Abstract
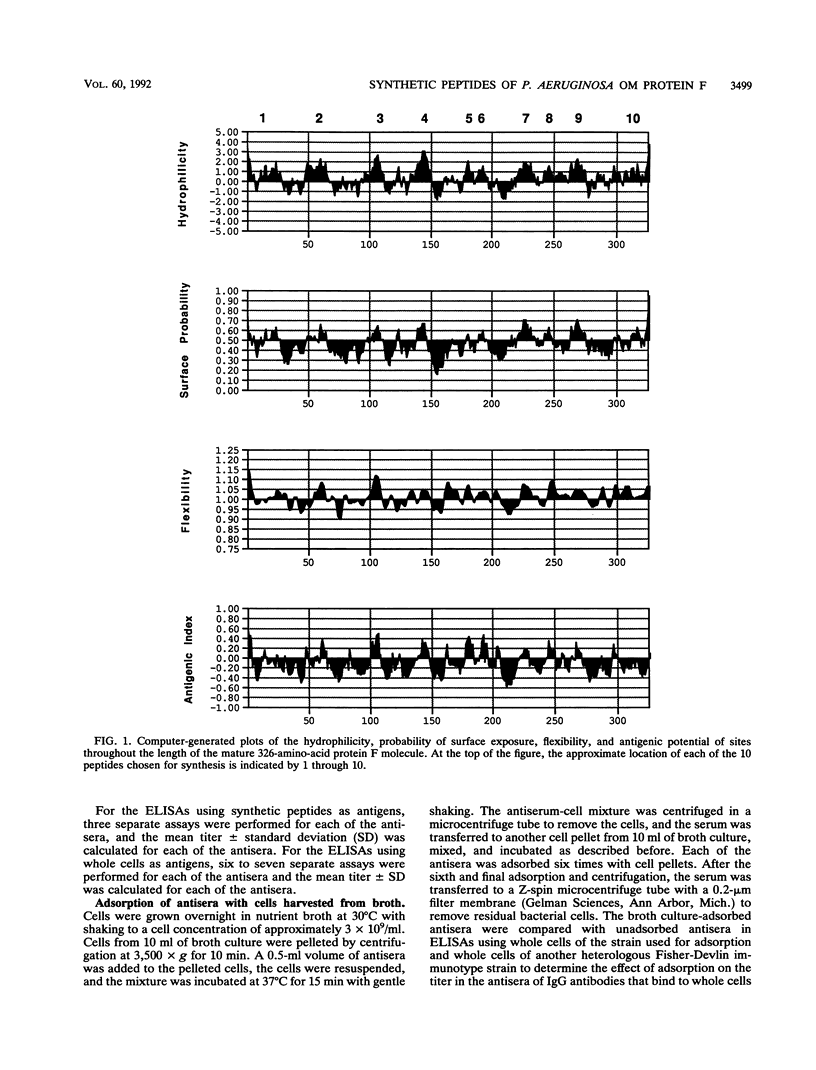
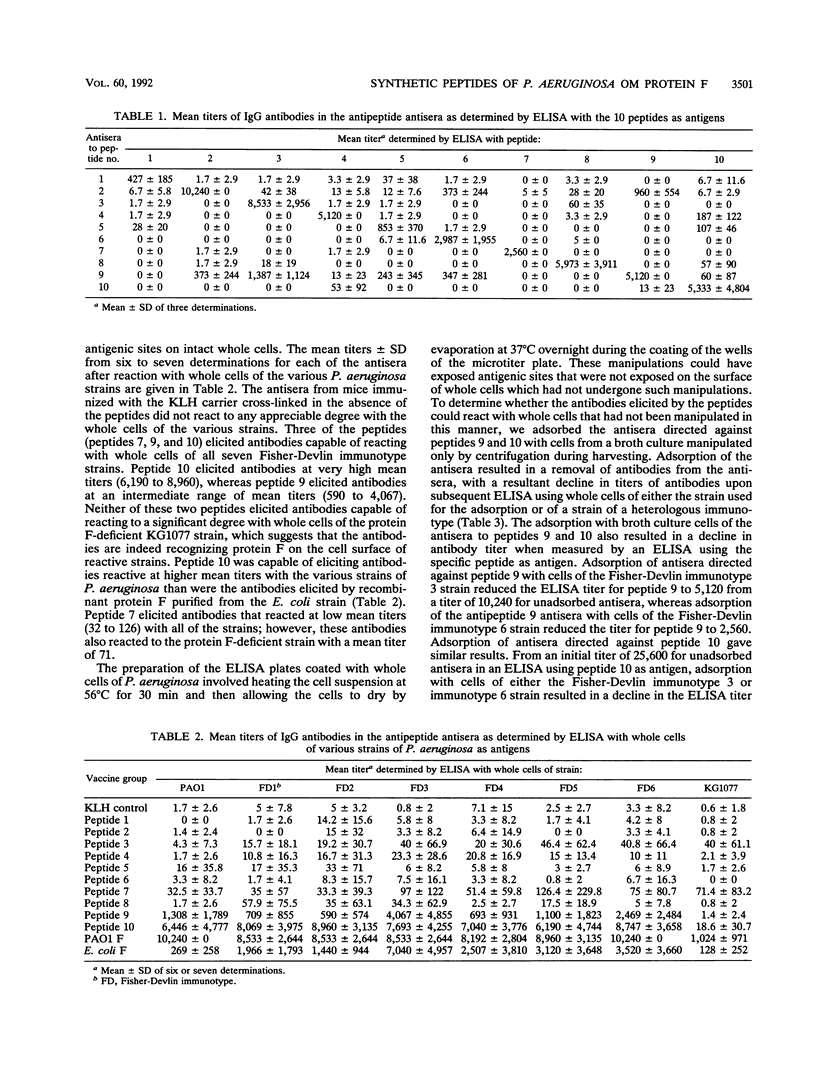
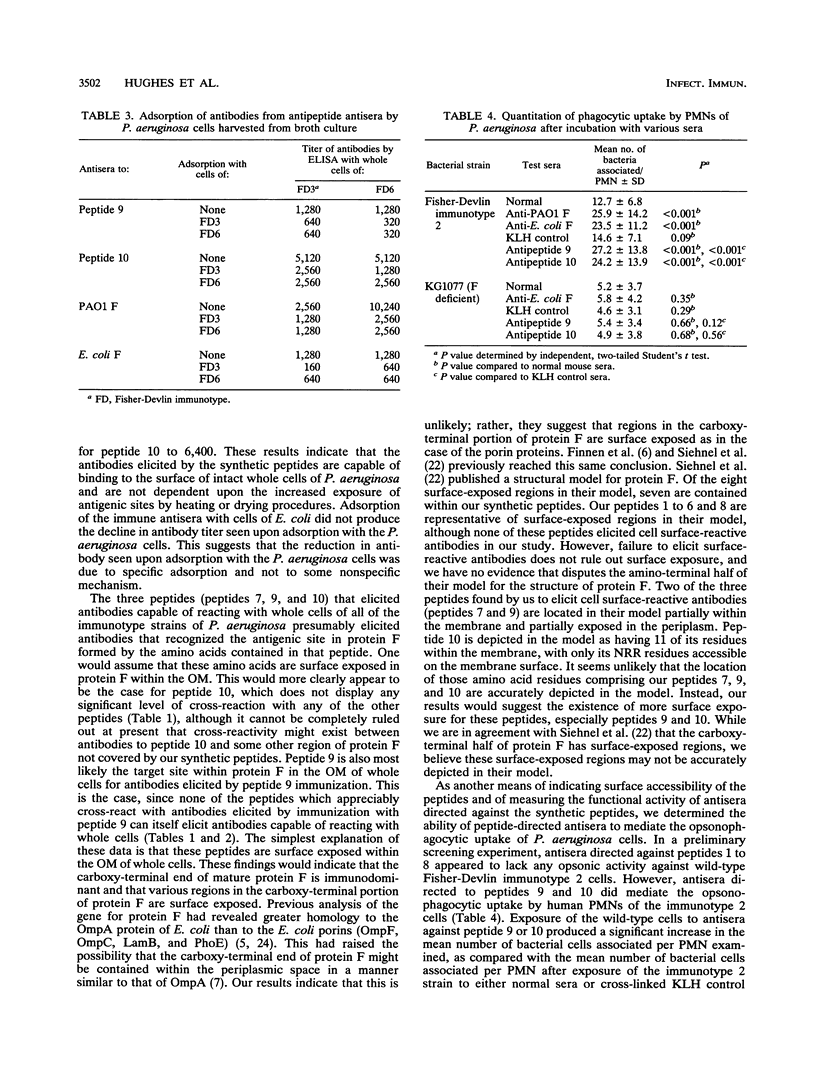
By using the published amino acid sequence for mature outer membrane protein F of Pseudomonas aeruginosa, a computer-assisted analysis was performed to identify sites with potential as surface-exposed, antigenic regions located throughout the length of the protein molecule. Synthetic peptides 13 to 15 amino acid residues in length were synthesized for 10 such regions. Mice were immunized with each of the 10 synthetic peptides conjugated to keyhole limpet hemocyanin. An enzyme-linked immunosorbent assay (ELISA) of the antisera was performed by using each of the synthetic peptides as the ELISA antigen to verify that immunoglobulin G (IgG) antibodies capable of reacting with the peptide used as immunogen were elicited by each peptide. Each of the antipeptide antisera was screened for the presence of IgG antibodies that could bind to the surface of intact cells of strains representing the seven heterologous Fisher-Devlin immunotypes of P. aeruginosa by use of an ELISA with whole cells of the various strains as the ELISA antigen. Three peptides elicited antibodies capable of reacting with whole cells of all seven immunotype strains. Peptide 10, corresponding to amino acid residues 305 to 318, elicited whole-cell-reactive antibodies at high titers. Peptide 9, corresponding to amino acid residues 261 to 274, elicited whole-cell-reactive antibodies at more intermediate titers. Peptide 7, corresponding to amino acid residues 219 to 232, elicited such antibodies only at low titers. The carboxy-terminal portion of the mature protein appears to be the immunodominant portion. In particular, peptides 10 (NATAEGRAINRRVE) and 9 (TDAYNQKLSERRAN) appear to have potential for use as immunogens in a synthetic vaccine for immunoprophylaxis against infections caused by P. aeruginosa. Antisera from mice immunized with either peptide 9 or 10 mediated opsonophagocytic uptake by human polymorphonuclear leukocytes of wild-type cells of P. aeruginosa but exhibited no opsonic activity against a protein F-deficient mutant of P. aeruginosa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battershill J. L., Speert D. P., Hancock R. E. Use of monoclonal antibodies to protein F of Pseudomonas aeruginosa as opsonins for phagocytosis by macrophages. Infect Immun. 1987 Oct;55(10):2531–2533. doi: 10.1128/iai.55.10.2531-2533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudet F., Thèze J., Zouali M. UV-treated polystyrene microtitre plates for use in an ELISA to measure antibodies against synthetic peptides. J Immunol Methods. 1991 Aug 28;142(1):73–82. doi: 10.1016/0022-1759(91)90294-p. [DOI] [PubMed] [Google Scholar]
- Counts G. W., Schwartz R. W., Ulness B. K., Hamilton D. J., Rosok M. J., Cunningham M. D., Tam M. R., Darveau R. P. Evaluation of an immunofluorescent-antibody test for rapid identification of Pseudomonas aeruginosa in blood cultures. J Clin Microbiol. 1988 Jun;26(6):1161–1165. doi: 10.1128/jcm.26.6.1161-1165.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchêne M., Schweizer A., Lottspeich F., Krauss G., Marget M., Vogel K., von Specht B. U., Domdey H. Sequence and transcriptional start site of the Pseudomonas aeruginosa outer membrane porin protein F gene. J Bacteriol. 1988 Jan;170(1):155–162. doi: 10.1128/jb.170.1.155-162.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudl R., MacIntyre S., Degen M., Henning U. Cell surface exposure of the outer membrane protein OmpA of Escherichia coli K-12. J Mol Biol. 1986 Apr 5;188(3):491–494. doi: 10.1016/0022-2836(86)90171-3. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Gilleland L. B., Matthews-Greer J. M. Outer membrane protein F preparation of Pseudomonas aeruginosa as a vaccine against chronic pulmonary infection with heterologous immunotype strains in a rat model. Infect Immun. 1988 May;56(5):1017–1022. doi: 10.1128/iai.56.5.1017-1022.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Parker M. G., Matthews J. M., Berg R. D. Use of a purified outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine in mice. Infect Immun. 1984 Apr;44(1):49–54. doi: 10.1128/iai.44.1.49-54.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N., Wakebe H., Yoshihara E., Nakae T., Nishino T. Role of protein F in maintaining structural integrity of the Pseudomonas aeruginosa outer membrane. J Bacteriol. 1989 Feb;171(2):983–990. doi: 10.1128/jb.171.2.983-990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Mouat E. C. Immunotherapeutic potential of monoclonal antibodies against Pseudomonas aeruginosa protein F. Eur J Clin Microbiol. 1985 Apr;4(2):224–227. doi: 10.1007/BF02013602. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Siehnel R., Martin N. Outer membrane proteins of Pseudomonas. Mol Microbiol. 1990 Jul;4(7):1069–1075. doi: 10.1111/j.1365-2958.1990.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Matthews-Greer J. M., Gilleland H. E., Jr Outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine against heterologous immunotype strains in a burned mouse model. J Infect Dis. 1987 Jun;155(6):1282–1291. doi: 10.1093/infdis/155.6.1282. [DOI] [PubMed] [Google Scholar]
- Matthews R. C., Burnie J. P., Tabaqchali S. Immunoblot analysis of serological response to Pseudomonas aeruginosa septicaemia in man. J Clin Pathol. 1986 Dec;39(12):1306–1312. doi: 10.1136/jcp.39.12.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon M. M., Hazlett L. D., Hancock R. E., Berk R. S., Barrett R. Monoclonal antibodies provide protection against ocular Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci. 1988 Aug;29(8):1277–1284. [PubMed] [Google Scholar]
- Mutharia L. M., Hancock R. E. Surface localization of Pseudomonas aeruginosa outer membrane porin protein F by using monoclonal antibodies. Infect Immun. 1983 Dec;42(3):1027–1033. doi: 10.1128/iai.42.3.1027-1033.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Nicas T. I., Hancock R. E. Outer membrane proteins of Pseudomonas aeruginosa serotype strains. J Infect Dis. 1982 Dec;146(6):770–779. doi: 10.1093/infdis/146.6.770. [DOI] [PubMed] [Google Scholar]
- Savoca R., Schwab C., Bosshard H. R. Epitope mapping employing immobilized synthetic peptides. How specific is the reactivity of these peptides with antiserum raised against the parent protein? J Immunol Methods. 1991 Aug 9;141(2):245–252. doi: 10.1016/0022-1759(91)90151-5. [DOI] [PubMed] [Google Scholar]
- Woodruff W. A., Hancock R. E. Pseudomonas aeruginosa outer membrane protein F: structural role and relationship to the Escherichia coli OmpA protein. J Bacteriol. 1989 Jun;171(6):3304–3309. doi: 10.1128/jb.171.6.3304-3309.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff W. A., Parr T. R., Jr, Hancock R. E., Hanne L. F., Nicas T. I., Iglewski B. H. Expression in Escherichia coli and function of Pseudomonas aeruginosa outer membrane porin protein F. J Bacteriol. 1986 Aug;167(2):473–479. doi: 10.1128/jb.167.2.473-479.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Zalman L. S., Nikaido H. Purification and properties of Pseudomonas aeruginosa porin. J Biol Chem. 1983 Feb 25;258(4):2308–2314. [PubMed] [Google Scholar]



