Abstract
The flavonoid baicalein inhibits fibrillation of α-synuclein, which is a major component of the Lewy bodies in Parkinson’s disease. It has been known that baicalein induces the formation of the α-synuclein oligomers and consequently prevents its fibrillation. In order to evaluate structural properties of the baicalein-stabilized oligomers, we purified the oligomer species by HPLC and examined their stability and structure by CD, FTIR, SEC-HPLC, SAXS, and AFM. Baicalein-stabilized oligomers are β-sheet-enriched according to CD and FTIR analysis. They did not form fibrils even after very prolonged incubation. From the SAXS data and AFM images, the oligomers were characterized as quite compact globular species. Oligomers were extremely stable, with a GdmCl Cm=3.3 M. This high stability explains the previously observed inhibition properties of baicalein toward α-synuclein fibrillation. These baicalein-stabilized oligomers, being added to the solution of the aggregating α-synuclein, were able to noticeably inhibit its fibrillation. After the prolonged co-incubation, short fibrils were formed, suggesting effective interaction of oligomers with the monomeric α-synuclein. Membrane permeability tests suggested that the baicalein-stabilized oligomers had a mild effect on the integrity of the membrane surface. This effect was rather similar to that of the monomeric protein, suggesting that the targeted stabilization of certain α-synuclein oligomers might offer a potential strategy for the development of novel Parkinson’s disease therapies.
Keywords: α-synuclein, baicalein, baicalein-stabilized oligomer, amyloid fibril
Introduction
Parkinson’s disease (PD)1 is the second most common neurodegenerative disorder, after Alzheimer’s disease, affecting approximately 0.2 % of the population. However, since it is predominantly an “aging” disease, the prevalence is much higher in the population over 60 years old. Clinically, it is a movement disorder characterized by tremor, rigidity, and bradykinesia 1. These symptoms are attributed to the progressive loss of dopaminergic neurons from the substantia nigra region of the brain. PD is characterized by cytosolic inclusions known as Lewy bodies 2. α-Synuclein has been shown to be a major fibrillar component of Lewy bodies 3, a variety of evidence implicates the aggregation of α-synuclein as a key step in the etiology of PD.
Human α-synuclein is a 140 residue, highly conserved presynaptic protein that is abundant in various regions of the brain 4;5. Structurally, purified α-synuclein is a natively unfolded protein at neutral pH 6-8. The aggregation of α-synuclein is thought to be a critical step in the pathogenesis of PD and several other neurodegenerative disorders 9. It has been suggested that the precursors of fibrils, oligomeric α-synuclein, might be more toxic than mature fibril 10-12. The molecular mechanisms underlying α-synuclein aggregation remain unknown.
Recently, to prevent the aggregation and fibrillation of α-synuclein, several small compounds have been examined 13-16. Dopamine and related catecholamines have been shown to inhibit α-synuclein aggregation 13;15. Additionally, flavonoid baicalein has markedly inhibited α-synuclein fibrillation 16. Baicalein is the main component of a traditional Chinese herbal medicine Scutellaria baicalensis and has multiple biological activities including antiallergic, anticarcinogenic, and anti-HIV properties 17-20. The inhibitory activity of dopamine, L-DOPA and baicalein is attributed predominantly to forming soluble oligomers of α-synuclein. The baicalein-induced oligomer of α-synuclein showed a partially structured form with a β-sheet structure by CD analysis and a stable globular form by AFM 16.
In this paper, we have examine the structural properties of the baicalein-stabilized oligomers, purified by SEC-HPLC, and its conformational stability using a set biophysical techniques such as CD, FTIR, SEC-HPLC, SAXS, and AFM. Our results indicate that baicalein-stabilized oligomers are extremely stable, with a GdmCl Cm=3.3 M, and do not form fibrils. Further, they have an inhibitory effect on the α-synuclein fibrillation.
Results and Discussion
Baicalein induces formation of stable oligomers by α-synuclein
α-Synuclein (1 mg/ml) was incubated with 100 μM baicalein at 37°C and pH 7.5, which are the typical fibrillation conditions for α-synuclein (Figure 1A). Min et al. showed that a presence of baicalein inhibited formation of α-synuclein amyloid fibrils completely by inducing oligomer formation 16. We confirmed that baicalein is able to effectively induce oligomerization of α-synuclein using SEC-HPLC (Figure 1B). After incubation for 2 days with 100 μM baicalein, the HPLC profile of α-synuclein showed a new peak with a retention time of 11.5 min, indicating formation of the stable oligomeric species. The peak corresponding to the monomeric protein was also observed in the elution profile. Purified samples eluting from the HPLC column were monitored by UV spectroscopy to confirm the baicalein binding (Figure 1C). The baicalein has three characteristic maxima in the absorption spectrum, at 216, 277, and 324 nm. Min et al. 16 showed that when the baicalein was oxidized, the absorbance at 324 nm disappeared, whereas when it was bound to α-synuclein, a new peak at ~360 nm was observed. The UV absorption spectrum of the oligomer showed an absorbance at around 360 nm, suggesting the effective baicalein binding. Interestingly, the monomer peak in the HPLC profile of the α-synuclein co-incubated with baicalein (see Figure 1B, peak (c)) seemed to be broader than that of α-synuclein alone (Figure 1B, peak (a)) and its UV absorption spectrum also showed an absorbance at 360 nm, indicating baicalein binding. Both baicalein-bound oligomers and monomers purified from HPLC showed yellow color originated from the flavonoid baicalein. We used these two samples for the detailed biophysical analysis. The purified baicalein-stabilized oligomers were incubated at 37°C for 1 month. No fibrils were formed and no dissociation was observed after this prolonged incubation, suggesting the high stability of the oligomers.
Figure 1.

Baicalein stabilizes high molecular weight oligomeric species. (A) Effect of baicalein on the α-synuclein fibrillation. (○) without and (□) with 100 μM baicalein. (B) SEC-HPLC profiles of monomeric α-synuclein (solid line), or incubated in the presence of 100 μM baicalein for 2 days (dotted line). Peak (a) corresponds to the momeric protein, whereas peaks (b) and (c) correspond to the baicalein-bound oligomers and monomers, respectively. (C) UV absorption spectra of the samples corresponding to the fractions collected from the corresponding peaks of the SEC-HPLC profiles.
Atomic Force Microscopy (AFM) and Electron Microscopy (EM) analyses of the baicalein-stabilized oligomers
To further characterize the baicalein-stabilized oligomer, atomic force microscopy (AFM) analysis was utilized in order to evaluate the oligomer size and shape (Figure 2A). Tapping mode AFM permits imaging at higher resolution than electron microscopy. The AFM image of baicalein-stabilized oligomer shows spherical oligomers with size varying in the range of 2.5 – 8.5 nm in height and 10 – 30 nm in width (Figure 2B). These data are consistent with the sizes of the baicalein-stabilized oligomers reported by Min et al. 16. The EM images of the baicalein-stabilized oligomer also showed multi-size oligomers with 8 – 22 nm in width (Figure 2C).
Figure 2.
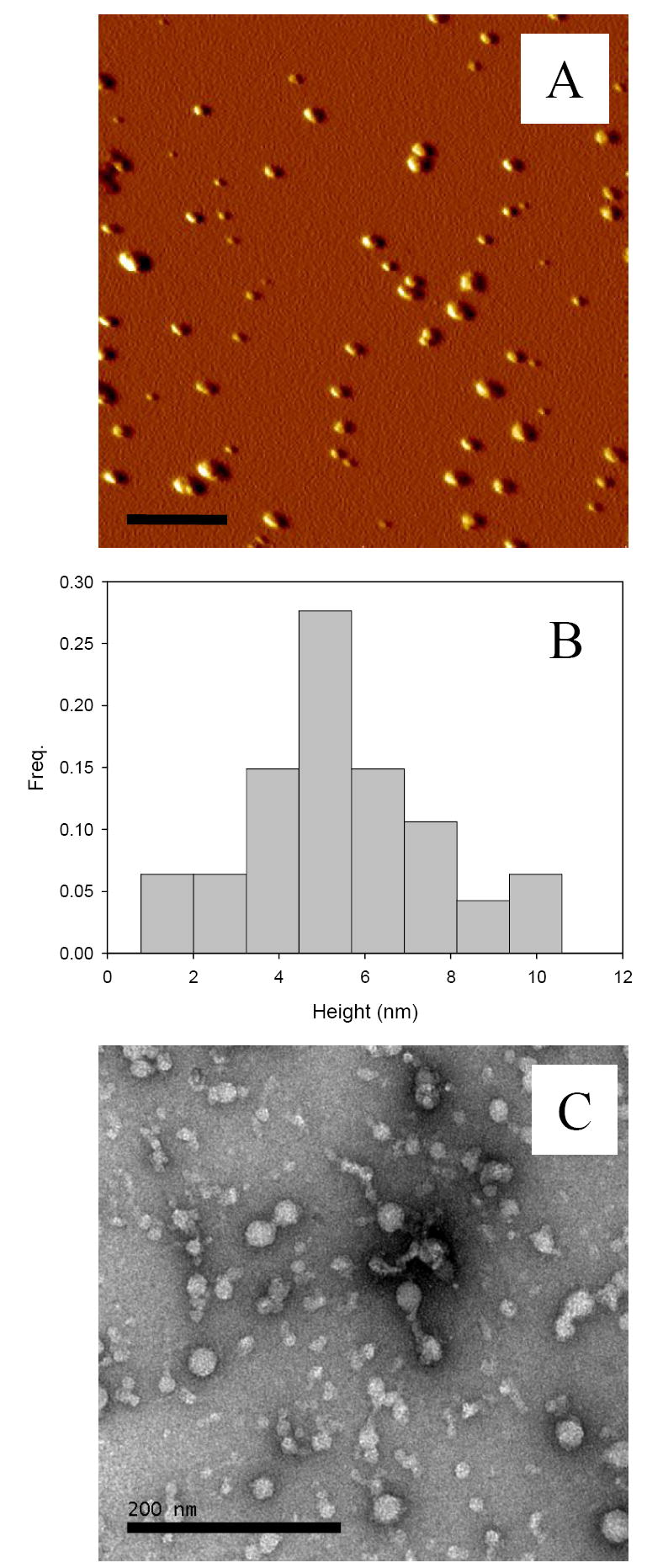
Baicalein-stabilized oligomers are spherical particles. (A) AFM image, (B) histogram of height of the oligomers analyzed by SPIP, and (C) EM image.
Small Angle X-ray Scattering (SAXS) analysis of the baicalein-stabilized oligomers
SAXS is an excellent technique for the investigation of conformation, shape, and association state of biopolymers in solution. Analysis of the scattering curves using the Guinier approximation gives information about the radius of gyration, Rg. Presentation of the scattering data in the form of Kratky plots provides information about the globularity (packing density) and conformation of a protein 21. For a native globular protein this plot has a characteristic maximum, whereas unfolded and partially folded polypeptides have significantly different-shaped Kratky plots.
Guinier analysis of the scattering data shows that a value of Rg for the baicalein-stabilized oligomer is 59 ± 2 Å (Figure 3A). At neutral pH, α-synuclein has significantly smaller Rg value, namely, 41 ± 1 Å 7;22. This value is much larger than that of a folded globular protein of the size of α-synuclein, because of the intrinsically disordered nature of this protein. In fact, the Rg for a native globular protein composed of n amino acid residues is known to be given by RgN = 2.9·n1/3 23. Application of this equation to the monomeric α-synuclein (140 residues) gave Rg of 15 Å 7;22. If the side-chain packing inside the baicalein-stabilized oligomers would resemble the packing of the globular proteins, then these oligomers would comprise of ~60 α-synuclein monomers. This value can be used as a highest estimate of the α-synuclein association state in the baicalein-stabilized oligomers.
Figure 3.
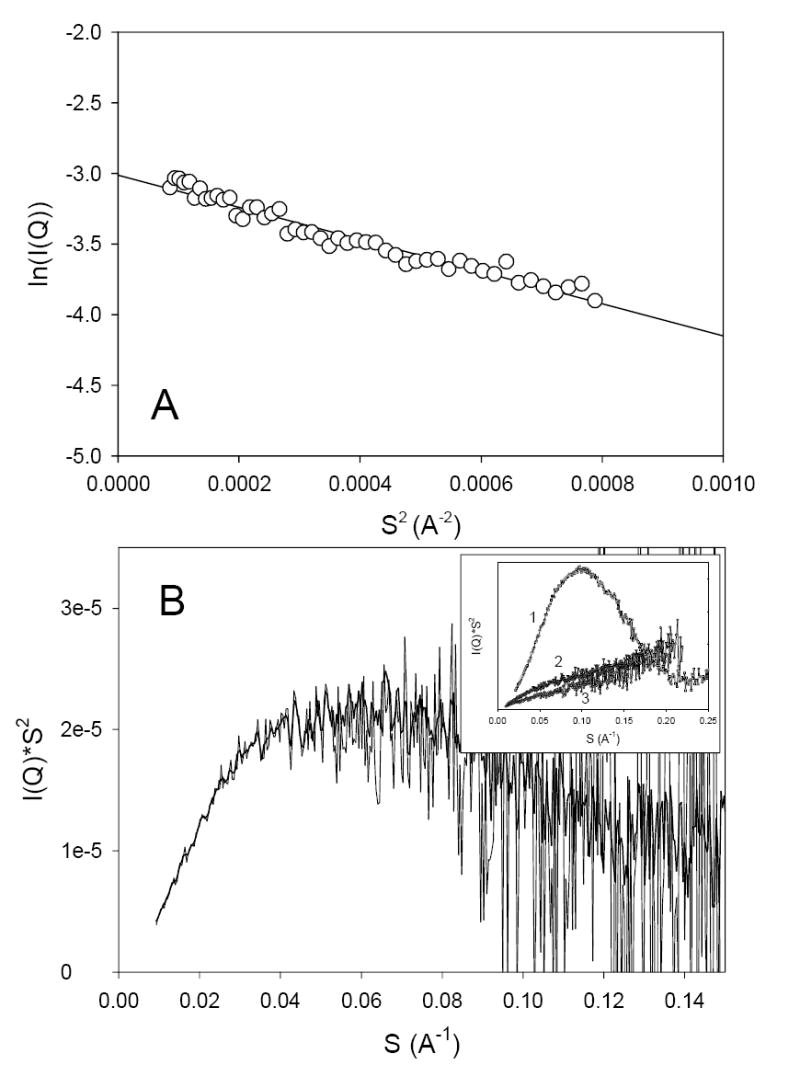
Guinier (A) and Kratky plot (B) representation of the results of small-angle X-ray scattering analysis of baicalein-stabilized oligomers of α-synuclein. Inset represents Kratky plots for staphylococcal nuclease (1) and monomeric α-synuclein in natively unfolded (3) and pre-molten globule-like conformations (2).
Analysis of the X-ray scattering in the form of a Kratky plot shows that the baicalein-stabilized oligomers have rather well-developed globular structure (Figure 3B), indicating packing density intermediate between that of pre-molten globules and typical globular proteins. This conclusion is confirmed by the data shown in inset to Figure 3B which represents Kratky plots for the typical globular protein, Staphylococcal nuclease (curve 1), and α-synuclein in its natively unfolded (curve 3) and partially-folded, pre-molten globule-like conformation (curve 2) stabilized at acidic pH.
Secondary structure of the baicalein-stabilized oligomers from FTIR and far-UV CD analyses
Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) analysis was used to evaluate the secondary structure of the baicalein-stabilized oligomers. FTIR spectra in the amide I region (1700-1600 cm-1), which is especially sensitive to the β-structure, were collected. Figure 4A shows the FTIR amide I region spectrum for the α-synuclein monomer. This spectrum is typical of a substantially unfolded protein. The major structural changes associated with the protein fibrillation occurred around 1630 cm-1, reflecting dramatic increase in so-called aggregation β-structure content (Figure 4B). Interestingly, the baicalein-stabilized oligomers showed an enlarged shoulder in the vicinity of 1630 cm-1, corresponding to the increased β-structure (Figure 4C). The secondary structure content of each species was estimated after curve fitting followed by the deconvolution with Fourier self-deconvolution and second derivatives. The analysis of the baicalein-stabilized oligomers showed a larger fraction of β-sheet/extended structure (38 ± 6 %), compared to monomer (20 ± 6 %). This contribution of β-sheet/extended structure further increased to 58 ± 7 % in the fibrillar form. Overall, the secondary structure of baicalein-stabilized oligomers was similar to that of amyloidogenic partially folded intermediate of α-synuclein formed at pH 3.0 reported in previous paper 7. The spectrum of baicalein-monomer also shows a small shoulder at 1630 cm-1 (Figure 4D), indicating a partially folded structure which may be induced by baicalein binding. The fraction of β-sheet/extended structure for baicalein-oligomer was 28 ± 4 %.
Figure 4.
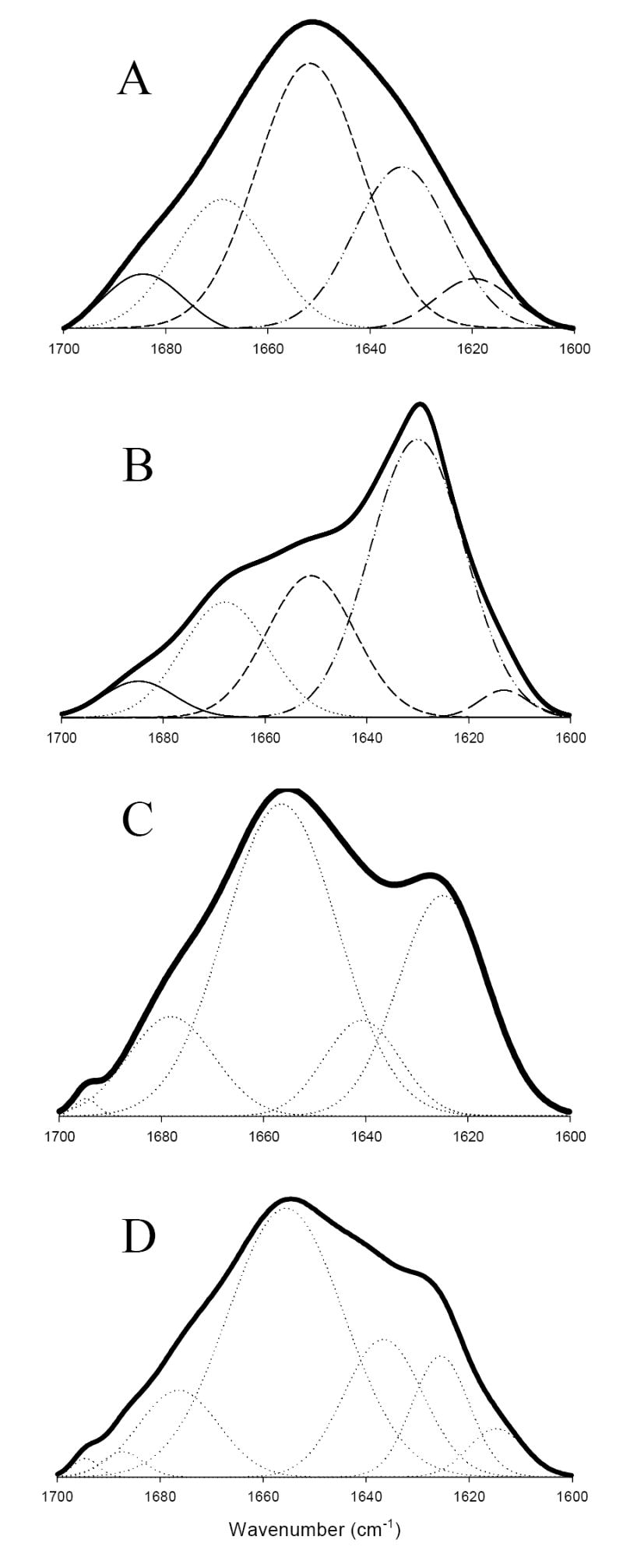
Baicalein-stabilized oligomers show β-sheet structure. FTIR spectra of (A) monomeric α-synuclein, (B) fibrils, (C) baicalein-stabilized oligomers, and (D) baicalein-bound monomer.
Analysis of the far-UV CD spectra for all these α-synuclein forms produced similar result (Figure 5A). We have previously showed that baicalein induced a noticeable change in the molar ellipticity, especially at 218 nm, indicating the presence of a partially folded conformation with significant β-structure 16. In this previous study, the baicalein-stabilized oligomers were not separated from the baicalein-bound monomers. Consistent with the FTIR results, Figure 5A shows that the far-UV CD spectrum of the purified baicalein-stabilized oligomers possessed shape typical for a β-structural protein, whereas the baicalein-bound monomers were characterized by a spectrum typical for a partially folded polypeptide.
Figure 5.

Baicalein-stabilized oligomers are thermodynamically stable. (A) far UV-CD spectra of (○) monomeric α-synuclein, (Δ) baicalein-bound monomer and (□) baicalein-stabilized oligomers. (B) GdmCl-induced conformational transition of baicalein-stabilized oligomers, measured by the ellipticity at 229 nm. (C) A linear dependence of the free energy change of denaturation upon GdmCl concentration.
Termodynamic stability of the baicalein-stabilized oligomers
To understand the thermodynamic stability of baicalein-stabilized oligomers, we examined their GdmCl-induced unfolding by monitoring the GdmCl-promoted changes in the ellipticity of the baicalein-stabilized oligomers at 229 nm. Figure 5B shows that it takes >2.0 M GdmCl to initiate the noticeable changes in secondary structure of the baicalein-stabilized oligomers. The unfolding transition is highly cooperative, being completed by 4.0 M GdmCl. The thermodynamic analysis of this process was performed assuming that the unfolding reaction occurred via a two-state mechanism. The Gibbs free energy change (ΔG) was then calculated for each data point using the equation ΔG = -RT lnK. ΔG was plotted against the concentration of GdmCl (Figure 5C) and fitted by the linear equation: ΔG = ΔG0 − m[GdmCl] where ΔG0 is the free energy of unfolding in the absence of denaturant and the slope (m) is a measure of the dependence of the free energy of unfolding on the concentration of GdmCl. The midpoint concentration (Cm) was calculated from the relationship Cm = ΔG0/m; i.e., as a GdmCl concentration where ΔG = 0. This analysis gave an m value of 1.2 kcal/mol·M, a ΔG0 value of 3.98 kcal/mol, and a Cm value of 3.3 M. These thermodynamic parameters reflect that the baicalein-stabilized oligomers of α-synuclein are rather stable entities. In agreement with this data, there was no change in the shapes of the oligomers and fibrils were not formed even after the prolonged incubation (up to one month) of α-synuclein with baicalein. This high conformational stability of the baicalein-bound oligomers can also be used to explain the inhibitory properties of baicalein toward α-synuclein fibrillation. A relatively low m value indicates that the surface area exposed to the solvent during unfolding is relatively small. This is consistent with the SAXS data, which shows that the baicalein-stabilized ologimers possess packing density intermediate between that of pre-molten globules and typical globular proteins.
The dissociation of the baicalein-stabilized oligomers at various GdmCl concentrations was investigated by SEC-HPLC (Figure 6). Amazingly, these oligomers never completely dissociated into the monomer even in the presence of high GdmCl concentrations. In fact, the peak corresponding to the monomeric α-synuclein was not observed even at the very high GdmCl concentrations. The only effect of the denaturant was the broadening of the elution peak. Samples from all the oligomer peaks still had baicalein molecules as assessed by the presence of the specific 360 nm peak in the UV absorption spectra.
Figure 6.

SEC-HPLC profiles of baicalein-stabilized oligomers in the presence of various GdmCl concentrations.
Baicalein-stabilized oligomers inhibit the α-synuclein fibrillation
Figure 7 represents an intriguing set of data on the effect of baicalein-stabilized oligomers on the α-synuclein fibrillation kinetics. The addition of 5-20% baicalein-stabilized oligomers substantially increased the duration of the lag time, decreased the fibril elongation rate, and decreased the maximum intensity of the ThT fluorescence. We have checked the amount of the non-fibrillar protein in supernatant after the fibrillation by SDS-PAGE. This analysis revealed that more protein remained in the supernatant after the addition of baicalein-stabilized oligomers (data not shown). This suggests that the baicalein-stabilized oligomers are able to inhibit the α-synuclein fibrillation likely via interaction with the monomers. The inhibition of the fibrillation by the baicalein-stabilized oligomers was further confirmed by EM analysis (Figure 8). Although 2-5 μm long fibrils, with a diameter of 9-12 nm were abundantly observed in a control sample where α-synuclein was incubated alone (Figure 8A), shorter and fewer fibrils were induced when the increasing amount of the baicalein-stabilized oligomers were added (Figures 8B-D). These results provided a strong support to the hypothesis that the thermodynamically stable baicalein-stabilized oligomers never transform to the fibrils as well as interact with monomeric α-synuclein and prevent it from fibrillation.
Figure 7.

Baicalien-stabilized oligomers inhibit the α-synuclein fibrillation. The fibrillation kinetics were studied for 70 μM α-synuclein solution in 20 mM Na-phosphate buffer, 100 mM NaCl, pH 7.5 in the absence (●) or the presence of baicalein-stabilized oligomers with (○) 5 %, (▼) 10 %, and (∇) 20 %.
Figure 8.

Electron microscope images of fibrils formed in the absence (A) and the presence of (B) 5 %, (C) 10 %, and (D) 20 % of baicalein-stabilized oligomers.
The baicalein-stabilized oligomers have a mild effect to lipid membrane
To understand the effect of baicalein-stabilized oligomers on the integrity of lipid membrane, we have prepared calcein encapsulated in large unilamellar vesicles (LUVs) of PA/PC. The encapsulated fluorophore has low fluorescence intensity because of self-quenching due to its high concentration in the vesicle. The fluorescence intensity increases on release of the dye from the vesicle core induced after the addition of an aliquot of a membrane-disruptive agent. Table I shows the extent of the calcein leakage induced by monomeric α-synuclein, its fibrils and baicalein-stabilized oligomers. Whereas monomeric α-synuclein resulted in 10 % release of the dye, the fibrils induced significantly greater release of the vesicle-encapsulated dye, indicating that α-synuclein fibrils were significantly more effective in membrane disruption than the monomeric α-synuclein. Interestingly, the baicalein-stabilized oligomers showed a mild effect on the membrane integrity, which was comparable to the effect of the monomeric α-synuclein.
Table I.
Baicalein-stabilized oligomer-induced membrane permeability. The concentration of lipid and protein were 0.05 and 0.0125 mg/ml, respectively.
| Phospholipid | Fibril | Monomer | Baicalein-stabilized oligomer |
|---|---|---|---|
| PA/PC (1:1) | 25.5 (± 4.2) | 9.8 (± 2.2) | 10.0 (± 2.6) |
The baicalein-controlled oligomerization of α-synuclein as a prototype of a new strategy for the development of therapies for Parkinson’s disease
For a long time it was believed that the amyloid fibrils are harmful. However, a novel emerging paradigm favors the idea that the deposited proteinacous inclusions (such as senile plaques in Alzheimer’s disease or Lewy bodies or Lewy neurites in Parkinson’s disease, etc.) are not toxic, but the formation of some small oligomers, different protofibrillar structures, are responsible for the neurotoxicity 10;24-26. This hypothesis is supported by the observation that the quantity of fibrillar deposits at autopsy does not typically correlate with the clinical severity of neurodegenerative disorderes such as Alzheimer’s or Parkinson’s disease 26. Furthermore, animal models of these pathological conditions develop disease-like phenotypes before fibrillar deposits can be detected 27;28. Finally, non-fibrillar oligomers of various amyloidogenic proteins (both disease-realted and non-disease-associated) were shown to be toxic in cell culture and to ne able to disrupt membrane inegrity in vitro 10;26;29-32. Special attention has been paid to the so-called amyloid pores; i.e., morphologically similar annular protofibrils, that resemble a class of pore-forming bacterial toxin, as these doughnut-shaped oligomers might cause the inappropriate membrane permealization leading to cell disfunction and finally cell death 10;26;32-34.
It is important to remember, however, that protein aggregates are highly heterogenious. This heterogeneity might be caused either by the heterogeneous starting materials or by multiple pathways of assembly, or by both. For example, α-synuclein was even termed protein-chameleon for its outstanding capability to adopt a wide range of structurally different conformations, including various monomeric species, small oligomers, large soluble oligomers with different morphologies (both spherical and annular), amorphous aggregates and fibrils 35. Therefore, it is difficult to believe that all the soluble oligomers, with their astonishing morphological variability, will be cytotoxic to the same degree. In agreement with previous studies 16, we are showing here that the flavonoid baicalein is able to inhibit the α-synuclein fibrillation via the stabilization of specific oligomers. These baicalein-stabilized oligomers possess very characteristic structural features, being spherical in shape (according to the AFM and EM data), having relatively globular structure with packing density intermediate between that of pre-molten globules and typical globular proteins (according to the Kratky plot analysis of the SAXS data), and having relatively well-developed secondary structure (according to the FTIR and far-UV CD analysis). These oligomers are characterized by high thermodynamic stability (as evidenced by the results of the unfolding analyses and by the fact that their shape did not change after the prolonged incubation) and are able to inhibit fibrillation of non-baicalein-treated α-synuclein. The most important finding of this paper is the fact that these highly stable and fibrillation-inhibiting oligomers do not disrupt the integrity of the biological membrane (at least they are not more disruptive than the monomeric protein). This is a very important observation, which suggests that the soluble oligomer formation does not always create harm and can be beneficial. We believe that these findings pave the road for the development of the novel therapies for Parkinson’s disease, whose molecular mechanism of action could be similar to that of baicalein – specific stabilization of the thermodynamically stable, non-fibrillating soluble oligomers that can inhibit α-synuclein fibrillation and do not cause the inappropriate membrane permealization.
Materials and methods
Expression and purification of human α-synuclein
Human wild type α-synuclein was expressed using Escherichia coli BL21 (DE3) cell line transfected with the pRK172/α-synuclein WT plasmid (generously donated by M. Goedert, MRC Cambridge). Expression and purification of human recombinant α-synuclein from E. coli were performed as previous described 36.
Materials
Baicalein and uranyl acetate dihydrate were obtained from Sigma (St. Louis, MO). Thioflavin T (ThT) was purchased from Fluka. All buffer and solution were prepared with nanopure water. 1,2-Dipalmitoyl-sn-glycero-3-phosphate (PA), and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (PC) were purchased from Avanti Polar Lipids as chloroform solutions.
ThT Fluorescence Assays for Fibrillation
The flavonoid baicalein were dissolved in Me2SO to make a stock solution at a concentration of 10 mM. Lyophilized α-synuclein was dissolved in 0.001 M NaOH and adjusted to pH 7.4 prior to centrifugation at 95,000 rpm with a Beckman Airfuge ultracentrifuge to remove any aggregated material. The protein solution (1 mg/ml; 70 μM) was mixed with flavonoid to give a final concentration of 100 μM with a final Me2SO concentration of 1%. A control α-synuclein solution containing 1% Me2SO was also prepared. All of the solutions were in the 20 mM Tris-HCl buffer at pH 7.4, 100 mM NaCl, and were stirred at 37 °C with a mini-Teflon stir bar. Aliquots of 5 μl were removed from the incubated solution and added to 1 ml of 10 μM ThT solutions in 50 mM Tris-HCl buffer (pH 8.0) as a function of time to monitor the fibrillation kinetics. ThT fluorescence was recorded at 482 nm with excitation at 450 nm and slits of 2.5 nm for both excitation and emission using a FluoroMax-3 spectrofluorometer (Jobin Yvon Horiba).
Circular Dichroism Measurements
CD spectra were measured with an AVIV 60DS spectrophotometer (Lakewood, NJ). Spectra were recorded in a 0.1-cm path length round cell from 250 to 190 nm. For all spectra, an average of 5 scans was obtained. CD spectra of the appropriate buffers were recorded and subtracted from the protein spectra.
Electron Microscopy
Transmission electron micrographs were collected using a JEOL JEM-100B microscope operating with an accelerating voltage of 80 kV. Typical nominal magnifications ranged from × 30,000 to 75,000. Samples were deposited on Formvar-coated 300 mesh copper grids and negatively stained with 1 % aqueous uranyl acetate.
Atomic Force Microscopy
For AFM imaging, aliquots of 5 μl of sample were placed on a freshly cleaved mica substrate. After incubation for 60 min, the substrate was rinsed with water to remove salt and loosely bound protein and blown dry with N2. AFM images were obtained with a PicoScan Plus microscopy (Molecular Imaging, Phoenix, AZ) equipped with the MAC mode in which the magnetically coated probe oscillates near its resonant frequency under an alternating magnetic field. Probes with a 2.8 newton/m force constant and a 75-kHz resonance frequency (Molecular Imaging) were used for the MAC mode imaging. The imaging was carried out at a scan rate of 1 line/s with 512 data points per line, at a drive current of 10 ± 4 Å. Height ranging from 0.5 – 100 nm were estimated by section analysis. At least four regions of the mica surface were examined to verify that similar structures existed through the sample. No filter treatment was used to modify the images. SPIP 4.0 (Image Metrology) was used to analyze the height, area and volume distribution of the α-synuclein oligomers.
ATR-FTIR Spectroscopy
Attenuated total reflectance Fourier transform infrared spectra were recorded on a ThermoNicolet Nexus 670 FTIR spectrometer equipped with an MCT detector. The samples were prepared as hydrated thin films as previously described 37;38 on the surface of an out-of-compartment germanium trapezoidal internal reflectance element (IRE). 512 interferograms were co-added at 1 cm-1 resolution to generate each spectrum. Spectra were deconvoluted to ascertain the percents of secondary structure using the program GRAMS32 (Galactic Industries). Fourier self deconvolution (FSD) and second derivatives were used to deconvolve the spectra. Peak positions identified by both were used for the curve-fitting routine. In curve-fitting, the initial peak positions found were fixed and the peak width was allowed to vary from 15 to 30 cm-1. Peak height, width, and percent Lorentzian were allowed to vary until the solution converged to a minimum, at which time the constraint on the peak positions was removed and curve-fitting continued until the minimum was reached. Percent secondary structure peak assignments were made as previously published 37;38.
Small Angle X-ray Scattering measurement
SAXS measurements were performed at the Beam Line 4-2 at Stanford Synchrotron Radiation Laboratory. X-ray energy was selected at 8980 eV (Cu edge) by a pair of Mo/B4C multilayer monochromator crystals 39. Scattering patterns were recorded by a linear position-sensitive proportional counter, which was filled with an 80% Xe/20% CO2 gas mixture. Scattering patterns were normalized by incident X-ray fluctuations, which were measured with a short length ion chamber before the sample. The sample-to-detector distance was calibrated to be 230 cm, using a cholesterol myristate sample. The measurements were performed in a 1.3-mm width capillary cell. To avoid radiation damage of the sample in SAXS measurements, the protein solution was continuously moved in the capillary cell. Background measurements were performed before and after each protein measurement and then averaged before being used for background subtraction. All SAXS measurements were performed at 37 ± 1 °C. The radius of gyration (Rg) was calculated according to the Guinier approximation 21:
| (1) |
where Q is the scattering vector given by Q = (4π sinθ)/λ, where 2θ is the scattering angle, and λ is the wavelength of X-ray. I(0), the forward scattering amplitude, is proportional to molecular mass of the scattering profile.
Size exclusion chromatography (SEC)
SEC data were collected on a Waters 2695 Separation Module (Milfold, MA) with a Waters 996 Photodiode Array Detector and Millennium 32 software. Protein was loaded onto a Tosoh Bioscience G2000SWXL and G4000SWXL columns. The elution was carried out at a flow rate of 0.5 – 1.0 mL/min and monitored by the absorbance at 275 nm.
UV absorption spectroscopy
Absorption spectra over the range from 220 to 500 nm were measured using a UV-2401 PC UV-visible recording spectrophotometer (Shimadzu, Japan) to detect the binding of baicalein molecules to monomer and oligomer of α-synuclein purified from SEC-HPLC.
Lipid Membrane Permeability
Calcein-loaded LUVs of PA/PC (molar ratio of 1:1) were prepared by hydrating lipid films in the presence of calcein, and separated from free dye using a Sepharose 4B column (Sigma) (1.5 × 12 cm) as described previously 40. The change in the fluorescence intensity of released calcein was monitored at 510 nm (excitation 490 nm) after incubating proteins and dye-containing vesicles at 25 °C in 20 mM Tris-HCl buffer, 100 mM NaCl, pH 7.4, until the dye release reached its end point, around 30 min. Total dye release was completed by the addition of 0.2 vol % Triton X-100. The percentage of probe release was calculated as follows,
| (2) |
where IF, IT, and IB are the fluorescence intensity of the dye released by the protein, total dye released, and control blank, respectively.
Acknowledgments
This research was supported in part by grants R01 NS39985 (to D.-P.H and A.L.F.), R01 LM007688-01A1 (V.N.U.) and GM071714-01A2 (V.N.U.) from the National Institutes of Health. We gratefully acknowledge the support of the IUPUI Signature Centers Initiative.
The abbreviations used are
- PD
Parkinson’s disease
- LUV
large unilamellar vesicle
- PA
1,2-dipalmitoyl-sn-glycero-3-phosphate
- PC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- AFM
atomic force microscopy
- EM
electron microscopy
- SAXS
small-angle X-ray scattering
- ThT
thioflavin T
- CD
circular dichroism
- ATR-FTIR
attenuated total reflectance Fourier transform infrared
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkinson J. An essay on shaking palsy. Sherwood, Neely and Jones; London: 1817. [Google Scholar]
- 2.Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 4.Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 5.Irizarry MC, Kim TW, McNamara M, Tanzi RE, George JM, Clayton DF, Hyman BT. Characterization of the precursor protein of the non-A beta component of senile plaques (NACP) in the human central nervous system. J Neuropathol Exp Neurol. 1996;55:889–95. doi: 10.1097/00005072-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 7.Uversky VN, Li J, Fink AL. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 8.Eliezer D, Kutluay E, Bussell R, Jr, Browne G. Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 9.Trojanowski JQ, Lee VM. Parkinson’s disease and related alpha-synucleinopathies are brain amyloidoses. Ann N Y Acad Sci. 2003;991:107–110. doi: 10.1111/j.1749-6632.2003.tb07468.x. [DOI] [PubMed] [Google Scholar]
- 10.Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT., Jr Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 11.Volles MJ, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 12.Volles MJ, Lansbury PT., Jr Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson’s disease. Biochemistry. 2003;42:7871–7878. doi: 10.1021/bi030086j. [DOI] [PubMed] [Google Scholar]
- 13.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Zhu M, Rajamani S, Uversky VN, Fink AL. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem Biol. 2004;11:1513–1521. doi: 10.1016/j.chembiol.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Zhu M, Manning-Bog AB, Di Monte DA, Fink AL. Dopamine and L-dopa disaggregate amyloid fibrils: implications for Parkinson’s and Alzheimer’s disease. Faseb J. 2004;18:962–964. doi: 10.1096/fj.03-0770fje. [DOI] [PubMed] [Google Scholar]
- 16.Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J Biol Chem. 2004;279:26846–26857. doi: 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]
- 17.Li BQ, Fu T, Gong WH, Dunlop N, Kung H, Yan Y, Kang J, Wang JM. The flavonoid baicalin exhibits anti-inflammatory activity by binding to chemokines. Immunopharmacology. 2000;49:295–306. doi: 10.1016/s0162-3109(00)00244-7. [DOI] [PubMed] [Google Scholar]
- 18.Ikezoe T, Chen SS, Heber D, Taguchi H, Koeffler HP. Baicalin is a major component of PC-SPES which inhibits the proliferation of human cancer cells via apoptosis and cell cycle arrest. Prostate. 2001;49:285–292. doi: 10.1002/pros.10024. [DOI] [PubMed] [Google Scholar]
- 19.Gao Z, Huang K, Xu H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol Res. 2001;43:173–178. doi: 10.1006/phrs.2000.0761. [DOI] [PubMed] [Google Scholar]
- 20.Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20:2861–2865. [PubMed] [Google Scholar]
- 21.Glatter O, Kratky O. Small-angle X-ray scattering. Academic Press; London, New York: 1982. [Google Scholar]
- 22.Li J, Uversky VN, Fink AL. Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein. Biochemistry. 2001;40:11604–11613. doi: 10.1021/bi010616g. [DOI] [PubMed] [Google Scholar]
- 23.Gast K, Damaschun H, Eckert K, Schulze-Forster K, Maurer HR, Muller-Frohne M, Zirwer D, Czarnecki J, Damaschun G. Prothymosin alpha: a biologically active protein with random coil conformation. Biochemistry. 1995;34:13211–13218. doi: 10.1021/bi00040a037. [DOI] [PubMed] [Google Scholar]
- 24.Urbanc B, Cruz L, Le R, Sanders J, Ashe KH, Duff K, Stanley HE, Irizarry MC, Hyman BT. Neurotoxic effects of thioflavin S-positive amyloid deposits in transgenic mice and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2002;99:13990–5. doi: 10.1073/pnas.222433299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–8. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 27.Lansbury PT., Jr Evolution of amyloid: what normal protein folding may tell us about fibrillogenesis and disease. Proc Natl Acad Sci U S A. 1999;96:3342–3344. doi: 10.1073/pnas.96.7.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg MS, Lansbury PT., Jr Is there a cause-and-effect relationship between alpha-synuclein fibrillization and Parkinson’s disease? Nat Cell Biol. 2000;2:E115–119. doi: 10.1038/35017124. [DOI] [PubMed] [Google Scholar]
- 29.Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19:8876–84. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–8. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 31.Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–11. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 32.Rochet JC, Outeiro TF, Conway KA, Ding TT, Volles MJ, Lashuel HA, Bieganski RM, Lindquist SL, Lansbury PT. Interactions among alpha-synuclein, dopamine, and biomembranes: some clues for understanding neurodegeneration in Parkinson’s disease. J Mol Neurosci. 2004;23:23–34. doi: 10.1385/jmn:23:1-2:023. [DOI] [PubMed] [Google Scholar]
- 33.Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774–9. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- 34.Lashuel HA, Lansbury PT., Jr Are amyloid diseases caused by protein aggregates that mimic bacterial pore-forming toxins? Q Rev Biophys. 2006;39:167–201. doi: 10.1017/S0033583506004422. [DOI] [PubMed] [Google Scholar]
- 35.Uversky VN. A protein-chameleon: conformational plasticity of alpha-synuclein, a disordered protein involved in neurodegenerative disorders. J Biomol Struct Dyn. 2003;21:211–234. doi: 10.1080/07391102.2003.10506918. [DOI] [PubMed] [Google Scholar]
- 36.Yamin G, Uversky VN, Fink AL. Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS Lett. 2003;542:147–152. doi: 10.1016/s0014-5793(03)00367-3. [DOI] [PubMed] [Google Scholar]
- 37.Oberg K, Chrunyk BA, Wetzel R, Fink AL. Nativelike secondary structure in interleukin-1 beta inclusion bodies by attenuated total reflectance FTIR. Biochemistry. 1994;33:2628–34. doi: 10.1021/bi00175a035. [DOI] [PubMed] [Google Scholar]
- 38.Oberg KA, Fink AL. A new attenuated total reflectance Fourier transform infrared spectroscopy method for the study of proteins in solution. Anal Biochem. 1998;256:92–106. doi: 10.1006/abio.1997.2486. [DOI] [PubMed] [Google Scholar]
- 39.Tsuruta H, Brennan S, Rek ZU, Irving TC, T WH, Hodgson KO. A wide-bandpass multilayer monochromator for biological small-angle scattering and fiber diffraction studies. J Appl Crystallogr. 1998;31:672–682. [Google Scholar]
- 40.Zhu M, Li J, Fink AL. The association of alpha-synuclein with membranes affects bilayer structure, stability, and fibril formation. J Biol Chem. 2003;278:40186–97. doi: 10.1074/jbc.M305326200. [DOI] [PubMed] [Google Scholar]


