Abstract
The eukaryotic translation initiation factor eIF4E is dysregulated in many cancers. eIF4E, through its mRNA export and translation functions, combinatorially modulates the expression of genes involved in Akt dependent survival signaling. For these activities, eIF4E must bind the 7-methyl guanosine (m7G) cap moiety on the 5’end of mRNAs. We demonstrate that a physical mimic of the m7G cap, ribavirin, inhibits eIF4E dependent Akt survival signaling. Specifically, ribavirin impairs eIF4E mediated Akt activation via inhibiting the production of an upstream activator of Akt, NBS1. Consequently, ribavirin impairs eIF4E dependent apoptotic rescue. A ribavirin analogue with distinct physico-chemical properties, tiazofurin, does not impair eIF4E activity indicating that only analogues that mimic the m7G cap will inhibit eIF4E function. Ribavirin represents a first-in-class strategy to inhibit eIF4E dependent cancers, through competition for m7G cap binding. Thus, ribavirin coordinately impairs eIF4E dependent pathways and thereby, potently inhibits its biological effects.
Keywords: eIF4E, Akt, m7G cap, ribavirin, RNA regulon, NBS1
Introduction
The eukaryotic translation initiation factor, eIF4E, is highly elevated in many cancers, and even moderate overexpression leads to cellular transformation, and tumorigenesis in mouse models [1–4]. eIF4E is found in both the nucleus and cytoplasm, functioning in mRNA export and translation initiation respectively. Both activities require eIF4E to bind the 7-methyl guanosine cap (m7G) found on the 5’ end of mRNAs [5]. Significantly, eIF4E does not affect mRNA export or translation of all transcripts to the same extent. For instance, eIF4E overexpression does not affect mRNA export or translational efficiency of GAPDH [5–8]. Importantly, eIF4E requires cap-binding, mRNA export, and translation activities for its oncogenic potential [1, 5, 8–10].
Recent findings indicate that eIF4E acts as a network node in an RNA regulon governing proliferation and survival signaling [10, 11]. The RNA regulon model describes how eukaryotic gene expression can be coordinately and combinatorially controlled through untranslated sequence elements for regulation (USER) codes, sensitizing them to specific levels of regulation [12]. For instance, RNAs sensitive to eIF4E at the mRNA export level contain a ~50 nucleotide region in the 3’ UTR known as the eIF4E sensitivity element (4E-SE) [5, 11]. Thus, any set of RNAs containing the 4E-SE can be coordinately exported to the cytoplasm by eIF4E, increasing cytoplasmic levels of these mRNAs and thus increased protein production. Similarly, mRNAs can contain a USER code in their 5’UTR for translational regulation [1, 5, 7]. These elements are not exclusive, for instance c-myc and ornithine decarboxylase expression are enhanced both by increasing their mRNA in the cytoplasm, and by loading that mRNA onto heavier (more efficient) polysomes [6]. Hence a given transcript can be sensitive to eIF4E at either one or both levels depending on its complement of USER codes.
An example of the central position eIF4E plays in its RNA regulon is its ability to coordinately enhance Akt survival signaling at two distinct levels [10]. First, eIF4E overexpression leads to enhanced Akt activation via upregulation of an activator of Akt, Nijmegen breakage syndrome 1 protein (NBS1) [10, 13]. Second, eIF4E, via its mRNA export and in some cases its translation activity, coordinately upregulates the expression of downstream effectors of Akt including cyclins B1, E1, D1, c-myc and MDM2 [11]. This two-tier effect likely explains the potent transforming effects of eIF4E, and underlies its position as a central node in this RNA regulon [10, 11].
Elevated eIF4E levels observed in several cancers suggest it is an important therapeutic target. However, initial concern with this strategy arose because eIF4E is required for cell survival. Yet studies of primary acute myeloid leukemia (AML) specimens with elevated levels of eIF4E (French-American-British (FAB) subtype M4/M5 AML) suggested that these cells developed an oncogene addiction to eIF4E [14, 15]. The proliferation of these specimens was more sensitive to eIF4E inhibition than normals or those derived from unrelated leukemias. Two recent studies using a mouse model of prostate cancer and human breast carcinoma cells support the idea of oncogene addiction to eIF4E [2, 3].
A clear means to impair eIF4E activity is to inhibit cap binding. Previous studies indicate that ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) acts as a physical mimic of the m7G cap and subsequently, inhibits eIF4E activity [15]. Both the m7G cap and ribavirin are positively charged at physiological pH, allowing these compounds to intercalate between two tryptophans in the cap-binding site of eIF4E [15, 16]. Ribavirin directly binds eIF4E and competes for m7G cap binding as observed by mass spectrometry, fluorescence, cap-competition chromatography and NMR as well as competition experiments in cells [15, 16]. Ribavirin treatment represses transformation in cancer models characterized by dysregulated eIF4E, including growth of M4/M5 AML primary patient specimens, xenograft mouse models of head and neck squamous cell carcinomas, and mouse models of metastatic disease [15, 17]. Ribavirin treatment is currently being tested in a phase I/II clinical trial for its efficacy in treating refractory M4/M5 AML, as a model of eIF4E dysregulated cancer (www.ribatrial.com). Thus, ribavirin is the first clinical intervention implemented for targeting eIF4E activity in cancers characterized by elevated eIF4E [15].
Given these previous findings, we investigated whether ribavirin impaired eIF4E dependent survival signaling, and we demonstrate that ribavirin treatment inhibits eIF4E mediated apoptotic rescue via inhibiting the Akt pathway. Further we show that a ribavirin analogue, tiazofurin (2-β-d-ribofuranosylthiazole-4-carboxamide) which is neutrally charged (Figure 1A) and unable to bind eIF4E, does not impair eIF4E function. Tiazofurin and ribavirin are considered to be functionally analogous compounds; however, our results suggest that in low micromolar range, they have distinct effects. Taken together, our studies demonstrate that ribavirin has network-wide effects suggesting strategies developed to target the eIF4E RNA regulon will be therapeutically valuable.
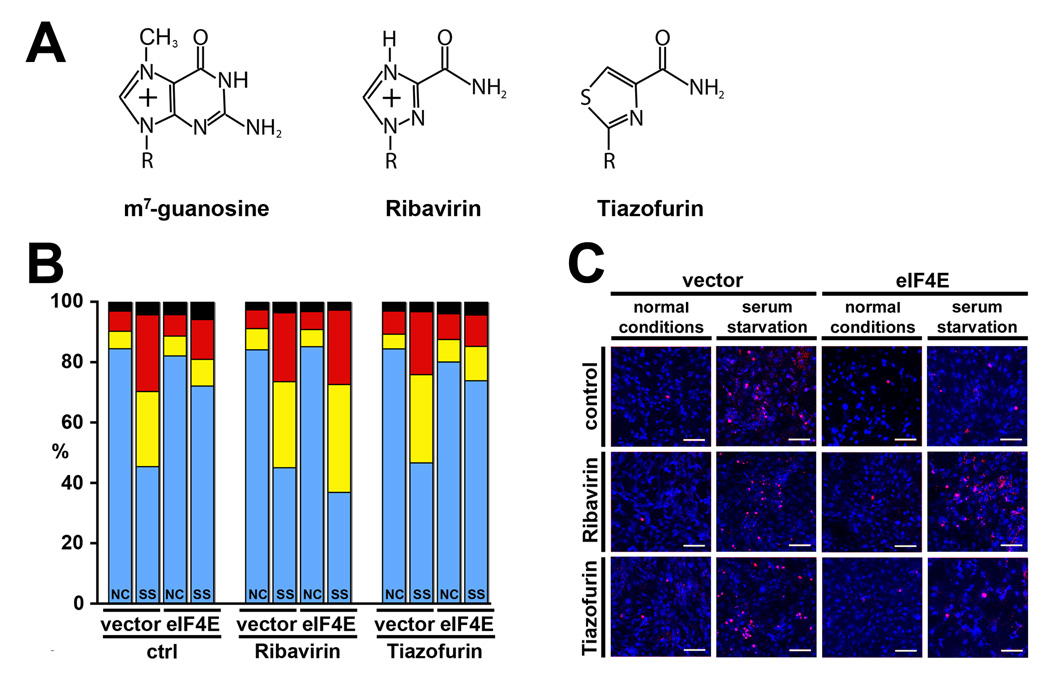
Figure 1.
Ribavirin impedes eIF4E mediated apoptotic rescue of serum-starved fibroblasts. (A) Chemical structures of m7-guanosine (m7G), ribavirin and tiazofurin. R represents the d-ribose sugar. (B) 1µM ribavirin, but not 1µM tiazofurin, inhibits eIF4E mediated rescue in murine fibroblasts as quantified by Annexin V – propidium iodide (PI) flow cytometry (bar color is as follows: blue – Annexin V−/PI−; yellow – Annexin V+/PI−; red – Annexin V+/PI+; black – Annexin V−/PI+; NC, normal controls; SS, serum starved; standard deviation is less than 9% for all measurements), and visualized by (C) TUNEL assays (blue – DAPI (viable), red – apoptotic; scale bar represents 100µm).
Materials and Methods
Cell culture and Treatments
U-2 OS lacZ and Akt1 wildtype derived murine fibroblast cell lines were as described [10, 18]. FaDu cells were maintained in 5% CO2 at 37°C in MEM with 10% FBS, 100units/ml penicillin G sodium and 100µg/ml streptomycin sulfate, supplemented with non-essential amino acids (all from GibcoBRL). Ribavirin (Kemprotec Ltd) and Tiazofurin (NCI, and kind gift from B. Mitchell, Stanford University, CA) were dissolved in PBS (GibcoBRL) at indicated concentrations and durations. Mass spectrometry analysis indicated that ribavirin and tiazofurin were >90% pure.
Anchorage dependent foci assays with ribavirin or tiazofurin treated FaDu cells were performed as described [15]. Briefly, cells seeded in a 10cm plate were treated for 10 – 12 days, then stained with Giemsa according to manufacturer’s conditions (Sigma). Average foci counts are represented graphically with errors shown from triplicates.
Apoptosis assays
Exponentially growing cell cultures were treated with ribavirin or tiazofurin for 48hrs, and then shifted to 0.1% serum conditions for the final 20hrs. Annexin V – propidium iodide staining and analyses, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), and laser-scanning confocal microscopy were as described [10]. Images were obtained using LSM510 software (Carl Zeiss, Inc) and were displayed using Photoshop CS 8.0 (Adobe).
Western analyses and Antibodies
Western analyses were performed as described [10]. Antibodies used for immunoblotting were as follows: anti-eIF4E (BD PharMingen); anti-NBS1, anti-Akt, anti-phospho T308Akt, anti-phospho S473Akt (all from Cell Signaling); anti-β-actin (AC-15), anti-α-tubulin (all from Sigma); anti-cyclin D1 (Santa Cruz); and anti-Xpress (for LacZ detection; Invitrogen).
Cellular Fractionation and qPCR
Fractionation, RNA isolation, primers, and conditions used for qPCR analyses were performed as described [10, 11]. All subsequent calculations were performed using the relative standard curve method described in Applied Biosystmes User Bulletin #2.
Results and Discussion
Ribavirin inhibits eIF4E mediated apoptotic rescue
eIF4E mediated rescue of fibroblasts from serum deprivation occurs via the Akt signaling pathway [10]. To examine the impact of ribavirin on eIF4E dependent survival signaling, we monitored apoptosis in serum deprived murine fibroblasts. In untreated control cells, serum deprivation reduces viability by about two fold (80% to 40%), whilst eIF4E overexpression leads to rescue from apoptosis (80% viable cells) as observed by both flow cytometry and TUNEL assays (Figure 1B–C).
For ribavirin treatment, we used 1µM concentrations as this level of ribavirin is readily achievable in patients and is not associated with significant toxicity [19]. The addition of ribavirin impeded the rescue activity of eIF4E, with levels of apoptosis similar to the untreated serum deprived controls (40% versus 80% viable cells) (Figure 1B). Results were confirmed by TUNEL (Figure 1C). Note that ribavirin, at this concentration, did not significantly impact on viability in non-serum starved controls (Figure 1B–C).
To establish whether ribavirin inhibited eIF4E mediated apoptotic rescue via modulating Akt signaling, Akt activation was assessed by monitoring the phosphorylation at S473Akt and T308Akt. As shown previously, levels of Akt phosphorylation are higher in eIF4E-overexpressing cells relative to vector controls (Figure 2A; [10]). However, ribavirin treatment leads to a dramatic reduction in Akt phosphorylation at both of these sites with no effect on total Akt levels (Figure 2A). Ribavirin has similar effects in vector control cells, indicating that it also impacts on Akt signaling via endogenous eIF4E.
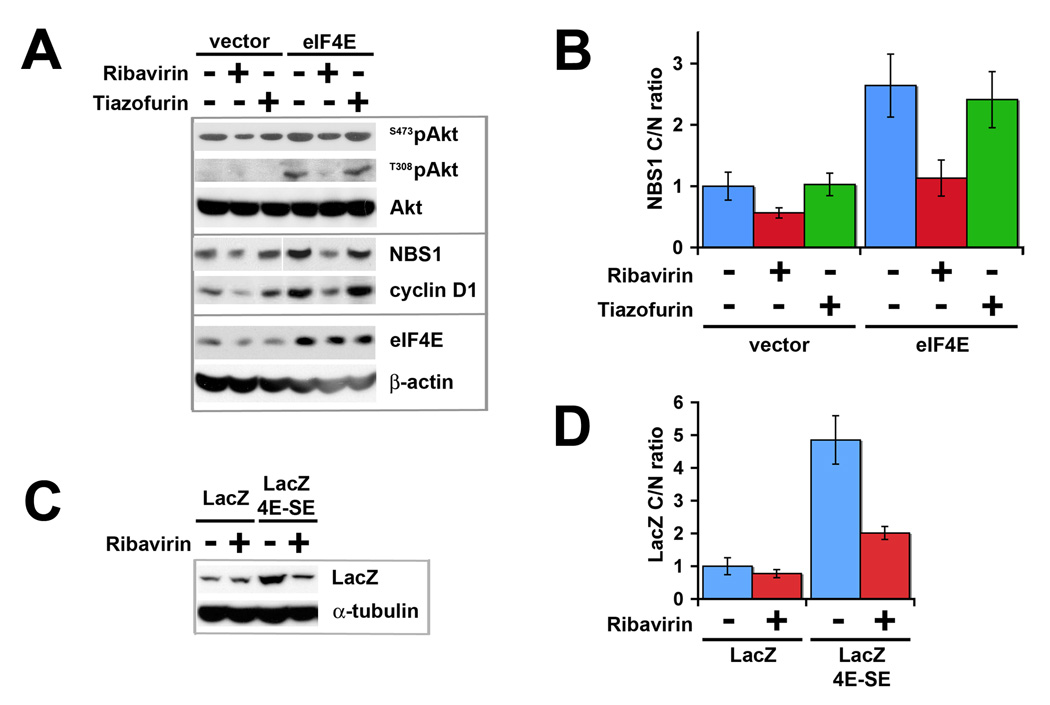
Figure 2.
Ribavirin inhibits Akt activation mediated by eIF4E overexpression. (A) Western blots analysis of murine fibroblasts overexpressing eIF4E treated with 1µM ribavirin or tiazofurin for 48hrs. Detected proteins are as indicated, with β-actin shown as a loading control. (B) RNA quantification from qPCR experiments from murine fibroblasts showing the relative fold difference for NBS1 mRNA cytoplasmic/nuclear (C/N) ratio. (C) Ribavirin inhibits the expression of a lacZ chimeric model construct, which contains a 4E-SE, but not the lacZ control. LacZ was detected using an antibody against the Xpress tag. α-tubulin is shown as a loading control. (D) RNA quantification from qPCR experiments showing the relative fold difference of lacZ mRNA C/N ratio. C/N ratios ± SD were normalized to vector control, arbitrarily set to 1. Averaged values were normalized to GAPDH levels. Fractionation controls (U6 snRNA – nuclear, tRNAlys – cytoplasmic) and total NBS1 and lacZ mRNA levels are shown in Supplementary Figure S1.
Ribavirin inhibits NBS1 dependent activation of the Akt pathway
Next, we determined if ribavirin inhibits Akt activation via NBS1. As observed previously, expression of the eIF4E-sensitive mRNAs, NBS1 and cyclin D1, are increased in eIF4E-overexpressing cells (Figure 2A; [10]). Consistent with the reduction in Akt activation, NBS1 protein levels were reduced by ribavirin treatment relative to untreated cells in both the context of eIF4E overexpression and of endogenous eIF4E (Figure 2A). Further, by fractionating cells into nuclear and cytoplasmic compartments and monitoring mRNA content using quantitative PCR (qPCR), we showed that ribavirin treatment impedes the nuclear export of NBS1 mRNA in cells with endogenous eIF4E and in cells overexpressing eIF4E, relative to untreated controls (Figure 2B). The quality of the fractionations were confirmed by monitoring the distribution of U6 snRNA (nuclear) and tRNAlys (cytoplasmic) (Supplementary Figure S1 A). Total NBS1 mRNA levels did not change (Supplementary Figure S1 B). Thus, ribavirin appears to inhibit Akt activation by inhibiting eIF4E’s ability to promote NBS1 expression, a factor that binds to PI3K and subsequently potentiates Akt activation [13].
Ribavirin as a general inhibitor of eIF4E dependent mRNA export
eIF4E modulates Akt signaling at two levels: at the level of activation of Akt and at the level of increased production of downstream effectors of Akt. Approximately 200 mRNAs are sensitive to eIF4E at the mRNA export level, many of which are downstream effectors of Akt (e.g. MDM2, cyclins B1, A2, E1 and D1) [11]. Thus, we sought to examine whether ribavirin was likely to globally modulate eIF4E dependent mRNA export. We studied the effects of ribavirin on a model chimeric construct, lacZ-4E-SE, which contains the 50 nucleotide element that sensitizes this transcript to eIF4E dependent mRNA export [11]. We confirmed fractionation quality by monitoring the distribution of U6 snRNA (nuclear) and tRNAlys (cytoplasmic) (Supplementary Figure S1 C). eIF4E overexpression leads to enhanced mRNA export of this transcript (without altering its stability as observed by no changes to total lacZ mRNA levels (Supplementary Figure S1 D)), and subsequently enhanced protein production as observed by western analysis (Figure 2C–D; [18]). Importantly, ribavirin treatment impedes the mRNA export of lacZ-4E-SE and thus the levels of lacZ protein, with no effect on lacZ alone (Figure 2C–D). These studies strongly suggest that all eIF4E target mRNAs that contain the 4E-SE will be sensitive to ribavirin treatment. In this way, ribavirin treatment will have a broad and coordinated impact on eIF4E dependent gene expression.
Ribavirin inhibits the eIF4E RNA regulon in cells with inherently high eIF4E levels
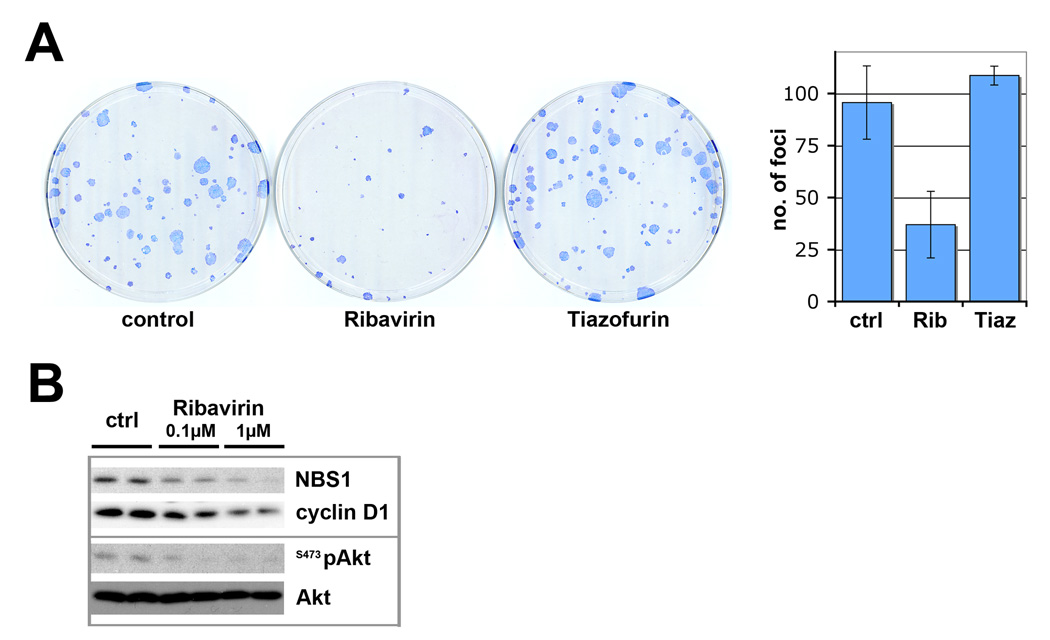
The above studies focused on systems with exogenously overexpressed eIF4E. Here, we examined the effects of ribavirin on FaDu cells, which are derived from a head and neck squamous cell carcinoma patient characterized by highly elevated eIF4E levels [20]. Consistently, ribavirin treatment led to reduced Akt activation at both S473Akt and T308Akt (duplicates are shown in Figure 3B; data not shown), whilst no changes in total Akt levels were observed. Further, NBS1 and cyclin D1 levels were also reduced (Figure 3B). These effects were even apparent at 0.1µM ribavirin. Next we tested the previously observed transformation potential for FaDu cells when treated with ribavirin [15]. As expected, there was a substantial decrease in anchorage dependent foci number and in overall foci size (Figure 3A) in controls versus ribavirin treated cells. These studies suggest that ribavirin acts similarly in FaDu cells as it did in fibroblasts overexpressing exogenous eIF4E. Thus ribavirin inhibits the eIF4E regulon and thereby the Akt signaling pathway in multiple contexts.
Figure 3.
Ribavirin inhibits Akt signaling and impedes transformation in FaDu cells. (A) FaDu cells were treated with 1µM ribavirin or 1µM tiazofurin and Giemsa-stained foci were compared to untreated controls. Quantification of foci is shown (error bars indicate SD). (B) Western blot analysis of FaDu cells treated with indicated amounts of ribavirin. Detected proteins are as indicated, with duplicates for each treatment shown.
Comparison of ribavirin and tiazofurin
Over the years, many analogues of ribavirin have been developed. One such analogue, tiazofurin, has been tested in clinical trials for blast crisis chronic myeloid leukemia (bcCML) [21, 22]. Interestingly, bcCML is characterized by high eIF4E levels [5, 14]. Tiazofurin is not positively charged (Figure 1A) and thus cannot bind eIF4E [15]. Instead, the anti-cancer effects of tiazofurin are attributed to its inhibition of inosine mono-phosphate dehydrogenase (IMPDH), a key enzyme in guanosine biosynthesis [21]. However, ribavirin and tiazofurin are often compared and considered to act through similar pathways. Thus, we examined the effects of tiazofurin on the eIF4E RNA regulon.
First, we examined whether tiazofurin inhibits eIF4E mediated apoptotic rescue. Using the same system described above, it is clear that 1µM tiazofurin does not affect eIF4E mediated apoptotic rescue as seen by flow cytometry or TUNEL (Figure 1B–C). Also at 1µM, tiazofurin did not markedly impact on cell viability of serum-starved cells relative to untreated controls or normal (not starved) cells (Figure 1B–C). Further, tiazofurin treatment did not modulate Akt activation or protein levels of either NBS1 or cyclin D1 (Figure 2A), nor did it modulate NBS1 mRNA export (Figure 2B). Finally tiazofurin did not impair anchorage dependent growth in FaDu cells (Figure 3A), nor did it impair transformation of eIF4E-overexpressing NIH3T3 cells (data not shown). We confirmed quality of tiazofurin by mass spectrometry, and that tiazofurin was active as an IMPDH inhibitor using an [2,8-3H]-inosine metabolism assay (data not shown; [21]). In summary, despite the fact tiazofurin and ribavirin are structurally related, at 1µM, tiazofurin does not impair eIF4E activities.
The differences in metabolism between ribavirin and tiazofurin likely underlie their distinct biological effects. Tiazofurin is metabolized into tiazofurin adenine dinucleotide (TAD), which binds the NAD binding site of IMPDH [23]. TAD inhibits IMPDH activity in vitro, in cell culture and in leukemia patients ([21] and references therein). Given the physico-chemical properties of TAD, one would not expect it to bind eIF4E, consistent with our results. In contrast, ribavirin is metabolized mainly into ribavirin tri-phosphate (RTP, about 85%) with some remaining di-phosphate (RDP) and mono-phosphate (RMP) forms. The RMP form (which is only about 10% of the ribavirin metabolites [24]), binds IMPDH [25]. In fact, RTP binds to eIF4E with higher affinity than ribavirin consistent with studies showing that m7GTP binds eIF4E more strongly than m7G [15]. Thus differences in the structures of these drugs, and their metabolized forms, likely underlie their different modes of action.
Conclusions
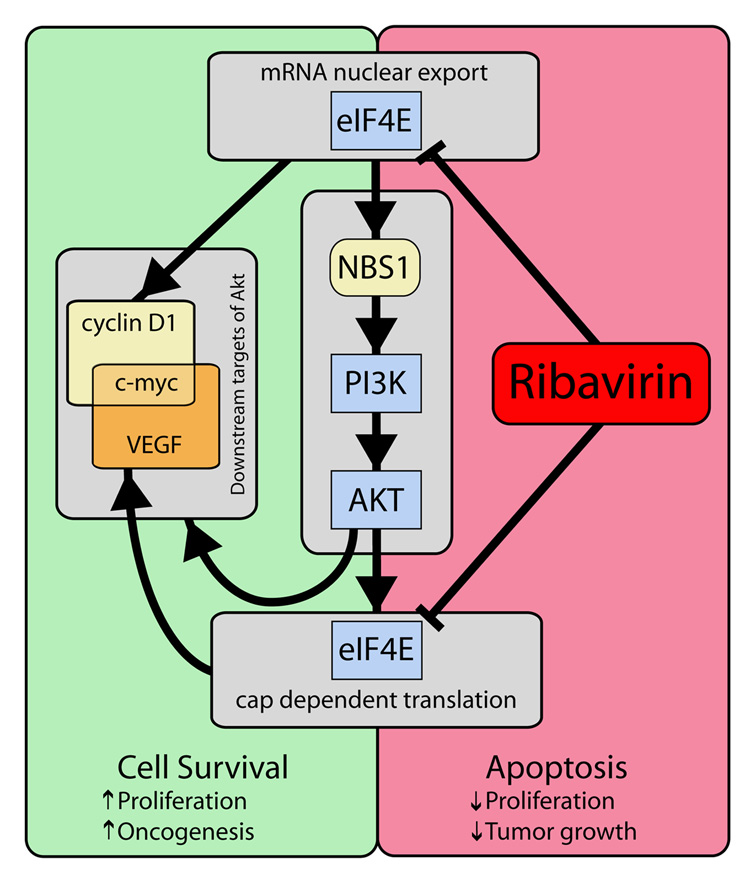
We present novel findings that the broad-spectrum anti-viral drug, ribavirin, impairs eIF4E mediated apoptotic rescue and eIF4E dependent Akt survival signaling (summarized in Figure 4). In particular, ribavirin impedes mRNA export and thus production of NBS1, thereby directly impacting on Akt activation, as well as impairing the production of downstream effectors of Akt. Our studies with the lacZ-4E-SE chimeric construct suggest that ribavirin is a general inhibitor of eIF4E dependent mRNA export. Further, ribavirin inhibits vascular endothelial growth factor (VEGF) translation [15], indicating eIF4E impacts on both levels of eIF4E dependent gene expression. Taken together, these findings suggest that ribavirin coordinately impairs eIF4E dependent modulation of gene expression. Thus ribavirin is positioned to coordinately inhibit the survival, proliferative, transforming and metastatic activities associated with eIF4E. Our studies with tiazofurin indicate that these effects of ribavirin relate to its effects on eIF4E. In this way, ribavirin provides a novel paradigm for drug design at two levels. It is a first-in-class as a physical mimic of the m7G cap and it targets network wide gene expression via inhibiting a node in an RNA regulon.
Figure 4.
Summary model of the two-tier modulation by ribavirin on the eIF4E RNA regulon and thus Akt signaling. Arrows do not necessarily represent a single-step process. In yellow are some of the known subset of mRNAs sensitive to eIF4E export activity (e.g. NBS1, cyclin D1, and c-myc), whilst in orange are mRNAs sensitive to eIF4E translation activity (e.g. VEGF, and c-myc), of which several are downstream effectors of Akt. Note that not every mRNA is affected by eIF4E (and thus ribavirin) at both the mRNA export and translation levels (see introduction). Thus, the model is not meant to depict this, but rather to show that both arms of eIF4E function impact on its abilities to modulate gene expression. In this way, ribavirin is positioned to potently impair the biological effects of eIF4E.
Supplementary Material
Acknowledgements
We are grateful for mass spectrometry assistance from Nadeem Siddiqui and technical assistance from Slobodanka Orolicki. We are grateful for the gift of tiazofurin from Dr Beverly Mitchell. K.L.B. Borden holds an equity position in Translational Therapeutics, Inc., K. Tan holds a Montreal Centre for Experimental Therapeutics in Cancer fellowship. K.L.B. Borden acknowledges support from the Leukemia and Lymphoma Society Translational Research Program, and holds a Canada Research Chair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graff JR, Zimmer SG. Translational control and metastatic progression: enhanced activity of the mRNA cap-binding protein eIF-4E selectively enhances translation of metastasis-related mRNAs. Clin Exp Metastasis. 2003;20:265–273. doi: 10.1023/a:1022943419011. [DOI] [PubMed] [Google Scholar]
- 2.Dong K, Wang R, Wang X, Lin F, Shen JJ, Gao P, Zhang HZ. Tumor-specific RNAi targeting eIF4E suppresses tumor growth, induces apoptosis and enhances cisplatin cytotoxicity in human breast carcinoma cells. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-9956-x. [DOI] [PubMed] [Google Scholar]
- 3.Graff JR, Konicek BW, Vincent TM, Lynch RL, Monteith D, Weir SN, Schwier P, Capen A, Goode RL, Dowless MS, Chen Y, Zhang H, Sissons S, Cox K, McNulty AM, Parsons SH, Wang T, Sams L, Geeganage S, Douglass LE, Neubauer BL, Dean NM, Blanchard K, Shou J, Stancato LF, Carter JH, Marcusson EG. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest. 2007;117:2638–2648. doi: 10.1172/JCI32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 5.Culjkovic B, Topisirovic I, Borden KL. Controlling gene expression through RNA regulons: the role of the eukaryotic translation initiation factor eIF4E. Cell Cycle. 2007;6:65–69. doi: 10.4161/cc.6.1.3688. [DOI] [PubMed] [Google Scholar]
- 6.Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci U S A. 1996;93:1065–1070. doi: 10.1073/pnas.93.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamane Y, Petroulakis E, Martineau Y, Sato TA, Larsson O, Rajasekhar VK, Sonenberg N. Epigenetic Activation of a Subset of mRNAs by eIF4E Explains Its Effects on Cell Proliferation. PLoS ONE. 2007;2:e242. doi: 10.1371/journal.pone.0000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonenberg N, Gingras AC. The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- 9.Polunovsky VA, Rosenwald IB, Tan AT, White J, Chiang L, Sonenberg N, Bitterman PB. Translational control of programmed cell death: eukaryotic translation initiation factor 4E blocks apoptosis in growth-factor-restricted fibroblasts with physiologically expressed or deregulated Myc. Mol Cell Biol. 1996;16:6573–6581. doi: 10.1128/mcb.16.11.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culjkovic B, Tan K, Orolicki S, Amri A, Meloche S, Borden KL. The eIF4E RNA regulon promotes the Akt signaling pathway. J Cell Biol. 2008;181:51–63. doi: 10.1083/jcb.200707018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol. 2006;175:415–426. doi: 10.1083/jcb.200607020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keene JD. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 2007;8:533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 13.Chen YC, Chiang HY, Yang MH, Chen PM, Chang SY, Teng SC, Vanhaesebroeck B, Wu KJ. Activation of phosphoinositide 3-kinase by the NBS1 DNA repair protein through a novel activation motif. J Mol Med. 2008;86:401–412. doi: 10.1007/s00109-008-0302-x. [DOI] [PubMed] [Google Scholar]
- 14.Topisirovic I, Guzman ML, McConnell MJ, Licht JD, Culjkovic B, Neering SJ, Jordan CT, Borden KL. Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis. Mol Cell Biol. 2003;23:8992–9002. doi: 10.1128/MCB.23.24.8992-9002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kentsis A, Topisirovic I, Culjkovic B, Shao L, Borden KL. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc Natl Acad Sci U S A. 2004;101:18105–18110. doi: 10.1073/pnas.0406927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kentsis A, Volpon L, Topisirovic I, Soll CE, Culjkovic B, Shao L, Borden KL. Further evidence that ribavirin interacts with eIF4E. RNA. 2005;11:1762–1766. doi: 10.1261/rna.2238705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeney A, Kenessey I, Timar F, Olah J, Pogany G, Babo I, Harisi R. Study of drugs against neoplastic metastasis. Magy Onkol. 2006;50:93–100. [PubMed] [Google Scholar]
- 18.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3′UTR. J Cell Biol. 2005;169:245–256. doi: 10.1083/jcb.200501019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koren G, King S, Knowles S, Phillips E. Ribavirin in the treatment of SARS: A new trick for an old drug? Cmaj. 2003;168:1289–1292. [PMC free article] [PubMed] [Google Scholar]
- 20.DeFatta RJ, Nathan CO, De Benedetti A. Antisense RNA to eIF4E suppresses oncogenic properties of a head and neck squamous cell carcinoma cell line. Laryngoscope. 2000;110:928–933. doi: 10.1097/00005537-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Malek K, Boosalis MS, Waraska K, Mitchell BS, Wright DG. Effects of the IMP-dehydrogenase inhibitor, Tiazofurin, in bcr-abl positive acute myelogenous leukemia. Part I. In vivo studies. Leuk Res. 2004;28:1125–1136. doi: 10.1016/j.leukres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Colovic M, Sefer D, Bogdanovic A, Suvajdzic N, Jankovic G, Atkinson HD, Milenkovic P. In vitro sensitivity of hematopoietic progenitors to tiazofurin in refractory acute myeloid leukemia and in the blast crisis of chronic myeloid leukemia. Cancer Lett. 2003;195:153–159. doi: 10.1016/s0304-3835(03)00152-6. [DOI] [PubMed] [Google Scholar]
- 23.Gebeyehu G, Marquez VE, Van Cott A, Cooney DA, Kelley JA, Jayaram HN, Ahluwalia GS, Dion RL, Wilson YA, Johns DG. Ribavirin, tiazofurin, and selenazofurin: mononucleotides and nicotinamide adenine dinucleotide analogues. Synthesis, structure, and interactions with IMP dehydrogenase. J Med Chem. 1985;28:99–105. doi: 10.1021/jm00379a018. [DOI] [PubMed] [Google Scholar]
- 24.Smee DF, Matthews TR. Metabolism of ribavirin in respiratory syncytial virus-infected and uninfected cells. Antimicrob Agents Chemother. 1986;30:117–121. doi: 10.1128/aac.30.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streeter DG, Witkowski JT, Khare GP, Sidwell RW, Bauer RJ, Robins RK, Simon LN. Mechanism of action of 1- -D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc Natl Acad Sci U S A. 1973;70:1174–1178. doi: 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.