Abstract
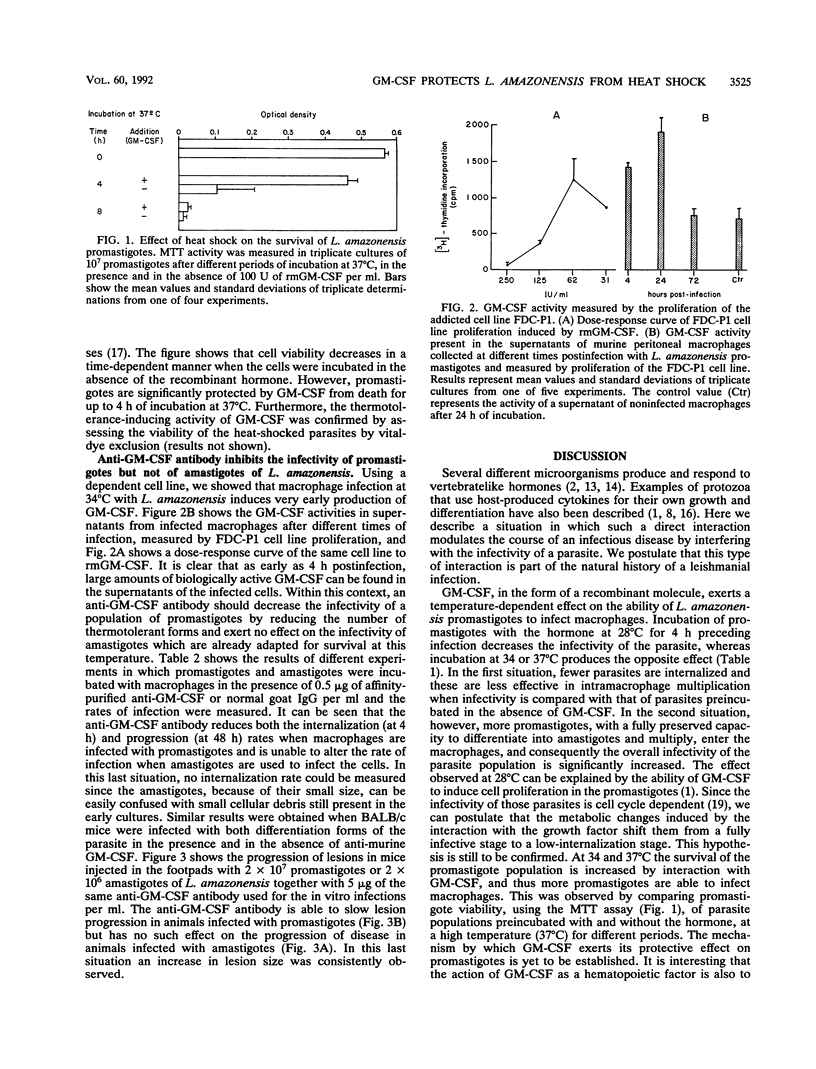
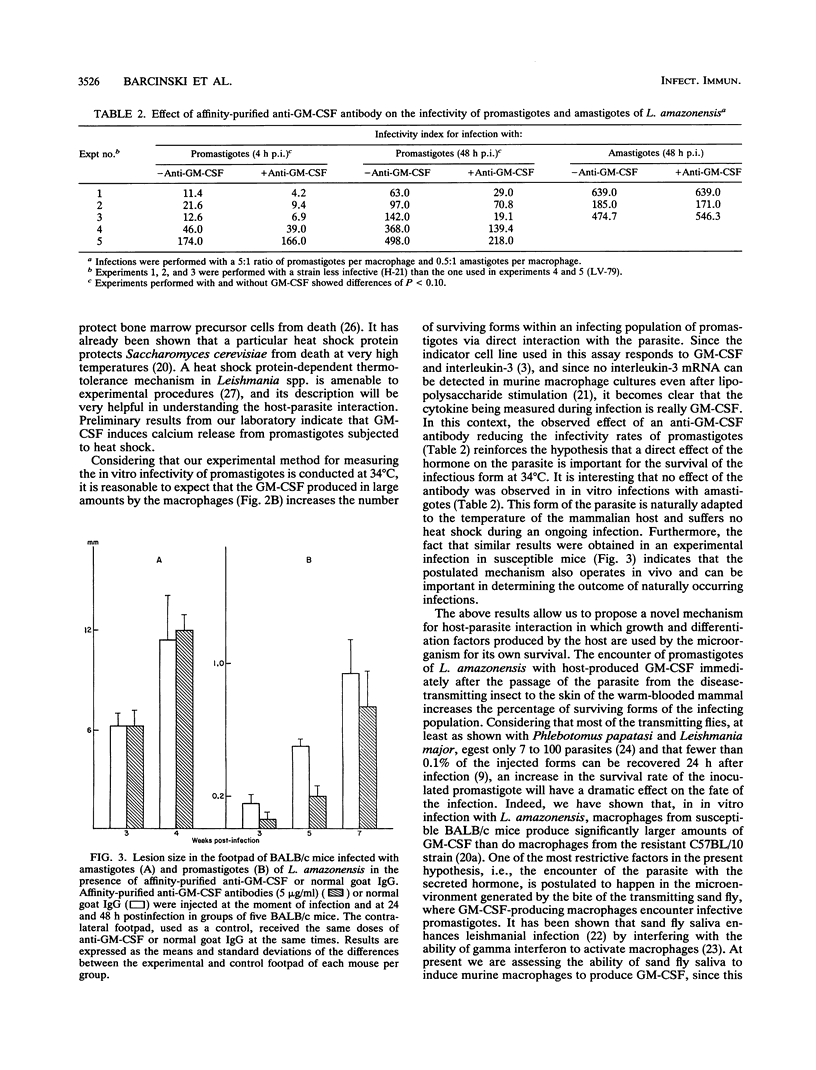
We have studied the effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on the infectivity of promastigotes of Leishmania amazonensis, an obligate intramacrophage parasite. We measured the capacity of the promastigotes to infect macrophages after preincubation at different temperatures (28, 34, and 37 degrees C) with recombinant murine GM-CSF, as well as the effect of an anti-murine GM-CSF antibody on the in vitro and in vivo infectivity of the parasite. GM-CSF increases the capacity of the promastigotes to infect cells when preincubated at 34 and 37 degrees C, whereas the anti-GM-CSF antibody exerts the opposite effect: it decreases the internalization rate and the progression of infection in macrophage cultures and slows the growth of the lesion in infected BALB/c mice. Neither of the described effects were observed when the in vitro and in vivo infections were made with amastigotes. Promastigotes die in a time-dependent manner when incubated at temperatures higher than 28 degrees C in the absence of GM-CSF. They are protected from this heat-induced death by incubation with the recombinant hormone. Our interpretation of these data is that the increase in the infectivity of promastigotes when incubated with GM-CSF at the temperatures at which infection occurs (34 and 37 degrees C) is due to the larger number of surviving forms within the infecting population. The decrease in infectivity when they are incubated with the antibody is due to inhibition of the protection conferred by the GM-CSF produced by the macrophages during the in vitro and in vivo infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charlab R., Blaineau C., Schechtman D., Barcinski M. A. Granulocyte-macrophage colony-stimulating factor is a growth-factor for promastigotes of Leishmania mexicana amazonensis. J Protozool. 1990 Sep-Oct;37(5):352–357. doi: 10.1111/j.1550-7408.1990.tb01157.x. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z. Y., Louis J., Kindler V., Pedrazzini T., Eliason J. F., Behin R., Vassalli P. Aggravation of experimental cutaneous leishmaniasis in mice by administration of interleukin 3. Eur J Immunol. 1988 Aug;18(8):1245–1251. doi: 10.1002/eji.1830180815. [DOI] [PubMed] [Google Scholar]
- Greil J., Bodendorfer B., Röllinghoff M., Solbach W. Application of recombinant granulocyte-macrophage colony-stimulating factor has a detrimental effect in experimental murine leishmaniasis. Eur J Immunol. 1988 Oct;18(10):1527–1533. doi: 10.1002/eji.1830181009. [DOI] [PubMed] [Google Scholar]
- Hancock W. W., Pleau M. E., Kobzik L. Recombinant granulocyte-macrophage colony-stimulating factor down-regulates expression of IL-2 receptor on human mononuclear phagocytes by induction of prostaglandin E. J Immunol. 1988 May 1;140(9):3021–3025. [PubMed] [Google Scholar]
- Handman E., Burgess A. W. Stimulation by granulocyte-macrophage colony-stimulating factor of Leishmania tropica killing by macrophages. J Immunol. 1979 Mar;122(3):1134–1137. [PubMed] [Google Scholar]
- Hide G., Gray A., Harrison C. M., Tait A. Identification of an epidermal growth factor receptor homologue in trypanosomes. Mol Biochem Parasitol. 1989 Aug;36(1):51–59. doi: 10.1016/0166-6851(89)90199-0. [DOI] [PubMed] [Google Scholar]
- Hill J. O., North R. J., Collins F. M. Advantages of measuring changes in the number of viable parasites in murine models of experimental cutaneous leishmaniasis. Infect Immun. 1983 Mar;39(3):1087–1094. doi: 10.1128/iai.39.3.1087-1094.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J. L., Reed S. G., Wick E. A., Giordano M. Granulocyte-macrophage and macrophage colony-stimulating factors activate intramacrophage killing of Leishmania mexicana amazonensis. J Infect Dis. 1990 Jul;162(1):224–230. doi: 10.1093/infdis/162.1.224. [DOI] [PubMed] [Google Scholar]
- Kiderlen A. F., Kaye P. M. A modified colorimetric assay of macrophage activation for intracellular cytotoxicity against Leishmania parasites. J Immunol Methods. 1990 Feb 20;127(1):11–18. doi: 10.1016/0022-1759(90)90334-r. [DOI] [PubMed] [Google Scholar]
- Le Roith D., Shiloach J., Roth J., Lesniak M. A. Evolutionary origins of vertebrate hormones: substances similar to mammalian insulins are native to unicellular eukaryotes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6184–6188. doi: 10.1073/pnas.77.10.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelchuk R., Graveley R., Liew F. Y. Susceptibility to murine cutaneous leishmaniasis correlates with the capacity to generate interleukin 3 in response to leishmania antigen in vitro. Cell Immunol. 1988 Jan;111(1):66–76. doi: 10.1016/0008-8749(88)90051-2. [DOI] [PubMed] [Google Scholar]
- Leroith D., Liotta A. S., Roth J., Shiloach J., Lewis M. E., Pert C. B., Krieger D. T. Corticotropin and beta-endorphin-like materials are native to unicellular organisms. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2086–2090. doi: 10.1073/pnas.79.6.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauël J. Macrophage-parasite interactions in Leishmania infections. J Leukoc Biol. 1990 Feb;47(2):187–193. doi: 10.1002/jlb.47.2.187. [DOI] [PubMed] [Google Scholar]
- Mazingue C., Cottrez-Detoeuf F., Louis J., Kweider M., Auriault C., Capron A. In vitro and in vivo effects of interleukin 2 on the protozoan parasite leishmania. Eur J Immunol. 1989 Mar;19(3):487–491. doi: 10.1002/eji.1830190312. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Hieny S., Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J Immunol. 1985 Jul;135(1):564–569. [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Identification of an infective stage of Leishmania promastigotes. Science. 1984 Mar 30;223(4643):1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Sanchez Y., Lindquist S. L. HSP104 required for induced thermotolerance. Science. 1990 Jun 1;248(4959):1112–1115. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- Soares L. R., Barcinski M. A. Differential production of granulocyte-macrophage colony-stimulating factor by macrophages from mice susceptible and resistant to Leishmania mexicana amazonensis. J Leukoc Biol. 1992 Mar;51(3):220–224. doi: 10.1002/jlb.51.3.220. [DOI] [PubMed] [Google Scholar]
- Thorens B., Mermod J. J., Vassalli P. Phagocytosis and inflammatory stimuli induce GM-CSF mRNA in macrophages through posttranscriptional regulation. Cell. 1987 Feb 27;48(4):671–679. doi: 10.1016/0092-8674(87)90245-5. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Ribeiro J. M. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988 Mar 11;239(4845):1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Ribeiro J. M. The role of vector saliva in transmission of arthropod-borne disease. Parasitol Today. 1990 May;6(5):157–160. doi: 10.1016/0169-4758(90)90338-5. [DOI] [PubMed] [Google Scholar]
- Warburg A., Schlein Y. The effect of post-bloodmeal nutrition of Phlebotomus papatasi on the transmission of Leishmania major. Am J Trop Med Hyg. 1986 Sep;35(5):926–930. doi: 10.4269/ajtmh.1986.35.926. [DOI] [PubMed] [Google Scholar]
- Weiser W. Y., Van Niel A., Clark S. C., David J. R., Remold H. G. Recombinant human granulocyte/macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. J Exp Med. 1987 Nov 1;166(5):1436–1446. doi: 10.1084/jem.166.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]



