Abstract
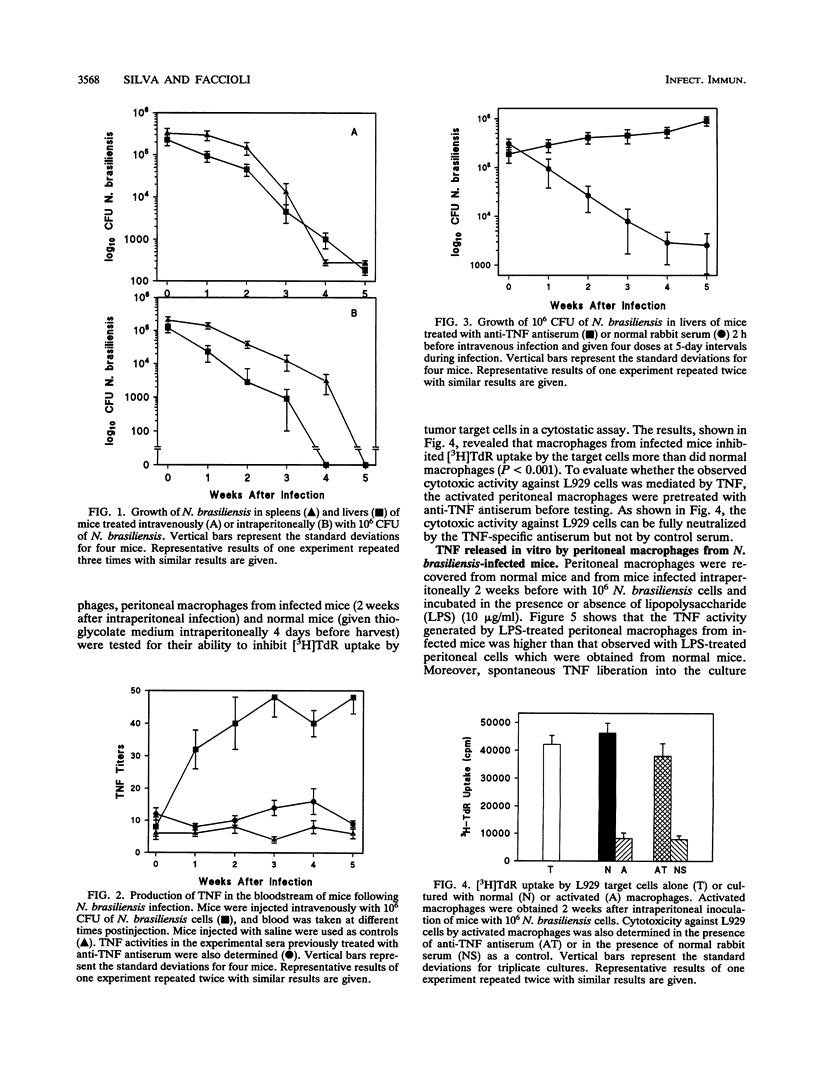
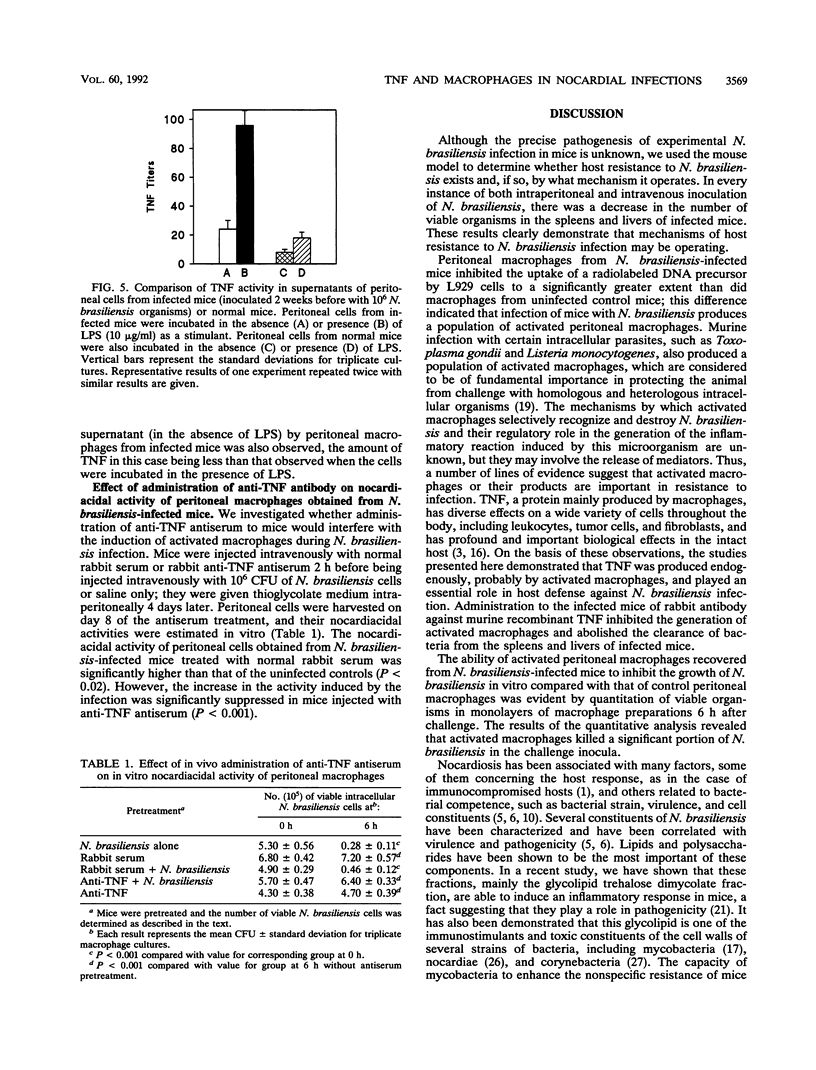
The roles of tumor necrosis factor (TNF) and macrophage activation in clearance of Nocardia brasiliensis from BALB/c mouse livers and spleens were evaluated. TNF activity was detectable in sera from animals at all stages of infection. Treatment of infected mice with an antiserum against TNF significantly enhanced the experimental infection as judged by enumeration of CFU in the spleens and livers of infected mice. In another set of experiments, a population of activated macrophages from the peritoneal cavities of N. brasiliensis-infected mice was studied by using a cytostatic assay. The observed cytotoxic activity of these activated macrophages against L929 cells was mediated by TNF, since this activity was inhibited by anti-TNF antiserum treatment. The level of TNF activity generated in vitro in the presence of lipopolysaccharide (LPS) by peritoneal macrophages from infected mice was higher than that of adherent peritoneal cells obtained from normal mice after challenge with LPS. When the nocardiacidal activity of peritoneal cells from N. brasiliensis-infected mice was estimated in vitro, a significant decrease in the number of CFU recovered was observed. Moreover, nocardiacidal activity of peritoneal cells obtained from N. brasiliensis-infected mice previously treated with anti-TNF antiserum was significantly reduced compared with the activity of cells obtained from infected mice previously treated with normal rabbit serum and that of cells from uninfected mice. These data suggest a role for TNF in resistance to N. brasiliensis infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. C., Adler J. L., Breman J., P'eng F. K., Sahyoun A., Schlesinger R. M., Madras P., Monaco A. P. Influence of rejection therapy on fungal and nocardial infections in renal-transplant recipients. Lancet. 1973 Jan 27;1(7796):180–184. doi: 10.1016/s0140-6736(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Bekierkunst A., Levij I. S., Yarkoni E. Suppression of urethan-induced lung adenomas in mice treated with trehalose-6,6-dimycolate (cord factor) and living bacillus Calmette Guérin. Science. 1971 Dec 17;174(4015):1240–1242. doi: 10.1126/science.174.4015.1240. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Kolonoski P., Young L. S. Natural killer cell activity and macrophage-dependent inhibition of growth or killing of Mycobacterium avium complex in a mouse model. J Leukoc Biol. 1990 Feb;47(2):135–141. doi: 10.1002/jlb.47.2.135. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Ekizlerian S. M., Brandão Filho S. L., Silva C. L. Mouse toxicity induced by lipids and cell walls isolated from actinomycetes. J Gen Microbiol. 1986 Sep;132(9):2647–2651. doi: 10.1099/00221287-132-9-2647. [DOI] [PubMed] [Google Scholar]
- Ekizlerian S. M., Brandão Filho S. L., Tincani I., Alves L. M., Silva C. L. Studies on the pathogenesis of actinomycotic mycetoma in animals injected with fractions isolated from Nocardia brasiliensis. Br J Exp Pathol. 1987 Feb;68(1):115–123. [PMC free article] [PubMed] [Google Scholar]
- Filice G. A., Beaman B. L., Remington J. S. Effects of activated macrophages on Nacardia asteroides. Infect Immun. 1980 Feb;27(2):643–649. doi: 10.1128/iai.27.2.643-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folb P. I., Jaffe R., Altmann G. Nocardia asteroides and Nocardia brasiliensis infections in mice. Infect Immun. 1976 May;13(5):1490–1496. doi: 10.1128/iai.13.5.1490-1496.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Ochoa A. Virulence of nocardiae. Can J Microbiol. 1973 Aug;19(8):901–904. doi: 10.1139/m73-144. [DOI] [PubMed] [Google Scholar]
- Hotez P. J., Le Trang N., Fairlamb A. H., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by a haematoprotozoan-induced mediator from peritoneal exudate cells. Parasite Immunol. 1984 May;6(3):203–209. doi: 10.1111/j.1365-3024.1984.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl J. L., Lambert L. H., Jr, Remington J. S. The effects of activated macrophages on tumor target cells: escape from cytostasis. Cell Immunol. 1976 Aug;25(2):279–293. doi: 10.1016/0008-8749(76)90118-0. [DOI] [PubMed] [Google Scholar]
- Krick J. A., Remington J. S. Resistance to infection with Nocardia asteroides. J Infect Dis. 1975 Jun;131(6):665–672. doi: 10.1093/infdis/131.6.665. [DOI] [PubMed] [Google Scholar]
- Lavalle P. Clinica y terapeutica de los micetomas. Dermatol Int. 1966 Apr-Jun;5(2):117–120. [PubMed] [Google Scholar]
- NOLL H., BLOCH H., ASSELINEAU J., LEDERER E. The chemical structure of the cord factor of Mycobacterium tuberculosis. Biochim Biophys Acta. 1956 May;20(2):299–309. doi: 10.1016/0006-3002(56)90289-x. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OCHOA A. G. Mycetomas caused by Nocardia brasiliensis; with a note on the isolation of the causative organism from soil. Lab Invest. 1962 Nov;11:1118–1123. [PubMed] [Google Scholar]
- Parant M., Parant F., Chedid L., Drapier J. C., Petit J. F., Wietzerbin J., Lederer Enhancement of nonspecific immunity to bacterial infection by cord factor (6,6'-trehalose dimycolate). J Infect Dis. 1977 May;135(5):771–777. doi: 10.1093/infdis/135.5.771. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Krahenbuhl J. L., Mendenhall J. W. A role for activated macrophages in resistance to infection with Toxoplasma. Infect Immun. 1972 Nov;6(5):829–834. doi: 10.1128/iai.6.5.829-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C. L., Ekizlerian S. M., Fazioli R. A. Role of cord factor in the modulation of infection caused by mycobacteria. Am J Pathol. 1985 Feb;118(2):238–247. [PMC free article] [PubMed] [Google Scholar]
- Silva C. L., Faccioli L. H., Rocha G. M. The role of cachectin/TNF in the pathogenesis of tuberculosis. Braz J Med Biol Res. 1988;21(3):489–492. [PubMed] [Google Scholar]
- Silva C. L., Faccioli L. H. Tumor necrosis factor (cachectin) mediates induction of cachexia by cord factor from mycobacteria. Infect Immun. 1988 Dec;56(12):3067–3071. doi: 10.1128/iai.56.12.3067-3071.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C. L., Figueiredo F. Tumor necrosis factor in paracoccidioidomycosis patients. J Infect Dis. 1991 Nov;164(5):1033–1034. doi: 10.1093/infdis/164.5.1033. [DOI] [PubMed] [Google Scholar]
- Silva C. L., Foss N. T. Tumor necrosis factor in leprosy patients. J Infect Dis. 1989 Apr;159(4):787–790. doi: 10.1093/infdis/159.4.787. [DOI] [PubMed] [Google Scholar]
- Silva C. L. Participation of tumor necrosis factor in the antitumor activity of mycobacterial trehalose dimycolate (cord factor). Braz J Med Biol Res. 1989;22(3):341–344. [PubMed] [Google Scholar]



