Abstract
As in many eukaryotic cells, fission yeast cytokinesis depends on the assembly of an actin ring. We cloned myp2+, a myosin-II in Schizosaccharomyces pombe, conditionally required for cytokinesis. myp2+, the second myosin-II identified in S. pombe, does not completely overlap in function with myo2+. The catalytic domain of Myp2p is highly homologous to known myosin-IIs, and phylogenetic analysis places Myp2p in the myosin-II family. The Myp2p sequence contains well-conserved ATP- and actin-binding motifs, as well as two IQ motifs. However, the tail sequence is unusual, since it is predicted to form two long coiled-coils separated by a stretch of sequence containing 19 prolines. Disruption of myp2+ is not lethal but under nutrient limiting conditions cells lacking myp2+ function are multiseptated, elongated, and branched, indicative of a defect in cytokinesis. The presence of salt enhances these morphological defects. Additionally, Δmyp2 cells are cold sensitive in high salt, failing to form colonies at 17°C. Thus, myp2+ is required under conditions of stress, possibly linking extracellular growth conditions to efficient cytokinesis and cell growth. GFP-Myp2p localizes to a ring in the middle of late mitotic cells, consistent with a role in cytokinesis. Additionally, we constructed double mutants of Δmyp2 with temperature-sensitive mutant strains defective in cytokinesis. We observed synthetic lethal interactions between Δmyp2 and three alleles of cdc11ts, as well as more modest synthetic interactions with cdc14ts and cdc16ts, implicating myp2+ function for efficient cytokinesis under normal conditions.
INTRODUCTION
Many eukaryotic cells divide by a process known as cytokinesis. In the best characterized systems, cell separation occurs via constriction of the plasma membrane mediated by a contractile ring (Satterwhite and Pollard, 1992; Fishkind and Wang, 1995). The contractile ring is composed of antiparallel actin filaments, which encircle the equator just inside the plasma membrane. In a variety of eukaryotic cells, myosin-II is the molecular motor, which provides the force to constrict the contractile ring. However, the molecules which activate myosin-II, thus initiating contraction or the molecules which direct the site of cleavage furrow formation remain mostly unknown. A model system which is genetically manipulatible would provide insight as to the regulators of cytokinesis.
The fission yeast, Schizosaccharomyces pombe, a genetically tractable unicellular organism, has proved to be an excellent model organism by which to study regulation of the cell cycle (Hayles and Nurse, 1989). Fission yeasts divide by medial fission. Actin is localized at the growing ends of the cells during interphase and in early M-phase actin concentrates at the center of the cell, where it forms a ring just inside the plasma membrane (Marks and Hyams, 1985). After completion of nuclear division and segregation, the actin ring constricts, closely followed by formation of a septum, which is deposited from the outside of the cell and moves inward. Digestion of the cell wall eventually separates the two daughter cells.
Genetic analysis has identified genes essential for cytokinesis, including cdc3+, which encodes a profilin, and cdc8+, which encodes a tropomyosin (Balasubramanian et al., 1992; Balasubramanian et al., 1994). Both proteins bind actin, and in their absence actin is mislocalized throughout the cell cycle (Balasubramanian et al., 1992; Balasubramanian et al., 1994; Chang et al., 1996). A few temperature-sensitive mutants, cdc4ts, cdc12ts, cdc15ts, and rng2ts, affect the distribution of actin only during mitosis (Chang et al., 1996). cdc4+ encodes a putative myosin light chain, which localizes to the actin ring during cytokinesis (McCollum et al., 1995), suggesting that a myosin may be involved in cytokinesis in S. pombe. During the course of this work, a myosin-II gene (myo2+) was cloned from the fission yeast, and the product was shown to localize to the actin ring during cytokinesis (Kitayama et al., 1997). Disruption of myo2+ is lethal. When cells are depleted of myo2+, cells arrest elongated, multiseptated, and occasionally branched. Overexpression of myo2+ is toxic to cell growth and leads to a large number of multinucleated cells with few septa and no apparent actin ring formation. Thus, as in many other eukaryotic cells, myosin-II is required for cytokinesis in fission yeast, demonstrating that S. pombe may provide a model system for the study of cytokinesis in general.
We identified a second myosin-II in S. pombe, which we named myp2+ for myosin-II of pombe. myp2+ may play a more specialized role in cytokinesis than myo2+. Under conditions of limiting nutrients, the absence of myp2+ function leads to multiseptated cells consistent with a role of myp2+ in cytokinesis. In addition, myp2+ is essential in the presence of 1 M KCl at 17°C. Thus myo2+ cannot replace myp2+ under all conditions. Overexpression of Myp2p is toxic, leading to multinucleated cells. In late mitotic cells, GFP-Myp2p localizes to either a ring or a dot in the middle of the cell, which precedes the site of septation. Together with the localization, genetic interactions under normal growth conditions between strains lacking myp2+ and other mutants defective in cytokinesis also suggest that myp2+ functions at all times during cytokinesis.
MATERIALS AND METHODS
Phylogenetic Analysis
Myosin sequences were obtained from GenBank (GB), Protein Identification Resource (PIR), and Swiss Prot (SP) databases. Names and accession numbers are as follows: chicken embryonic skeletal muscle myosin-II, SP: P02565; chicken nonmuscle myosin-IIa, SP: P14105; chicken smooth muscle myosin-II, SP: P10587; chicken skeletal muscle myosin-II, SP: P13538; chicken brush border myosin-I, GB: X58479; chicken myosin-V, GB: Z11718; Acanthamoeba myosin-Ib, SP: P19706; Acanthamoeba myosin-Ic, SP: P10569; Acanthamoeba high-molecular-weight myosin-I, PIR: A23662; Acanthamoeba myosin-II, SP: P05659; Arabidopsis ATM2, GB: Z34292; Arabidopsis Mya1, GB: Z28389; Arabidopsis Mya2, GB: Z34294; Caenorhabditis elegans myosin-Ia, GB: Z75564; bovine brush border myosin-I, SP: P10568; bovine myosin-X, GB: U55210; Dictyostelium myosin-Ic, GB: L35323; Dictyostelium myosin-Ia, SP: P22467; Dictyostelium myosin-II, SP: P08799; Dictyostelium MyoJ, GB: L35322; Drosophila myosin-Ia, PIR S45573; Drosophila myosin-Ib, PIR: S45574; Drosophila muscle myosin-II, GB: M61229; Drosophila 95F, SP: Q01989; Drosophila ninaC, SP: P10676; Entamoeba myosin-II, GB: L03534; frog myosin-Iβ, GB: U14549; human myosin-Ic, GB: U14391; mouse myosin-Iα, GB: L00923; mouse dilute, GB: X57377; pig myosin-VI, PIR: A54818; rat myr1a, PIR: A45439; rat myr3, GB: X74815; rat myr4, PIR: A53933; rat myr5, GB: X77609; Saccharomyces cerevisiae MYO1, PIR: S46773; S. cerevisiae MYO2, SP: P19524; S. cerevisiae MYO4, SP: P32492; S. pombe myo2+, GB: U75357. We used the program Clustal W (Thompson et al., 1994) to create a multiple-sequence alignment of these sequences and to build the tree from the alignment. Bootstrapped distance matrix analysis was performed using Clustal W with 1000 bootstrapping trials. We used the PHYLIP program DRAWTREE to draw the tree from the output of the Clustal W program.
Strains Media and Transformation
S. pombe strains used in this study are listed in Table 1. Yeast culture, methods, media, and genetic manipulations were carried out by standard methods (Moreno et al., 1991). Transformation of S. pombe was achieved by electroporation (Moreno et al., 1991) or by a lithium acetate method (Okazaki et al., 1990).
Table 1.
Strains used in this study
| Strain | Genotype |
|---|---|
| FY435 | h+ his7–366 leu1–32 ura4-Δ18 ade6-M210 |
| FY436 | h− his7–366 leu1–32 ura4-Δ18 ade6-M216 |
| TP5 | h− Δmyp2::his7+ his7–366 leu1–32 ura4-Δ18 ade6-M210 |
| TP50 | h+ Δmyp2::his7+ his7–366 leu1–32 ura4-Δ18 ade6-M216 |
| TP47 | h− cdc11–136 his7–366 leu1–32 ura4-Δ18 |
| TP49 | h− cdc11–123 his7–366 leu1–32 |
| TP36 | h− cdc11–19 his7–366 leu1–32 ura4-Δ18, ade6-M216 |
| TP37 | h+ cdc14–118 his7–366 leu1–32 |
| TP9 | h− cdc8–27 his7–366 leu1–32 ura4-Δ18, ade6-M216 |
| TP32 | h+ cdc16–116 his7–366 leu1–32 ade6-M210 |
| TP51 | h+ cdc11–136 Δmyp2::his7+ his7–366 leu1–32 ura4-Δ18 ade6-M216 |
| TP52 | h− cdc11–123 Δmyp2::his7+ his7–366 leu1–32 ura4-Δ18 |
| TP53 | h− cdc11–19 Δmyp2::his7+ his7–366 leu1–32 ura4-Δ18 ade6-M216 |
| TP54 | h− cdc14-118 Δmyp2::his7+ his7–366 leu1–32 ura4-Δ18 |
| TP55 | h− cdc16–116 Δmyp2::his7+ his7–366 leu1–32 ura4-Δ18 ade6-M210 |
| TP23 | h+ cdc8–27 Δmyp2::his7+ his7–366 leu1–32 ura4-Δ18 ade6-M216 |
Identification of myp2+, Cloning, and Sequencing
We used the TBlastN algorithm (Altschul et al., 1990) to search the Sanger genome S. pombe database (http://www.sanger.ac.uk/Projects/S_pombe/) with the chicken skeletal muscle myosin II sequence. We obtained one open reading frame that had a score of 359 and smallest sum probability of 2.6 × 10−215 for N = 13. We obtained the nucleotide sequence for the potential myosin-II open reading frame by FTP from the collection of completely sequenced cosmids at the Sanger S. pombe genome database (ftp://ftp.sanger.ac.uk/pub/yeast/sequences/pombe/).
Using the sequence information from the Sanger genome S. pombe database, we designed primers to amplify the myp2+ gene by polymerase chain reaction (PCR). Two PCR products were obtained. One is 2.2 kilobases (kb) and comprises the catalytic domain of myp2+. The second is the full length myp2+ and is 6.4 kb. These PCR products were subcloned into pBluescript by conventional methods using a BamHI restriction site designed into the 5′ PCR primer and a SalI restriction site designed into the 3′ PCR primer. The PCR products were sequenced by automated sequencing (Applied Biosystems Inc., Foster City, CA). The catalytic domain was intact and had no sequence differences with respect to the sequence obtained from the Sanger database. The original full-length PCR product had several mutations in the 5′ end, including a 700-base pair (bp) deletion but was intact from the unique AccI site to the 3′ end. The region from the AccI site to the 3′ end was sequenced on both strands, and eight nucleotide differences were found with respect to the sequence obtained from the Sanger database, resulting in three amino acid changes. Because multiple PCR clones contain the same nucleotide differences, we assume that the unfinished sequence provided by the Sanger database was incorrect. To construct an intact myp2+ clone, a BamHI/AccI fragment from the catalytic domain was ligated into the full-length construct replacing the incorrect 5′ end. This construct is pmyp2BS. The sequence of myp2+ has been deposited into GenBank with accession number AF029788.
To determine the 5′ end of myp2+, we isolated RNA from wild-type cells in midlog growth in yeast extract and supplements media following the manufacturer’s recommendations (RNeasy Midi Prep, Qiagen, Chatsworth, CA). We performed 5′ rapid amplification of cDNA ends (5′ RACE) following the manufacturer’s recommendations (Life Technologies, Inc. 5′ RACE system). The 5′ RACE PCR products were amplified with Taq polymerase and subcloned into the TA-cloning vector (Invitrogen, San Diego, CA). The RACE products were sequenced by automated sequencing (ABI).
Disruption of myp2+
PCR primers were designed based on the sequence obtained from the Sanger database to amplify a 1.4-kb region upstream and overlapping the 5′ of myp2+ and a 1-kb region downstream and overlapping the 3′ of myp2+ from genomic DNA. The 5′ PCR product contains 411 bp of the coding sequence, and the 3′ PCR product contains the last 42 bp of coding sequence (Figure 4B). The 5′ and 3′ PCR products were ligated to an EcoRI fragment of pEA2 (Apolinario et al., 1993) containing the his7+ gene, resulting in the linear knockout construct deleting 5858 bp of myp2+ (Figure 4B). The linear fragment was transformed into a his7−366 wild-type diploid constructed from FY435 and FY436. His+ transformants were sporulated on Edinburgh minimal medium, and stably transformed His+ haploids were analyzed by colony PCR (C. Troxell, personal communication) to determine the position of integration of the his7+ gene. The forward PCR primer is located within the knockout construct, and the reverse PCR primer is located outside the knockout construct (Figure 4B). Amplification of the disrupted locus results in a PCR product that is 2.5 kb smaller than the wild-type locus.
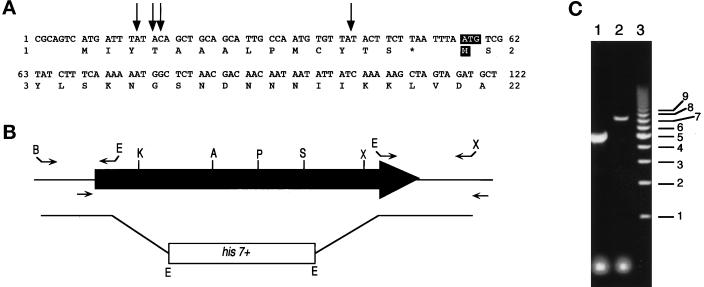
Figure 4.
Cloning 5′ of myp2+ and construction of the myp2-disrupted strain. (A) The DNA sequence for the 5′ region of myp2+ in the pmyp2BS construct is shown along with the deduced amino acid sequence. The arrows indicate the position of the beginning of four separate 5′ RACE PCR products that were sequenced. The putative start codon and methionine are highlighted with white lettering against black. (B) Schematic representation of the genomic locus of myp2+. The shaded arrow represents the open reading frame of 6312 bp. The primers used to amplify the 5′ and 3′ regions of myp2+ that were used to generate the knockout construct are represented above the locus. The restriction sites used to ligate together the knockout construct are indicated at the ends of the primers. B, BamHI; E, EcoRI; X, XhoI. The primers used to amplify the genomic locus from His+ haploids generated from the diploid transformed with the knockout construct are indicated below the locus. (C) Amplification of the genomic locus by colony PCR from His+ haploids. PCR products were separated on an 0.8% agarose gel. Lane 1 represents a homologous recombination event resulting in disruption of the myp2+ locus by his7+. The disrupted locus is 2.5 kb smaller than the wild-type locus. Lane 2 represents a heterologous recombination event in which his7+ has been integrated elsewhere in the genome thus leaving the myp2+ locus intact. Lane 3 is the molecular weight marker with sizes in kilobases indicated to the right.
Complementation and Overexpression
pSGP573 is a GFP-tagging vector that carries the thiamine-repressible nmt1+ promoter (gift of S.G. Pasion). To express myp2+ from the nmt1+ promoter and fused to GFP at the N terminus, a PCR product of the 5′ 2.2 kb of myp2+ was amplified from genomic DNA with a primer that anneals immediately downstream of the start methionine. A NotI site was engineered into the 5′ primer. We verified the sequence of this PCR product and using the unique AccI site within this fragment, we ligated it to the 3′ of myp2+, creating pmyp2BS-1. The NotI/SalI fragment from pmyp2BS-1 containing myp2+ without the start methionine was ligated into pSGP573 to construct pGFPmyp2. pGFPmyp2 was transformed into FY436 and TP5 strains. Ura+ transformants were selected and streaked on selective EMM ± 1 M KCl with and without thiamine at 36, 32, and 25°C.
Microscopy
For analysis of the phenotype of Δmyp2, cells were either removed from plates and resuspended in buffer or visualized directly on plates. We used a 40× long-working-distance bright-field objective (Nikon) to visualize cells on plates. Cells removed from plates and resuspended in buffer were either directly deposited on a glass slide and then visualized by phase contrast microscopy or stained with calcofluor and 4′-6-diamidino-2-phenylindole (DAPI). To stain cells, the cells were spun down and then resuspended in 70% ethanol at room temperature for 10 min. The cells were resuspended in phosphate-buffered saline containing 0.5 μg/ml calcofluor and 0.5 μg/ml DAPI for 10 min at room temperature. The cells were washed twice with phosphate-buffered saline and then resuspended in phosphate-buffered saline and deposited onto a glass microscope slide and covered with a coverslip. Cells were observed with a 100× objective on a Leitz microscope using a filter appropriate for calcofluor and DAPI.
To determine the localization of GFP-Myp2p, cells were grown in culture to exponential phase. Cells were harvested by centrifugation and resuspended in water containing 0.5 μg/ml DAPI and incubated at room temperature for 10 min. Cells were pelleted and then resuspended in water and deposited onto a glass microscope slide, dried down, and covered with 1 mg/ml phenylenediamine in 90% glycerol and a coverslip. Cells were observed with a 63× objective on a Leitz microscope using filters appropriate for DAPI and GFP. All images were photographed with 35-mm slide film, which were digitally scanned and printed using the Macintosh program Adobe Photoshop.
RESULTS
Identification of myp2+ Sequence
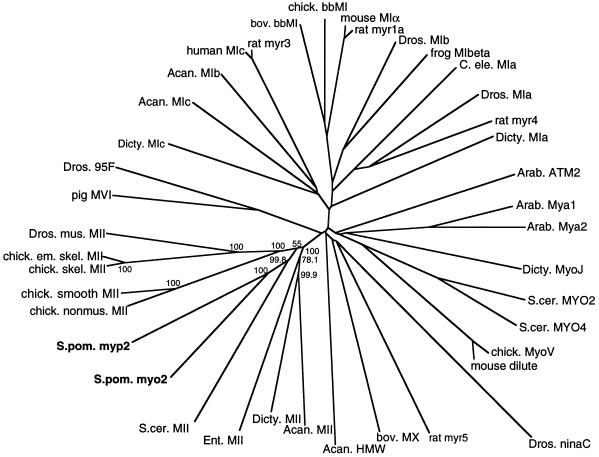
A search of the Sanger S. pombe genome database using the TBlastN algorithm (Altschul et al., 1990) yielded one large open reading frame (ORF) with significant homology to chicken skeletal myosin-II protein sequence. We named this ORF myp2+ for myosin-II of pombe. The gene myp2+ encodes a polypeptide of 2104 amino acids and predicted molecular weight of 240,000. The first 824 amino acids are highly homologous to the catalytic domains of myosin heavy chains. To classify Myp2p, we used Clustal W (Thompson et al., 1994), to align the putative catalytic domain of Myp2p with a panel of myosins from several distinct families. From this alignment, we built a phylogenetic tree. The S. pombe myosins Myp2p and Myo2p and the S. cerevisiae myosin-II MYO1p are related to each other (Figure 1) and as a group join the main branch of myosin-IIs with a bootstrapping value of 55%, which indicates that of the 1000 bootstrapping trials, 55% of the time the yeast myosin-IIs grouped with the main branch and 45% of the time they joined the amoebae myosin-IIs. However, the node of the amoebae myosin-IIs joins the main myosin-II branch 100% of the time and is more distant than the node of the yeast myosin-IIs. In addition, both Acanthamoeba and Dictyostelium myosin-II are well-characterized type II myosins by various criteria. Thus, this analysis places Myp2p as a member of the myosin-II family. An alignment of the catalytic domains of the yeast and amoebae myosin-IIs (Figure 2) illustrates that Myp2p has a well-conserved putative ATP-binding motif (underline) as well as a putative actin-binding motif (asterisks). In addition, Myp2p has two putative IQ motifs (underlined twice).
Figure 1.
Phylogenetic analysis of myp2+. An unrooted phylogenetic tree was generated from an alignment of catalytic domains of myosins from several families using Clustal W (Thompson et al., 1994). The numbers indicate the bootstrapping value in percentage of 1000 bootstrapping trials for the type II myosin subfamily. In this analysis, the lengths of the branches joining two proteins are proportional to the percentage of amino acid sequence divergence between the two proteins. The myosin-catalytic domain sequences do not include the light chain-binding region, since they were defined as ending 18 residues after the conserved threonine in the TKVFF sequence. The accession numbers for the sequences are given in MATERIALS AND METHODS. The abbreviations used are as follows: Acan., Acanthamoeba; Arab., Arabidopsis; bov., bovine; chick., chicken; em., embryonic; skel., skeletal; mus., muscle; nonmus., nonmuscle; Dicty., Dictyostelium; Dros., Drosophila; C. ele., C. elegans; S. cer., S. cerevisiae; S. pom., S. pombe.
Figure 2.
Sequence alignment of myp2+ with six myosin-IIs from other organisms. The catalytic domain of myp2+ was aligned with myosin-IIs from S. pombe, S. cerevisiae, amoebae, and chicken using Clustal W (Thompson et al., 1994). From top to bottom: S. pombe myp2+, S. pombe myo2+, S. cerevisiae myosin-II (MYO1p), Entamoeba myosin-II, Acanthamoeba myosin-II, Dictyostelium myosin-II, and chicken skeletal muscle myosin-II. The sequence accession numbers are given in MATERIALS AND METHODS. The numbers to the left of the amino acid sequence indicate amino acid position. Amino acid identities are shown in white lettering against black, conserved residues are shown in black lettering against gray. The putative ATP-binding motif is underlined. The putative actin-binding site has asterisks underneath. The putative IQ motifs are underlined twice.
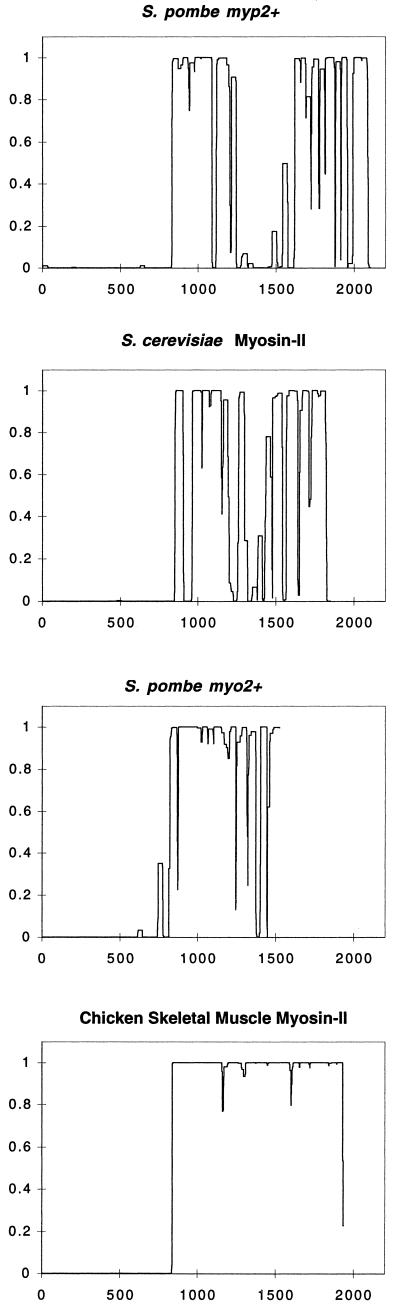
The C-terminal tail of Myp2p consists of 1277 residues, including 25 prolines. The coiled-coil prediction algorithm Coils (Lupas et al., 1991) predicts two distinct regions in the Myp2p tail with a high probability to form a coiled-coil (Figure 3). These two regions are separated by a stretch of 270 amino acids containing 19 of the 25 prolines. Myosin-II tails from other organisms rarely contain prolines, because the tail forms a single coiled-coil rod and prolines disrupt α-helices. However, the algorithm Coils predicts that the tail of S. cerevisiae myosin-II (MYO1p), which consists of 1000 residues containing 6 prolines, also has two regions of coiled-coil, much like Myp2p (Figure 3). Thus, Myp2p and MYO1p may represent a subset of myosin-IIs that contain a bipartite coiled-coil tail.
Figure 3.
Coiled-coil predictions of myosin-IIs from different organisms. The predictions were generated by the Coils program (Lupas et al., 1991) for S. pombe Myp2p, S. cerevisiae myosin-II (MYO1p), S. pombe Myo2p, and chicken skeletal muscle myosin-II. x-axis, amino acid position. y-axis, probability of forming a coiled-coil. A window of 28 amino acids was used to generate the profiles shown.
For comparison, the tail of myosin-II from chicken skeletal muscle has no prolines in its tail of 1098 residues. The prediction for chicken skeletal muscle myosin-II indicates that the entire tail forms one coiled-coil (Figure 3), which has been established by physical measurement (Lowey et al., 1969). Similarly, the tail of the other myosin-II in S. pombe, Myo2p, is predicted to form a single region of coiled-coil (Figure 3). However, the tail of Myo2p contains 9 prolines. Also the tail is significantly shorter than Myp2p only having 711 residues. Toward the end of the Myo2p tail, there are several regions of low coiled-coil propensity, reflecting the presence of the prolines, but no large break like Myp2p and MYO1p, possibly because the tail of Myo2p is shorter than the Myp2p tail. Alignment of the coiled-coil predictions, such that each myosin-II tail begins roughly at the same position on the x-axis (Figure 3), demonstrates that the reduction of the coiled-coil propensity occurs in the same region of the tail for each yeast myosin-II. Consistent with this, when the yeast myosin-II tails are aligned, the prolines are located in the same region of sequence. Thus, the yeast myosin-II tails have a unique coiled-coil structure. Myp2p and MYO1p have two domains of coiled-coil separated by a stretch of sequence of ∼200 amino acids containing the majority of the prolines and the Myo2p tail has only the first coiled-coil domain, which ends with a stretch of sequence containing prolines.
Cloning of myp2+
We cloned myp2+ by amplifying two overlapping sections of myp2+ from genomic DNA. These segments were each subcloned into pBluescript KS+ by conventional methods. We verified the sequence of myp2+. There were eight nucleotide differences from the sequence obtained from the Sanger genome database resulting in three different amino acid residues (see MATERIALS AND METHODS). We obtained a full-length clone (pmyp2BS) by ligating together the amplified overlapping clones using a unique restriction site in the myp2+ ORF (see MATERIALS AND METHODS).
The 5′ end of pmyp2BS has a small ORF of 14 amino acids just upstream and out of frame with the putative start methionine of myp2+ (Figure 4A). We used 5′ RACE PCR (Frohman et al., 1988) to determine the 5′ end of myp2+. The sequenced RACE PCR products all start within the small 14-amino acid ORF (Figure 4A, arrows), suggesting that the first methionine, which is in frame with the myp2+ ORF, is the start methionine. The RACE PCR also demonstrates that the 5′ of myp2+ is transcribed in wild-type cells at the time the RNA was isolated. In addition, we amplified a 1.4-kb region of the 3′ of myp2+ from a cDNA library. Together with the RACE result, this shows that the myp2+ RNA is present in wild-type cells during midlog growth in rich media (YES).
Disruption of myp2+
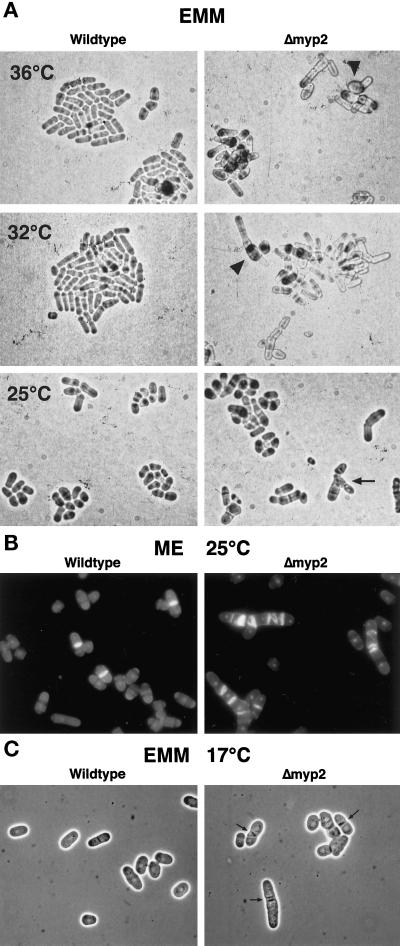
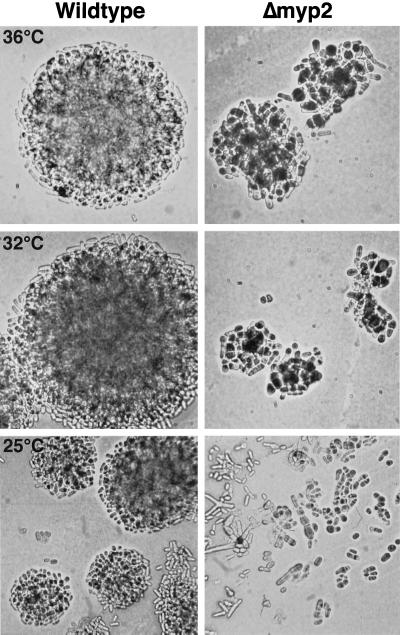
To examine myp2+ function, we disrupted myp2+ in S. pombe by replacing 80% of the coding sequence with the his7+ gene (Figure 4B). Sporulation of the diploid heterozygous for myp2+ resulted in four viable spores. We verified that myp2+ was disrupted in His+ haploids by amplifying the genomic locus with specific primers (Figure 4B). Amplification of the disrupted locus results in a smaller fragment than of the wild-type locus (Figure 4C). Wild-type and Δmyp2 strains form colonies of similar size at the same rate at all temperatures tested on YES, minimal media (EMM), and malt extract (ME). For example, at 32°C Δmyp2 single colonies form after 2 days on YES. The Δmyp2 strain had no apparent phenotype on YES media. However, on EMM (Figure 5A) or ME (Figure 5B), Δmyp2 colonies are composed of a heterogeneous mix of cells. Δmyp2 cells are often multiseptated, elongated, branched, or slightly swollen. The swollen cells are more prevalent at higher temperatures. Calcofluor staining reveals that the septa are generally abnormal (Figure 5B). In addition, at 17°C Δmyp2 cells have a very pronounced septum, which can be seen by phase contrast microscopy (Figure 5C, arrows). Because multiply septated cells have a nucleus in each compartment (Figure 5B), the deletion of myp2+ probably does not affect the nuclear division cycle. We do not know which components in the EMM or ME trigger the morphological differences in the Δmyp2 cells. The phenotype observed on EMM and ME is most penetrant when the cells are grown on agar. In liquid EMM culture, Δmyp2 cells grow at the same rate as wild-type at 32° and 25°C and appear normal except for an elevated number of septated cells. In an exponentially growing EMM liquid culture at 32°C, 10% of wild-type cells are septated, but 20% of Δmyp2 cells are septated.
Figure 5.
Phenotype of Δmyp2 strain. (A) Wild-type and Δmyp2 strains on EMM-agar grown at 36°C (top), 32°C (middle), and 25°C (bottom). Cells were taken from YES plates and streaked onto EMM plates. Cells were visualized using a 40× long-working-distance bright-field objective and photographed directly off the plate. Both strains form groups of cells that will eventually form colonies of roughly the same size. Many of Δmyp2 cells have multiple septa, and a few are branched (arrow). Δmyp2 cells are slightly wider than wild-type and are occasionally swollen (arrowheads) at the higher temperatures. (B) Fluorescence micrographs of wild-type and Δmyp2 cells grown on ME agar, removed from the plate, and stained with DAPI and calcofluor. (C) Phase contrast micrographs of wild-type and Δmyp2 cells grown on EMM agar at 17°C smeared on a microscope slide. Δmyp2 cells have very pronounced and misshapen septa (arrows).
The most striking effects of the myp2+ deletion are seen when the Δmyp2 cells are grown on plates with 1 M KCl. The morphological phenotype, including multiseptated cells, is more penetrant in YES or EMM with 1 M KCl than on EMM or ME. Δmyp2 cells also grow more slowly than wild-type on YES-agar +1 M KCl or EMM-agar + 1 M KCl (Table 2), although growth rates in liquid media with 1 M KCl are the same. By the time wild-type cells have formed colonies on YES-agar + 1 M KCl, Δmyp2 colonies are smaller and have a large number of elongated, wider cells, with multiple septa (Figure 6, 32°C and 36°C). Lower temperatures enhance the slow-growth phenotype. For example, at 25°C by the time that wild-type forms small colonies, no Δmyp2 colonies are visible (Figure 6, 25°C). At 17°C wild-type forms colonies after 10 days, but Δmyp2 forms no colonies even after 21 days (Table 2). The cells at 17°C on 1 M KCl die highly multiseptated and branched, indicative of a defect in cytokinesis. Thus in the presence of 1 M KCl, Δmyp2 cells are cold sensitive. Together with the Δmyp2+ phenotype observed with EMM and ME, the phenotype observed in 1 M KCl suggests that myp2+ is required for growth when the cells are stressed, either by limiting nutrients (EMM or ME) or by salt (1 M KCl).
Table 2.
Rate of colony formation in YESa + 1 M KCl
| Temperature (°C) | Wild-type | Δmyp2 |
|---|---|---|
| 36 | 3 d | 5 d |
| 32 | 3 d | 7 d |
| 25 | 6 d | 12 d |
| 17 | 10 d | No colonies |
Colony formation in EMM + 1 M KCl is the same for each strain at each temperature.
Figure 6.
Phenotype of Δmyp2 cells grown on YES + 1 M KCl at 36°C (top), 32°C (middle), and 25°C (bottom). Cells were streaked onto YES + 1 M KCl plates form YES plates. Cells were visualized using a 40× long-working-distance bright-field objective and photographed directly off the plate. Δmyp2 cells eventually form colonies at all three temperatures but are slower than wild-type, as apparent from the smaller colonies at 36°C and 32°C.
Complementation of Δmyp2 Strain
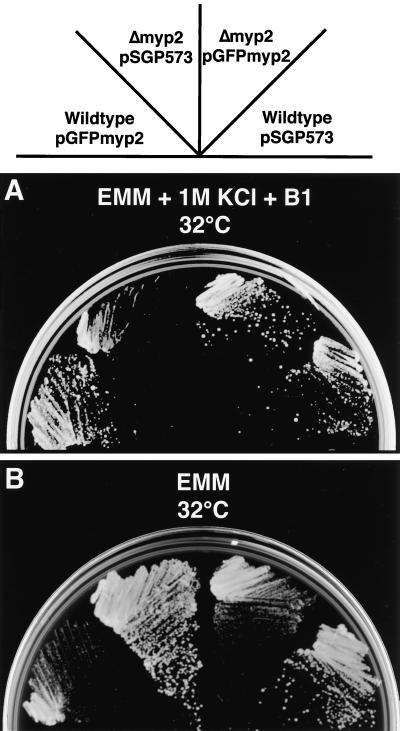
We verified that the cloned myp2+ could complement the Δmyp2 disruption phenotype. We constructed a fusion of GFP to the N terminus of Myp2p (see MATERIALS AND METHODS). Expression of GFP-Myp2p is controlled by the nmt1+ promoter, which allows low levels of expression in the presence of thiamine, and is induced at least 100-fold by removing thiamine from the media (Basi et al., 1993; Forsburg, 1993). The pGFPmyp2 plasmid restores wild-type growth to Δmyp2 cells on selective EMM with 1 M KCl at 32°C in the presence of thiamine (Figure 7A). The Δmyp2 transformants have normal morphology, and the colony size is the same as wild-type. Thus, GFP-Myp2p is functional. Expression of GFP-Myp2p was verified by microscopy (see below). Additionally, low levels of expression of GFP-Myp2p have no effect on colony formation of wild-type cells (Figure 7A).
Figure 7.
Complementation by pGFPmyp2 and overexpression of pGFPmyp2. Ura+ transformants of strains carrying the indicated plasmid (pSGP573 or pGFPmyp2) were streaked to (A) EMM-Ura + 1 M KCl + thiamine (B1) (this plate shows colonies after 4 days at 32°C) and to (B) EMM-Ura (this plate shows colonies after 3 days at 32°C).
Overexpression of GFP-Myp2p
Overexpression of GFP-Myp2p is accomplished by removing thiamine from the medium. On EMM plates without 1 M KCl, both wild-type and Δmyp2 cells are able only to form pinprick colonies in the absence of thiamine (Figure 7B). The cells present in the pinprick colonies are multinucleated and highly elongated. Like the phenotype of Δmyp2 cells, overexpression of GFP-Myp2p results in a defect in cytokinesis. However, the two phenotypes differ in that overexpressing GFP-Myp2p results in longer cells with very few septa and no branches. Thus overexpression of functional Myp2p is toxic to cell growth possibly by inhibition of cytokinesis and septation.
Localization of GFP-Myp2p
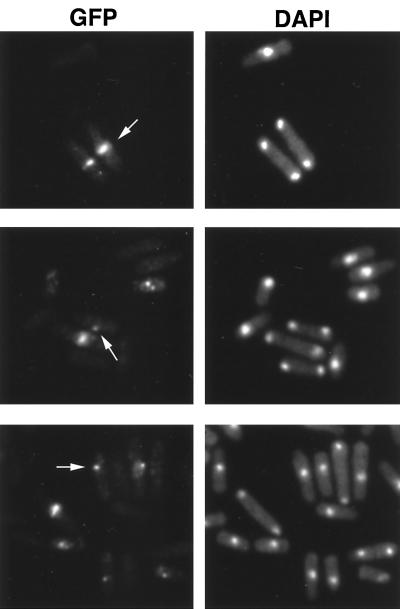
To observe the subcellular localization of GFP-Myp2p, we grew wild-type cells and Δmyp2 cells transformed with pGFPmyp2 in selective EMM culture with thiamine to exponential phase. Cells in late mitosis contain either a ring (Figure 8, top, arrow) or a bright spot (Figure 8, middle, arrow) of GFP-Myp2p in the middle of the cell. The GFP-Myp2p ring is consistent with actin ring localization during cytokinesis and precedes the site of septation. The spot may represent the localization either before ring formation or after contraction of the ring. In interphase, GFP-Myp2p mostly appears concentrated in a dot in the cytoplasm, usually near the nucleus (Figure 8, bottom, arrow). The distribution of GFP-Myp2p was similar in wild-type and Δmyp2 cells. The intensity of GFP fluorescence is variable between cells, which may reflect variable expression from the episome. However, our complementation results show that sufficient functional GFP-Myp2p is expressed to complement the Δmyp2 phenotype.
Figure 8.
Localization of GFP-Myp2p in Δmyp2 cells. Δmyp2 cells were grown in liquid with thiamine and harvested during exponential growth. Cells were stained with DAPI and observed immediately after staining. GFP-Myp2p localizes to a ring in the middle of late mitotic cells (arrow, top). Some late mitotic cells only show a bright spot in the middle of the cell (arrow, middle). Cells in interphase, show GFP-Myp2p localizing to a bright dot near the nucleus (arrow, bottom).
Genetic Interactions of Δmyp2 with Cytokinesis Temperature-sensitive Mutants
From the phenotypic analysis of the Δmyp2 strain, we know that myp2+ function is important for cytokinesis under specific growth conditions. To determine whether the loss of myp2+ function abrogates cytokinesis under normal growth conditions in YES media, we investigated genetic interactions between Δmyp2 and known regulators of cytokinesis. Double mutant strains were constructed between Δmyp2 and strains with temperature-sensitive mutations in cdc3+, cdc4+, cdc8+, cdc11+, cdc14+, and cdc16+. cdc3+ encodes a profilin (Balasubramanian et al., 1994), cdc8+ encodes a tropomyosin (Balasubramanian et al., 1992), and cdc4+ encodes a putative myosin light chain (McCollum et al., 1995). These three genes are localized at the contractile ring during cytokinesis, and the temperature-sensitive alleles are characterized as late septation mutants with defects in the organization of the septum and the actin contractile ring (Balasubramanian et al., 1992; Balasubramanian et al., 1994; McCollum et al., 1995; Chang et al., 1996). cdc11ts and cdc14ts are early septation mutants given that they are unable to form a septum and arrest with multiple nuclei. cdc11+ and cdc14+ are cloned and encode novel proteins (Fankhauser and Simanis, 1994b). cdc16+ is homologous to S. cerevisiae BUB2; it functions in the mitotic checkpoint as well as in regulation of septum formation (Fankhauser et al., 1993). We did not observe any interactions between the late septation mutants (cdc3ts, cdc4ts, and cdc8ts) and Δmyp2. However, we did observe synthetic interactions between Δmyp2 and the early septation mutants (cdc11ts and cdc14ts) and cdc16ts.
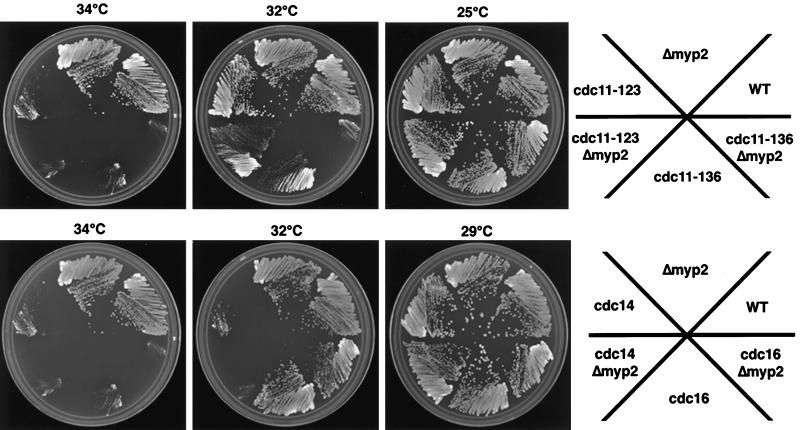
All three alleles of cdc11ts we tested (cdc11–19, cdc11–123, and cdc11–136) were synthetically lethal with Δmyp2. Δmyp2 cdc11–123 can only form pinprick colonies at 32°C, while both parent strains grow like wild-type (Figure 9, top). We observed a similar phenotype for Δmyp2 cdc11–19. cdc11–136 can form pinprick colonies at 32°C, but Δmyp2 cdc11–136 cannot form colonies at all (Figure 9, top). For Δmyp 2cdc14ts and Δmyp2 cdc16ts, there was a modest reduction in colony size (Figure 9, bottom) and the double mutants were darker pink on phloxin-B than any of the single mutants. Given the strong interaction of Δmyp2 with cdc11ts and the modest interaction with cdc14ts and cdc16ts, we conclude that myp2+ functions in normal growth conditions and contributes to septation.
Figure 9.
Genetic interactions observed with Δmyp2 strain and several temperature-sensitive mutant strains that affect cytokinesis. Cells of the indicated strains were streaked onto YES + phloxin-B plates at the indicated temperatures and then scanned in once the wild-type colonies have formed. In all cases, the double mutants were darker pink than the single mutants indicating that the double mutants are sicker.
DISCUSSION
We have identified and characterized a second myosin-II from fission yeast, myp2+. Phylogenetic analysis is useful to categorize myosins into subfamilies based on the sequences of the catalytic domain (Mooseker and Cheney, 1995). Having no assay for myp2+ function, we used phylogenetic analysis to determine its subfamily. Myp2p groups with the yeast myosin-IIs which join the main branch of myosin-IIs near the node of the amoebae myosin-IIs. We are confident that Myp2p is a type II myosin. The catalytic domain contains a well-conserved ATP-binding motif, an actin-binding motif, and like most myosin-IIs two IQ motifs that mediate light chain binding. The first IQ motif is relatively well-conserved except that it does not contain the first isoleucine and the second glutamine residues. Like most myosin-IIs, the second IQ motif of Myp2p is degenerate. The second IQ motif, however, does contain the invariant arginine, involved in light chain binding, in the middle of the motif (IQXXRGXXXR), which Myo2p, another S. pombe myosin-II, does not. Further biochemical and genetic analyses are required to determine the light chain content for both S. pombe myosin-IIs.
Although the catalytic domain places Myp2p as a myosin-II, the Myp2p tail differs from most other myosin-IIs, having a long sequence containing 17 prolines that divides two regions predicted to form coiled-coils. We used the most stringent conditions for the Coils prediction algorithm by choosing a window of 28 amino acids to ascertain the probability of forming a coiled coil (Lupas et al., 1991). The Paircoil algorithm (Berger et al., 1995) predicted a similar coiled-coil profile. S. cerevisiae myosin-II, MYO1p, has a nonhelical gap containing 6 prolines in a similar position in the tail. MYO1, like myp2+, is not essential and the absence of MYO1 leads to long chains of budded cells (Watts et al., 1987). S. pombe Myo2p contains 9 prolines and several of these are restricted to an analogous region as MYO1p and Myp2p. However, the Myo2p tail is shorter and does not appear to contain the second coiled-coil-rod domain. Biochemical studies of the tails from these myosins will be necessary to determine the coiled-coil content of each and whether the differences in the tails may have some relevance to the function of each myosin.
Insight as to the functions of these type II myosins, myp2+ and myo2+, in S. pombe can be obtained from studying the phenotypes of strains disrupted for each myosin. Disruption of myp2+ is conditional, whereas disruption of myo2+ is lethal. Δmyp2 cells exhibit a phenotype including multiseptated cells, which is consistent with a decreased efficiency of cytokinesis under nutrient limiting conditions (EMM or ME). If the Δmyp2 cells are given an additional stress by adding 1 M KCl to the media, then in addition to the multiseptated phenotype, the growth rate is impaired, and at 17°C the Δmyp2 cells die multiseptated. This suggests that myp2+ is activated under conditions of stress. In response to high salt, several MAP kinases are activated in S. pombe (Shiozaki and Russell, 1995; Millar et al., 1995; Degols et al., 1996; Kato et al., 1996; Zaitsevskaya-Carter and Cooper, 1997). The downstream targets of these kinases are not known. Interestingly, the phenotype of the disruption of one of these kinases, spm1+, is similar to Δmyp2: Δspm1 cells are viable and wild-type in appearance in rich media but suffer morphological defects similar to the Δmyp2 phenotype on minimal media. Similarly, this phenotype is enhanced under high-salt conditions (Zaitsevskaya-Carter and Cooper, 1997). One intriguing possibility is that myp2+ activity under these conditions may be affected by the spm1+ pathway.
Low level episomal expression of GFP-Myp2p complements the disruption phenotype of Δmyp2. In late mitotic (binucleate) cells, GFP-Myp2p localizes to a ring at the center of the cell, similar to the localization of the actin ring and GFP-myo2 during cytokinesis. In interphase cells, GFP-Myp2p was apparent as a dot near the nucleus, much like GFP-myo2 (Kitayama et al., 1997).
Overexpression of GFP-Myp2p causes a defect in cytokinesis that differs clearly from the defect in Δmyp2 cells. Understanding the mechanism of the defect caused by overexpression will require more work, but one possibility is that the excess Myp2p may compete with Myo2p for a limiting pool of shared light chains. Consistent with this, overexpression of Myo2p causes a very similar phenotype as overexpression of Myp2p (Kitayama et al., 1997).
Regulation of cytokinesis in S. pombe requires the product of the cdc15+ gene, which is thought to activate cdc16+ (Marks et al., 1992). The cdc16ts mutant has a unique phenotype, in which cells arrest as binucleates with multiple septa. This suggests that cdc16+ is involved in coupling the nuclear cycle to septation (Fankhauser et al., 1993). Mutants defective in the early stage of septum formation, cdc7ts and cdc14ts, are synthetically lethal with each other and with cdc16ts. cdc7+ encodes a protein kinase and cdc14+ encodes a novel protein (Fankhauser and Simanis, 1994a,b). Several alleles of cdc11ts [encoding another novel protein (Fankhauser and Simanis, 1994b)] are also synthetically lethal with cdc16ts. It has been suggested based on these observations that these gene products interact with one another to initiate the formation of the septum and to couple this process to the cell cycle (Marks et al., 1992). Septum formation occurs after the assembly of an actin ring. Activation of a myosin motor may provide the force of contraction. Cdc4p, an apparent myosin light chain, associates with the actin ring, as does Myp2p and Myo2p (Kitayama et al., 1997; McCollum et al., 1995).
The localization of Myp2p during late mitosis, together with the genetic interactions of Δmyp2 with cdc11ts and to a lesser extent cdc14ts and cdc16ts, suggests that Myp2p acts during normal cytokinesis, as well as under conditions of stress. Interestingly, Δmyp2 does not interact with any of the late septation mutants (cdc3ts, cdc4ts, and cdc8ts) that affect actin ring formation. Possibly, the interactions observed with the early septation mutant cdc11ts may provide a link between the actin-mediated events at cytokinesis and initiation of septation, since Myp2p localizes to the same area as actin in late mitotic cells.
Why does fission yeast need two myosin-II genes? In both S. pombe and S. cerevisiae, there is precedent for multiple genes encoding proteins with overlapping function that may allow the cell to fine tune its response to different growth conditions. For example, in S. pombe there are two α-tubulin genes (Hiraoka et al., 1984; Toda et al., 1984). Both are expressed and present in the α-tubulin pool, although possibly because of levels of expression, only one is essential (Adachi et al., 1986). We note that Myo2p and Myp2p are not completely redundant, but they may overlap in function. Δmyo2 spores die with a multiseptate phenotype. This could reflect that a limiting amount of Myo2p is packaged from the parent diploid. Alternatively, it could reflect a limited ability of Myp2p to function in lieu of Myo2p during germination of the Δmyo2 spore. In contrast, deletion of myp2+ causes defects in cytokinesis that are specific to media and cell stress. Thus, Myo2p is not sufficient under these conditions for normal cell growth in the absence of Myp2p. Interestingly, the lethality of Δmyp2 cells at 17°C in 1 M KCl may reflect that the catalytic activity of Myo2p is highly sensitive to the growth temperature as is known for muscle myosin-IIs (White and Taylor, 1976). Perhaps these myosins are used selectively to regulate cytokinesis under different growth conditions. This could occur via differential expression, association with different light chains, or responding to unique signaling pathways in the cell. Further characterization of myp2+, including analysis of myp2+ and myo2+ double mutants, will help us to determine how the second myosin-II functions in fission yeast cytokinesis.
ACKNOWLEDGMENTS
This study is part of the thesis work for M.B., who is a graduate student in the Biochemistry, Cellular, and Molecular Biology training program at Johns Hopkins University. We are indebted to Sally G. Pasion for the gift of pSGP573 and helpful suggestions for microscopy. We thank Richard Cheney for expert advice regarding the phylogenetic analysis. We thank members of the Forsburg and Pollard laboratories who have made helpful suggestions about the manuscript. We are grateful to members of the Thomas Kelly laboratory for providing valuable advice on experimental technique and design. This work was supported by grant GM-26132 from the Natinal Institutes of Health to T.P. and by a National Science Foundation predoctoral fellowship to M.B.
Footnotes
Abbreviations used: GB, GenBank; PIR, Protein Identification Resource; SP, Swiss Prot; PCR, polymerase chain reaction; kb, kilobases; bp, base pair; 5′ RACE, 5′ rapid amplification of cDNA ends; DAPI, 4′-6-diamidino-2-phenylindole; ORF, open reading frame; ME, malt extract.
REFERENCES
- Adachi Y, Toda T, Niwa O, Yanagida M. Differential expressions of essential and nonessential alpha-tubulin genes in Schizosaccharomyces pombe. Mol Cell Biol. 1986;6:2168–2178. doi: 10.1128/mcb.6.6.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Apolinario E, Nocero M, Jin M, Hoffman CS. Cloning and manipulation of the Schizosaccharomyces pombe his7+ gene as a new selectable marker for molecular genetic studies. Curr Genet. 1993;24:491–495. doi: 10.1007/BF00351711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian MK, Helfman DM, Hemmingsen SM. A new tropomyosin essential for cytokinesis in the fission yeast S. pombe. Nature. 1992;360:84–87. doi: 10.1038/360084a0. [DOI] [PubMed] [Google Scholar]
- Balasubramanian MK, Hirani BR, Burke JD, Gould KL. The Schizosaccharomyces pombe cdc3+ gene encodes a profilin essential for cytokinesis. J Cell Biol. 1994;125:1289–1301. doi: 10.1083/jcb.125.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basi G, Schmid E, Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- Berger B, Wilson DB, Wolf E, Tonchev T, Milla M, Kim PS. Predicting coiled coils by use of pairwise residue correlations. Proc Natl Acad Sci USA. 1995;92:8259–8263. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Woollard A, Nurse P. Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J Cell Sci. 1996;109:131–142. doi: 10.1242/jcs.109.1.131. [DOI] [PubMed] [Google Scholar]
- Degols G, Shiozaki K, Russell P. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:2870–2817. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Marks J, Reymond A, Simanis V. The S. pombe cdc16 gene is required both for maintenance of p34cdc2 kinase activity and regulation of septum formation: a link between mitosis and cytokinesis? EMBO J. 1993;12:2697–2704. doi: 10.1002/j.1460-2075.1993.tb05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Simanis V. The cdc7 protein kinase is a dosage dependent regulator of septum formation in fission yeast. EMBO J. 1994a;13:3011–3019. doi: 10.1002/j.1460-2075.1994.tb06600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Simanis V. Cold fission: splitting the pombe cell at room temperature. Trends Cell Biol. 1994b;4:96–101. doi: 10.1016/0962-8924(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Fishkind DJ, Wang YL. New horizons for cytokinesis. Curr Opin Cell Biol. 1995;7:23–31. doi: 10.1016/0955-0674(95)80041-7. [DOI] [PubMed] [Google Scholar]
- Forsburg SL. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993;21:2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman MA, Dush MK, Martin GR. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayles J, Nurse P. A review of mitosis in the fission yeast Schizosaccharomyces pombe. Exp Cell Res. 1989;184:273–286. doi: 10.1016/0014-4827(89)90327-3. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y, Toda T, Yanagida M. The NDA3 gene of fission yeast encodes beta-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984;39:349–358. doi: 10.1016/0092-8674(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Kato T, Jr, Okazaki K, Murakami H, Stettler S, Fantes PA, Okayama H. Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 1996;378:207–212. doi: 10.1016/0014-5793(95)01442-x. [DOI] [PubMed] [Google Scholar]
- Kitayama C, Sugimoto A, Yamamoto M. Type II myosin heavy chain encoded by the myo2 gene composes the contractile ring during cytokinesis in Schizosaccharomyces pombe. J Cell Biol. 1997;137:1309–1319. doi: 10.1083/jcb.137.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S, Slayter HS, Weeds AG, Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969;42:1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Marks J, Fankhauser C, Simanis V. Genetic interactions in the control of septation in Schizosaccharomyces pombe. J Cell Sci. 1992;101:801–808. doi: 10.1242/jcs.101.4.801. [DOI] [PubMed] [Google Scholar]
- Marks J, Hyams JS. Localization of F-actin through the cell division cycle of Schizosaccharomyces pombe. Eur J Cell Biol. 1985;39:27–32. [Google Scholar]
- McCollum D, Balasubramanian MK, Pelcher LE, Hemmingsen SM, Gould KL. Schizosaccharomyces pombe cdc4+ gene encodes a novel EF-hand protein essential for cytokinesis. J Cell Biol. 1995;130:651–660. doi: 10.1083/jcb.130.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JB, Buck V, Wilkinson MG. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Mooseker MS, Cheney RE. Unconventional myosins. Annu Rev Cell Dev Biol. 1995;11:633–675. doi: 10.1146/annurev.cb.11.110195.003221. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Okazaki N, Kume K, Jinno S, Tanaka K, Okayama H. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 1990;18:6485–6489. doi: 10.1093/nar/18.22.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterwhite LL, Pollard TD. Cytokinesis. Curr Opin Cell Biol. 1992;4:43–52. doi: 10.1016/0955-0674(92)90057-j. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Adachi Y, Hiraoka Y, Yanagida M. Identification of the pleiotropic cell division cycle gene NDA2 as one of two different α-tubulin genes in Schizosaccharomyces pombe. Cell. 1984;37:233–242. doi: 10.1016/0092-8674(84)90319-2. [DOI] [PubMed] [Google Scholar]
- Watts FZ, Shiels G, Orr E. The yeast MYO1 gene encoding a myosin-like protein required for cell division. EMBO J. 1987;6:3499–3505. doi: 10.1002/j.1460-2075.1987.tb02675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HD, Taylor EW. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976;15:5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]
- Zaitsevskaya-Carter T, Cooper JA. Spm1, a stress-activated MAP kinase that regulates morphogenesis in S. pombe. EMBO J. 1997;16:1318–1331. doi: 10.1093/emboj/16.6.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]