation between strains of Ae. albopictus.
Recent investigations have suggested that the 2005 La Réunion outbreak may have been caused by an increased transmission of CHIKV by Ae. albopictus, making the variation in susceptibility of mosquitoes to this strain of CHIKV a timely and pertinent question.24,25 Furthermore, the size of the ongoing outbreak in the Indian Ocean region and the widespread invasion by Ae. albopictus of the United States increase the risk of introduction and transmission of CHIKV in North America. For these reasons, we tested the susceptibility of current Florida and well-characterized laboratory strains of Ae. aegypti and Ae. albopictus to a La Réunion isolate of CHIKV.
MATERIALS AND METHODS
Mosquitoes
Four mosquito strains were tested in this experiment: F1 Ae. aegypti from F0 field collections made from December 2006 to January 2007 in Palm Beach County (added biweekly for a total initial collection of approximately 100 female mosquitoes); F1 Ae. albopictus from F0 field collections made from December 2006 to January 2007 in Palm Beach County (added biweekly for a total initial collection of approximately 250 female mosquitoes); Rockefeller strain Ae. aegypti maintained at the Florida Medical Entomology Laboratory (FMEL) since 2003; and Lake Charles strain Ae. albopictus, also maintained at FMEL since 2003. Both Lake Charles and Rockefeller strains have been in colony for many years, and their history has been described elsewhere.26 Colonies were given 20% sucrose ad libitum, and blood fed on live chickens. Chicken care followed the animal use and care policies of the National Institutes of Health (University of Florida, IACUC Protocol VB-17). Colonies were kept in 14-hour light:10-hour dark cycle at 28 ± 1°C and a relative humidity > 80% at all times. To produce adults, eggs were hatched in tap water and put in groups of 50 in one liter of tap water and fed 0.3 g 1:1 yeast:albumin. Larvae were reared at 28°C with a 14-hour light:10-hour dark cycle.
Virus
We used CHIKV strain LR2006-OPY1, which was isolated from a febrile patient in France who had been infected in La Réunion,12 then passaged once in African green monkey kidney (Vero) cells. The virus stock titer was determined (log10 7.2 plaque-forming units [pfu]/mL) by plaque assay as described previously.27 Virus stocks were stored at −80°C. To produce fresh virus for mosquito infection, a T-75 tissue culture flask with a confluent monolayer of Vero cells in 10 mL of cell culture medium (M199 medium supplemented with 10% fetal bovine serum, antibiotics, and anti-mycotics; Invitrogen, Carlsbad, CA) was inoculated with 100 µL of stock virus and incubated in a atmosphere of 5% CO2 at 35°C for 24 hours.
Oral infection of mosquitoes
Mosquitoes were exposed to blood mixed with virus in artificial membrane feeders.28 Three to six day-old mosquitoes were placed in cylindrical, waxed cardboard containers (height = 14 cm, diameter = 11 cm) (Dade Paper Co., Miami, FL) in groups of approximately 50 and placed in an incubator (14-hour light:10-hour dark cycle, with an automated dusk/dawn period of 1 hour at 28°C and a relative humidity of approximately 95%) within a certified Biosafety Level 3 containment facility. Sugar was removed 24 hours prior to feeding. Mosquitoes were exposed to blood meals consisting of a 1:10 dilution of freshly propagated CHIKV in citrated bovine blood (Hemostat Laboratories, Dixon, CA). Infectious blood was heated at 35°C for 20 minutes and placed on mosquito cages for one hour. After feeding, mosquitoes were cold anesthetized and only fully engorged mosquitoes were kept. Because of low feeding rates for Ae. albopictus (approximately 10%), female mosquitoes were exposed daily for three consecutive days. Samples of each blood meal were assayed using real-time, quantitative, reverse transcription–polymerase chain reaction (qRT-PCR) with copy number standardized to pfu by a plaque assay performed on 10-fold serial dilutions of virus stock.29 Blood meal titers ranged between log10 5.9 pfu/mL and log10 6.5 pfu/mL, as measured by qRT-PCR.
Determination of infection kinetics and status
Blood fed Ae. aegypti females were killed (by freezing) 3, 7, and 10 days post-exposure (pe), and Ae. albopictus females at 7 days pe to check for infection and dissemination. Mosquito legs were separated from bodies and individual body and legs were triturated separately in 2-mL tubes with 900 µL of BA-1 media30 and two zinc-plated BBs at 25 Hz for 3 minutes using a Tissuelyzer® tissue homogenizer (Qiagen Inc., Valencia, CA) and clarified by centrifugation (3,148 × g for 4 minutes). Samples were stored at −80°C until further processing no more than 10 days after freezing. Nucleic acid was then extracted using the MagNA Pure Instrument with the MagNA Pure LC Total Nucleic Acid Isolation Kit (Roche Diagnostics, Chicago, IL) following the manufacturer’s instructions.
A one-step qRT-PCR was used to determine infection status and body titer of samples. Protocols have been described elsewhere.31 Primers were designed from the E1 gene (Genbank accession no. DQ443544) and had the following sequences: forward: 5′-ACC CGG TAA GAG CGA TGA ACT-3′; reverse: 5′-ACG CCG CAT CCG GTA TGT-3′. The probe sequence was: 5′-/5Cy5/CCG TAG GGA ACA TGC CCA TCT CCA /3BHQ_2/-3′ (IDT DNA, Coralville, IA).
Statistical analysis
All statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). Proportion of mosquitoes infected (no. positive bodies/no. tested) or with disseminated infections (no. positive legs/no. positive bodies) were compared using contingency table analysis. If significant effects were detected, pairwise comparisons were made with an alpha adjusted to account for multiple comparisons (α = 0.05/n, where n = no. of comparisons). For strain comparisons, only data from 7 days pe were used because there were insufficient mosquitoes from either Ae. albopictus group to harvest on days 3 or 10. Only body titers of mosquitoes with disseminated infections were compared. Transformations failed to make body titer data normally distributed, and rank tests (Kruskal-Wallis or Wilcoxon tests with a normal approximation) were used to detect significant variation in body titers between strains and between days pe for Ae. aegypti strains.32
RESULTS
Effect of different mosquito strains: infection/dissemination
Mosquito strain significantly affected the proportion of mosquitoes infected (χ2 = 10.867, degrees of freedom [df] = 3, P = 0.0125; Table 1) and disseminated (χ2 = 58.054, df = 3, P < 0.0001; Table 1) on day 7 pe. The F1 Ae. aegypti from Florida was significantly less likely to be disseminated than mosquitoes from other strains, and Rockefeller Ae. aegypti was significantly less likely to be disseminated than either strain of Ae. albopictus, with no difference between the two Ae. albopictus strains (adjusted α = 0.0083). The only significant difference in infection status was between F1 Ae. aegypti and Lake Charles Ae. albopictus (χ2 = 9.4826, df = 1, P = 0.0021).
TABLE 1.
Percent infected, percent disseminated, and body titer for mosquitoes seven days post-exposure to chikungunya virus for each strain tested
| Mosquito strain | % Infected | % Disseminated | Mean ± SE log10 body titer* |
|---|---|---|---|
| F1 Aedes aegypti | 93 (98/105) | 67 (66/98) | 5.63 ± 0.10 |
| F1Ae. albopictus | 100 (29/29) | 100 (29/29) | 5.53 ± 0.08 |
| Rockefeller Ae. aegypti | 98 (76/77) | 80 (69/76) | 5.57 ± 0.11 |
| Lake Charles | |||
| Ae. Albopictus | 100 (99/99) | 98 (98/99) | 6.21 ± 0.05 |
Plaque-forming units/mL.
Effect of different mosquito strains: body titer
Different mosquito strains had significantly different body titers for disseminated infections at day 7 pe (χ2 = 61.47, df = 3, P < 0.0001; Table 1). Lake Charles Ae. albopictus had significantly higher titers than the other three strains, between which there were no differences in body titer at day 7 pe (adjusted α = 0.0083).
Effect of time for Ae. aegypti strains: infection/dissemination
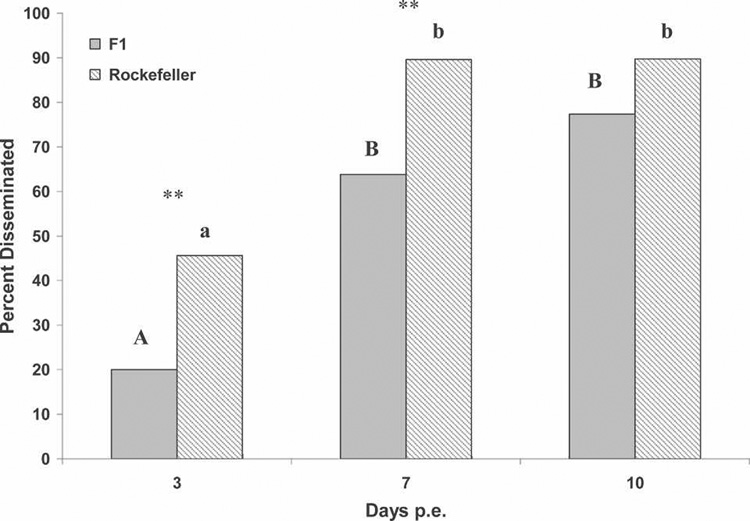
Time since exposure significantly affected the proportion of disseminated infections among Rockefeller Ae. aegypti (χ2 = 36.19, df = 2, P < 0.0001; Figure 1), but not the proportion infected (χ2 = 2.44, df = 2, P = 0.29). The F1 Ae. aegypti had a similar pattern, with a significant difference between days since exposure in dissemination (χ2 = 50.31, df = 2, P < 0.0001; Figure 1), but not infection (χ2 = 1.051, df = 2, P = 0.59). On days 3 and 7, but not day 10, there were significantly more Rockefeller Ae. aegypti with disseminated infections than F1 Ae. aegypti when each day was compared separately (adjusted α = 0.0166, day 3: χ2 = 8.07, df = 1, P < 0.0045; day 7: χ2 = 13.53, df = 1, P < 0.0002; day 10: χ2 = 0.136, df = 1, P < 0.72; Figure 1).
FIGURE 1.
Percent disseminated versus days post-exposure for Aedes aegypti strains. **significant pairwise differences between strains (α = 0.0166) by chi-square test. Letters denote homogenous groups by post hoc pairwise comparisons (uppercase for F1 Ae. aegypti; lowercase for Rockefeller Ae. aegypti).
Effect of time for Ae. aegypti strains: body titer
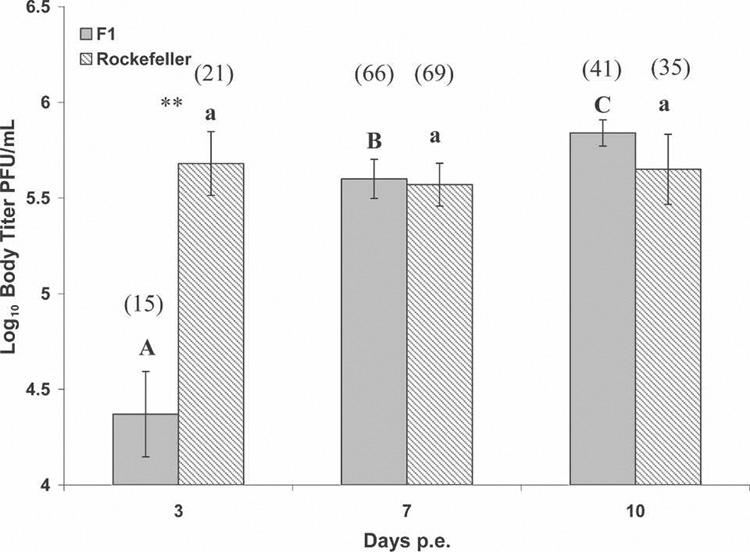
Body titer varied significantly as a function of days since exposure for F1 Ae. aegypti with disseminated infections (χ2 = 25.94, df = 2, P < 0.0001; Figure 2), but not Rockefeller Ae. aegypti (χ2 = 1.18, df = 2, P < 0.55; Figure 2). Body titer was significantly different on all days pe for F1 Ae. aegypti (adjusted α = 0.0166; Figure 2). On day 3 pe, but not day 7 pe or 10 pe, F1 Ae. aegypti had significantly lower body titer than Rockefeller Ae. aegypti (Z = −3.63, df = 1, P < 0.0003).
FIGURE 2.
Body titer (mean ± SE log10 plaque-forming units/0.1 mL) for Aedes aegypti mosquitoes with disseminated infections. Letters denote homogenous groups comparing days post-exposure within strain by all possible pairwise comparisons using Wilcoxon’s two-sample rank test (α = 0.0166; uppercase for F1 Ae. aegypti; lowercase for Rockefeller Ae. aegypti). **significant post hoc differences in body titer between strains by Wilcoxon’s two-sample rank test (α = 0.0166). Numbers in parentheses are the number of disseminated mosquitoes tested for each strain at each day.
DISCUSSION
All four mosquito strains tested were highly susceptible to infection with CHIKV. The F1 Ae. aegypti from Florida was less susceptible, with slightly lower infection rates and significantly lower dissemination rates relative to Rockefeller Ae. aegypti and both Ae. albopictus strains. The course of infection in F1 Ae. aegypti was slower than in the Rockefeller strain, and had a lower average body titer three days pe. There were no differences between the Rockefeller Ae. aegypti and the two Ae. albopictus strains in infection or dissemination rates, although Lake Charles Ae. albopictus had a higher average body titer than any other strain after seven days. Although the differences we observed were statistically significant, the biologic relevance of these differences is unknown.
The findings for Ae. albopictus, especially the F1 strain, were hampered by small sample sizes. This was a result of very low feeding rates (approximately 10%) using the artificial membrane system. The low numbers of blood fed individuals limited our ability to estimate extrinsic incubation period (EIP) for Ae. albopictus, although based upon the complete infection and dissemination after 7 days at 28°C, we suspect the course of infection to be rapid.
The estimation of EIP for Ae. aegypti is important for parameterizing epidemiologic models and therefore predicting the speed and severity of an epidemic.33 The F1 Ae. aegypti had a higher percent disseminated and increased body titer over time, with decreasing differences in body titer between days 7 pe and 10 pe. Therefore, it is likely that under the conditions tested here, Ae. aegypti would be infectious within seven days of biting an infected human.
There were large statistical differences in dissemination (on all days) and body titer (on day 3 pe) between F1 Ae. aegypti and Rockefeller Ae. aegypti demonstrates the importance of examining the course of infection at various times pe in different strains. This variation in the EIP between strains could impact epidemiologic risk.
The susceptibility of Florida mosquitoes of both species to CHIKV suggests the potential for emergence of this virus in Florida. However, other mitigating factors might limit this risk, specifically the behavior of Florida mosquitoes with regard to blood feeding, and the lower likelihood of human-vector contact in the United States, relative to less wealthy nations.34 Furthermore, although Ae. albopictus was more susceptible in this study, the differences in ecology between these species, especially differences in their human biting rate, suggests more detailed information is needed before conclusions as to the vectorial capacity of each species can be made in a given location.
The spread of Ae. albopictus in the last 30 years19 and the importation of numerous, active cases of chikungunya fever to the United States9–11,35 emphasize the importance of knowing the relative vector competence of North American mosquitoes to this arbovirus. This knowledge, coupled with enhanced understanding of the ecology of the major vectors will enable locally specific, efficient deployment of public health resources in the event of an outbreak of CHIKV in North America.
Acknowledgments
We thank Stephanie Richards, Bridget Farrell, Heather Robinson, and Krystle van Sickler for assistance with maintaining cells and mosquitoes and processing samples. We also thank Phil Lounibos and Stephanie Richards for reading earlier versions of this manuscript and three anonymous reviewers. The isolate of CHIKV used in this study was graciously provided by Robert Tesh (World Reference Center for Emerging Viruses and Arboviruses, through the University of Texas Medical Branch in Galveston, Texas).
Financial support: Michael H. Reiskind and Catherine J. Westbrook were supported by National Institutes of Health (NIH) grant AI-044793 (Invasion Biology of Aedes albopictus) to L. Phil Lounibos, and Kendra Pesko was supported by a University of Florida Alumni Fellowship, and NIH grant AI-042164.
REFERENCES
- 1.Enserink M. Massive outbreak draws fresh attention to little known virus. Science. 2006;311:1085. doi: 10.1126/science.311.5764.1085a. [DOI] [PubMed] [Google Scholar]
- 2.Bessaud M, Peyrefitte CN, Pastorino BAM, Tock F, Merle O, Colpart JJ, Dehecq JS, Girod R, Jaffar-Bandjee MC, Glass PJ, Parker M, Tolou HJ, Grandadam M. Chikungunya virus strains, Reunion Island outbreak. Emerg Infect Dis. 2006;12:1604–1606. doi: 10.3201/eid1210.060596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous. Outbreak news: chikungunya, India. Wkly Epidemiol Rec. 2006;81:409–410. [PubMed] [Google Scholar]
- 4.Chretien J, Anyamba A, Bedno S, Breiman R, Sang R, Sergon K, Powers A, Onyango C, Small J, Tucker C, Linthicum K. Drought-associated chikungunya emergence along coastal east Africa. Am J Trop Med Hyg. 2007;76:405–407. [PubMed] [Google Scholar]
- 5.Ross R. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond) 1956;54:177–191. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jupp PG, McIntosh B. Chikungunya disease. In: Monath T, editor. The Arboviruses: Epidemiology and Ecology. Boca Raton, FL: CRC Press; 1988. pp. 137–157. [Google Scholar]
- 7.Laras K, Sukri N, Larasati R, Bangs M, Kosin R, Djauzi WT, Master J, Koasaih H, Hartati S, Beckett C, Sedyaningsih E, Beecham H, III, Corwin A. Tracking the re-emergence of epidemic chikungunya virus in Indonesia. Trans R Soc Trop Med Hyg. 2005;99:128–141. doi: 10.1016/j.trstmh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 8.AbuBakar S, Sam I, Wong P, MatRahim N, Hooi P, Roslan N. Reemergence of endemic chikungunya, Malaysia. Emerg Infect Dis. 2007;13:147–149. doi: 10.3201/eid1301.060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farnon E. Update: chikungunya fever diagnosed among international travelers–United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:276–277. [PubMed] [Google Scholar]
- 10.Hochedez P, Jaureguiberry SB, Debruyne M, Bossi P, Hausfater P, Brucker G, Bricaire F, Caumes E. Chikungunya infection in travelers. Emerg Infect Dis. 2006;12:1565–1567. doi: 10.3201/eid1210.060495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanciotti RS, Kosoy O, Laven J, Panella A, Velez J, Lambert A, Campbell G. Chikungunya virus in US travelers returning from India, 2006. Emerg Infect Dis. 2007;13:764–767. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parola P, de Lamballerie X, Jourdan J, Rovery C, Vaillant V, Minodier P, Brouqui P, Flahault A, Raoult D, Charrel RN. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis. 2006;12:1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiter P, Fontenille D, Paupy C. Aedes albopictus as an epidemic vector of chikungunya virus: another emerging problem? Lancet Infect Dis. 2006;6:463–464. doi: 10.1016/S1473-3099(06)70531-X. [DOI] [PubMed] [Google Scholar]
- 14.Zeller HG. Dengue, arboviruses and migrations in the Indian Ocean. Bull Soc Pathol Exot. 1998;91:56–60. [PubMed] [Google Scholar]
- 15.Fontenille D, Rodhain F. Biology and distribution of Aedes albopictus and Aedes aegypti in Madagascar. J Am Mosq Control Assoc. 1989;5:219–225. [PubMed] [Google Scholar]
- 16.Christophers S. Aedes aegypti, the Yellow Fever Mosquito: Its Life History, Bionomics and Structure. Cambridge, UK: Cambridge University Press; 1960. [Google Scholar]
- 17.Darsie R, Ward G. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. Gainesville, FL: University Press of Florida; 2005. [Google Scholar]
- 18.Hawley W, Reiter P, Copeland RS, Pumpuni C, Craig GB. Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- 19.Benedict M, Levine R, Hawley W, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Meara GF, Evans L, Gettman A, Cuda J. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- 21.Mangiafico J. Chikungunya virus infection and transmission in five species of mosquito. Am J Trop Med Hyg. 1971;20:642–645. doi: 10.4269/ajtmh.1971.20.642. [DOI] [PubMed] [Google Scholar]
- 22.Tesh R, Gubler D, Rosen L. Variation among geographic strains of Aedes albopictus in susceptibility to infection with chikungunya virus. Am J Trop Med Hyg. 1976;25:326–335. doi: 10.4269/ajtmh.1976.25.326. [DOI] [PubMed] [Google Scholar]
- 23.Turell M, Beaman J, Tammariello R. Susceptibility of selected strains of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) to chikungunya virus. J Med Entomol. 1992;29:49–53. doi: 10.1093/jmedent/29.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Mishra B, Ratho RK. Chikungunya re-emergence: possible mechanisms. Lancet. 2006;368:918. doi: 10.1016/S0140-6736(06)69370-7. [DOI] [PubMed] [Google Scholar]
- 25.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, Lavenir R, Pardigon N, Reynes JM, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel MP, Brehin AC, Cubito N, Despres P, Kunst F, Rey FA, Zeller H, Brisse S. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:1058–1070. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alto BW, Lounibos LP, Higgs S, Juliano SA. Larval competition differentially affects arbovirus infection in Aedes mosquitoes. Ecology. 2005;81:3279–3288. doi: 10.1890/05-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gargan T, Bailey C, Higbee G, Gad A, El-Said S. The effect of laboratory colonization on the vector-pathogen interactions of Egyptian Culex pipiens and Rift Valley fever virus. Am J Trop Med Hyg. 1983;32:1154–1163. doi: 10.4269/ajtmh.1983.32.1154. [DOI] [PubMed] [Google Scholar]
- 28.Alto BW, Lounibos LP, Juliano SA. Age-dependent blood-feeding of Aedes aegypti and Aedes albopictus on artificial and living hosts. J Am Mosq Control Assoc. 2003;19:347–352. [PubMed] [Google Scholar]
- 29.Bustin S. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 30.Lanciotti R, Kerst A, Nasci R, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from clinical specimens, field-collected mosquitoes and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards S, Pesko K, Alto B, Mores C. Reduced infection in mosquitoes exposed to blood meals containing previously frozen flaviviruses. Virus Res. 2007;129:224–227. doi: 10.1016/j.virusres.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sokal R, Rohlf F. Biometry. New York: W.H. Freeman and Company; 1995. [Google Scholar]
- 33.Dye C. Vectorial capacity: must we measure all its components? Parasitol Today. 1986;2:203–209. doi: 10.1016/0169-4758(86)90082-7. [DOI] [PubMed] [Google Scholar]
- 34.Reiter P, Lathrop S, Bunning M, Biggerstaff B, Singer D, Tiwari T, Baber L, Amador M, Thirion J, Hayes J, Seca C, Mendez J, Ramirez B, Robinson J, Rawlings J, Vorndam V, Waterman S, Gubler D, Clark G, Hayes E. Texas lifestyle limits transmission of dengue virus. Emerg Infect Dis. 2003;9:86–89. doi: 10.3201/eid0901.020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC) Chikungunya fever diagnosed among international travelers–United States, 2005–2006. MMWR Morb Mortal Wkly Rep. 2006;55:1040–1042. [PubMed] [Google Scholar]