Abstract
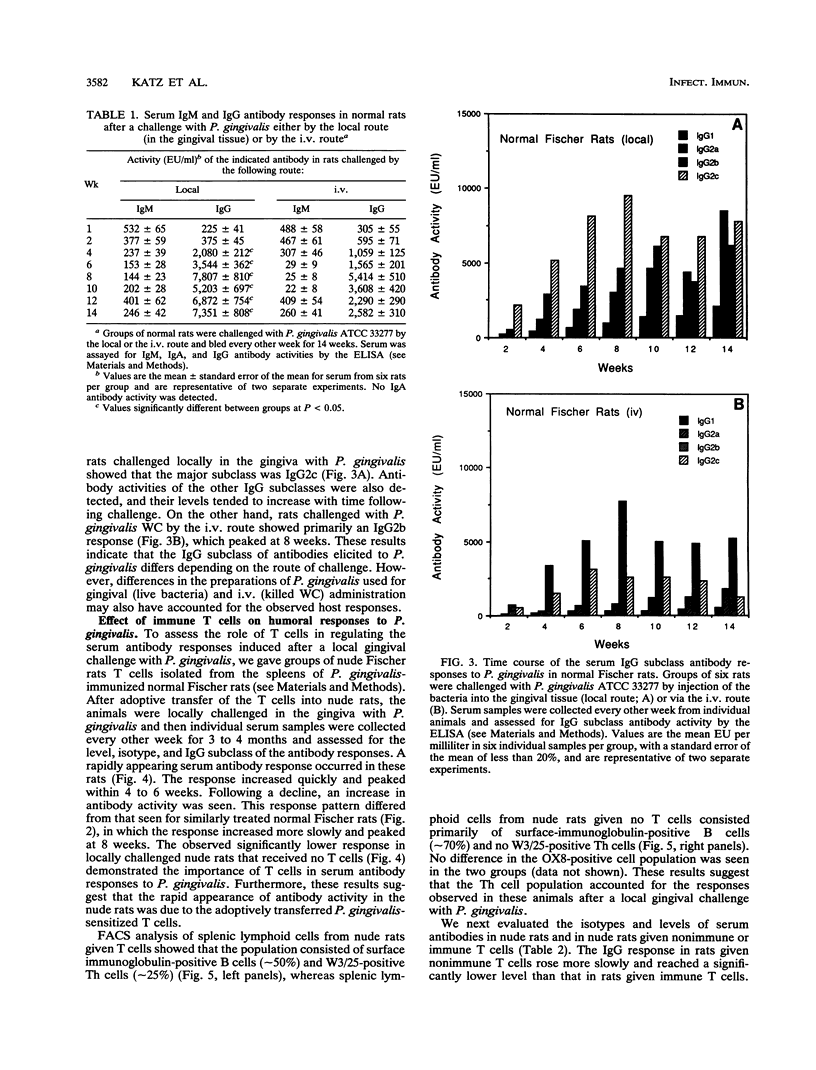
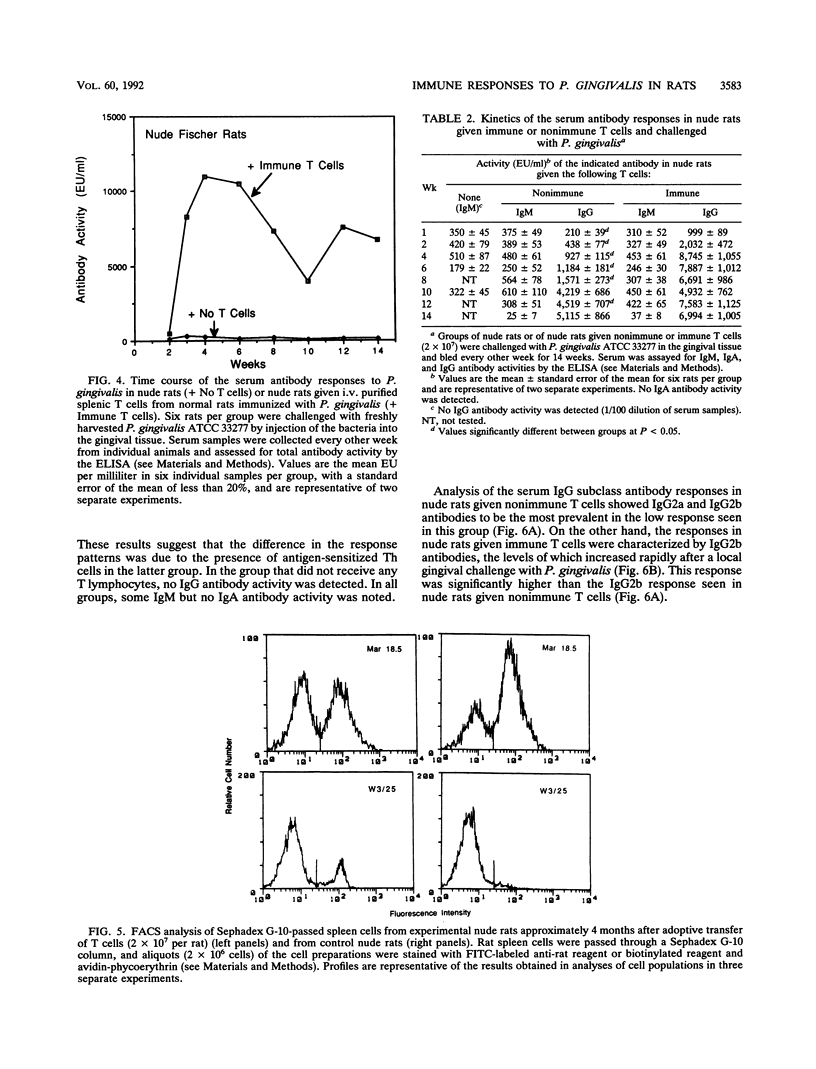
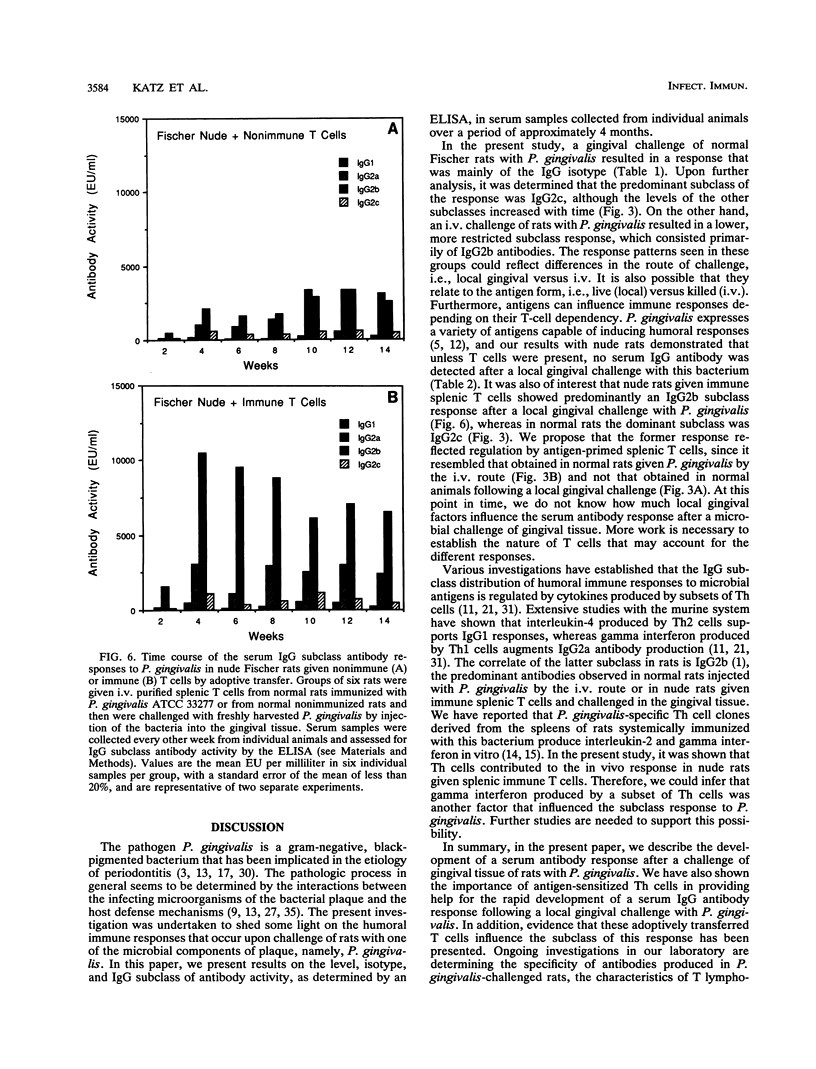
In this study, we describe the time course and T-cell dependence of the serum antibody response to the periodontopathogen Porphyromonas (Bacteroides) gingivalis in an experimental rat model. Normal Fischer rats were challenged by a local injection of P. gingivalis (2 x 10(8) bacteria) into gingival tissue or by the administration of a similar number of bacteria by the intravenous (i.v.) route on days 0, 2, and 4. Serum antibody activity was detected within 1 week and peaked at 8 weeks after gingival challenge. A similar but lower response was seen for rats challenged by the i.v. route. The response in both groups of rats was mainly of the immunoglobulin G (IgG) isotype; some IgM but no IgA antibody activity was detected. Analysis of the IgG subclass revealed mainly IgG2c in animals challenged locally in the gingiva with P. gingivalis, whereas IgG2b predominated in rats challenged by the i.v. route. The importance of T cells in the response was established by demonstrating the absence of serum IgG antibodies in nude rats after a local challenge of gingival tissue with P. gingivalis. Nude rats given purified splenic T cells from normal rats immunized systemically with P. gingivalis prior to a local gingival challenge showed a rapid appearance of serum antibody activity that peaked between 4 and 6 weeks. This initial peak occurred 2 to 4 weeks earlier than that seen in normal animals. Fluorescence-activated cell sorter analysis of splenic lymphoid cells from these nude rats revealed a helper T-cell population. The levels of serum IgG antibodies in nude rats given nonimmune T cells rose slowly, and the antibodies were mainly of the IgG2a and IgG2b subclasses. Nude rats given immune T cells showed a rapid increase primarily in IgG2b antibody levels following a local gingival challenge. These findings suggest that the immune helper T-cells contributed to the rapid development of the response to P. gingivalis. Furthermore, it is likely that the IgG subclass response to P. gingivalis in these nude rats was related to the splenic origin of the T cells used for adoptive transfer.
Full text
PDF

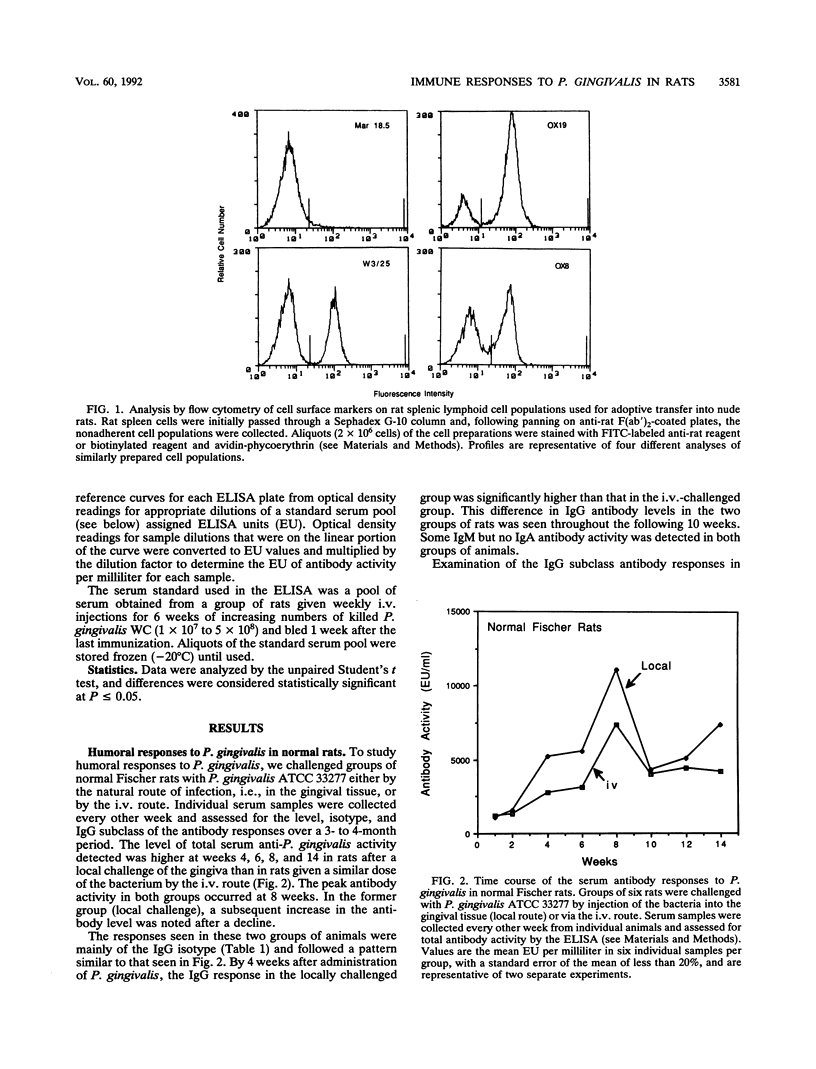

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celenligil H., Kansu E., Ruacan S., Eratalay K., Caglayan G. Immunohistological analysis of gingival lymphocytes in adult periodontitis. J Clin Periodontol. 1990 Sep;17(8):542–548. [PubMed] [Google Scholar]
- Colwell D. E., Michalek S. M., Briles D. E., Jirillo E., McGhee J. R. Monoclonal antibodies to Salmonella lipopolysaccharide: anti-O-polysaccharide antibodies protect C3H mice against challenge with virulent Salmonella typhimurium. J Immunol. 1984 Aug;133(2):950–957. [PubMed] [Google Scholar]
- Ebersole J. L., Frey D. E., Taubman M. A., Haffajee A. D., Socransky S. S. Dynamics of systemic antibody responses in periodontal disease. J Periodontal Res. 1987 May;22(3):184–186. doi: 10.1111/j.1600-0765.1987.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Ebersole J. L. Systemic humoral immune responses in periodontal disease. Crit Rev Oral Biol Med. 1990;1(4):283–331. doi: 10.1177/10454411900010040601. [DOI] [PubMed] [Google Scholar]
- Ebersole J. L., Taubman M. A., Smith D. J., Frey D. E. Human immune responses to oral microorganisms: patterns of systemic antibody levels to Bacteroides species. Infect Immun. 1986 Feb;51(2):507–513. doi: 10.1128/iai.51.2.507-513.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge J. H., Kimura S., Morisaki I., Michalek S. M., Hamaoka T., Hamada S., McGhee J. R. Immunoregulation in the rat: cellular and molecular requirements for B cell responses to types 1, 2, and T-dependent antigens. J Immunol. 1985 Apr;134(4):2236–2246. [PubMed] [Google Scholar]
- Genco R. J., Slots J. Host responses in periodontal diseases. J Dent Res. 1984 Mar;63(3):441–451. doi: 10.1177/00220345840630031601. [DOI] [PubMed] [Google Scholar]
- Gmür R., Hrodek K., Saxer U. P., Guggenheim B. Double-blind analysis of the relation between adult periodontitis and systemic host response to suspected periodontal pathogens. Infect Immun. 1986 Jun;52(3):768–776. doi: 10.1128/iai.52.3.768-776.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Bramanti T. E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2(2):177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Ebersole J., Felton J., Brunsvold M., Kornman K. S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988 Jan 1;239(4835):55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- Katz J., Michalek S. M. A method for generating antigen-specific rat T helper cell clones. J Immunol Methods. 1991 Apr 8;138(1):77–86. doi: 10.1016/0022-1759(91)90066-o. [DOI] [PubMed] [Google Scholar]
- Katz J., Michalek S. M., Beagley K. W., Eldridge J. H. Characterization of rat T helper cell clones specific for Bacteroides gingivalis antigen. Infect Immun. 1990 Sep;58(9):2785–2791. doi: 10.1128/iai.58.9.2785-2791.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen B. Microbiological and immunological aspects of experimental periodontal disease in rats: a review article. J Periodontol. 1991 Jan;62(1):59–73. doi: 10.1902/jop.1991.62.1.59. [DOI] [PubMed] [Google Scholar]
- Kornman K. S. Nature of periodontal diseases: assessment and diagnosis. J Periodontal Res. 1987 May;22(3):192–204. doi: 10.1111/j.1600-0765.1987.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Gutman G. A., Lewis D. E., Griswold S. T., Warner N. L. Monoclonal antibodies against rat immunoglobulin kappa chains. Hybridoma. 1982;1(2):125–131. doi: 10.1089/hyb.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., Morisaki I., Harmon C. C., Hamada S., McGhee J. R. Effective immunity to dental caries: gastric intubation of Streptococcus mutans whole cells or cell walls induces protective immunity in gnotobiotic rats. Infect Immun. 1983 Feb;39(2):645–654. doi: 10.1128/iai.39.2.645-654.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki I., Eldridge J. H., Michalek S. M., Hamada S., McGhee J. R. Immunoregulation in the rat: requirements for in vitro B cell responses to classical TI-1 and TI-2 antigens. J Immunol. 1983 Sep;131(3):1131–1137. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Murray P. A., Burstein D. A., Winkler J. R. Antibodies to Bacteroides gingivalis in patients with treated and untreated periodontal disease. J Periodontol. 1989 Feb;60(2):96–103. doi: 10.1902/jop.1989.60.2.96. [DOI] [PubMed] [Google Scholar]
- Naito Y., Okuda K., Takazoe I. Detection of specific antibody in adult human periodontitis sera to surface antigens of Bacteroides gingivalis. Infect Immun. 1987 Mar;55(3):832–834. doi: 10.1128/iai.55.3.832-834.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Okuda K., Takazoe I. Immunoglobulin G response to subgingival gram-negative bacteria in human subjects. Infect Immun. 1984 Jul;45(1):47–51. doi: 10.1128/iai.45.1.47-51.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., McGhee M. L., Moldoveanu Z., Hamada S., Mestecky J., McGhee J. R., Kiyono H. Bacteroides-specific IgG and IgA subclass antibody-secreting cells isolated from chronically inflamed gingival tissues. Clin Exp Immunol. 1989 Apr;76(1):103–110. [PMC free article] [PubMed] [Google Scholar]
- Scher I. CBA/N immune defective mice; evidence for the failure of a B cell subpopulation to be expressed. Immunol Rev. 1982;64:117–136. doi: 10.1111/j.1600-065x.1982.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Seymour G. J. Possible mechanisms involved in the immunoregulation of chronic inflammatory periodontal disease. J Dent Res. 1987 Jan;66(1):2–9. doi: 10.1177/00220345870660010401. [DOI] [PubMed] [Google Scholar]
- Seymour G. J., Powell R. N., Davies W. I. Conversion of a stable T-cell lesion to a progressive B-cell lesion in the pathogenesis of chronic inflammatory periodontal disease: an hypothesis. J Clin Periodontol. 1979 Oct;6(5):267–277. doi: 10.1111/j.1600-051x.1979.tb01930.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Syed S. A. Characteristics of Bacteroides asaccharolyticus from dental plaques of beagle dogs. J Clin Microbiol. 1980 May;11(5):522–526. doi: 10.1128/jcm.11.5.522-526.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Bacteriology of human experimental gingivitis: effect of plaque age. Infect Immun. 1978 Sep;21(3):821–829. doi: 10.1128/iai.21.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman M. A., Buckelew J. M., Ebersole J. L., Smith D. J. Periodontal bone loss and immune response to ovalbumin in germfree rats fed antigen-free diet with ovalbumin. Infect Immun. 1981 Apr;32(1):145–152. doi: 10.1128/iai.32.1.145-152.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman M. A., Yoshie H., Ebersole J. L., Smith D. J., Olson C. L. Host response in experimental periodontal disease. J Dent Res. 1984 Mar;63(3):455–460. doi: 10.1177/00220345840630031801. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Marshall D. R., Moore W. E., Best A. M., Palcanis K. G., Ranney R. R. Serum antibody reactive with predominant organisms in the subgingival flora of young adults with generalized severe periodontitis. Infect Immun. 1985 May;48(2):303–311. doi: 10.1128/iai.48.2.303-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



