Abstract
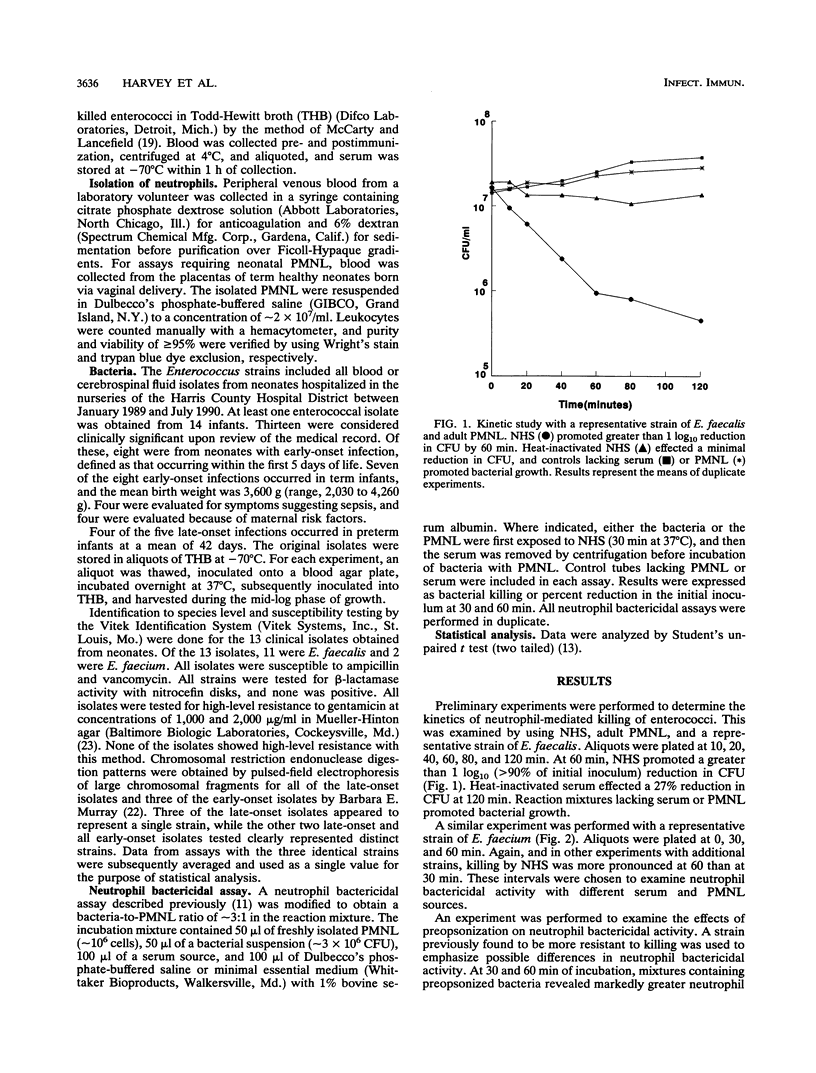
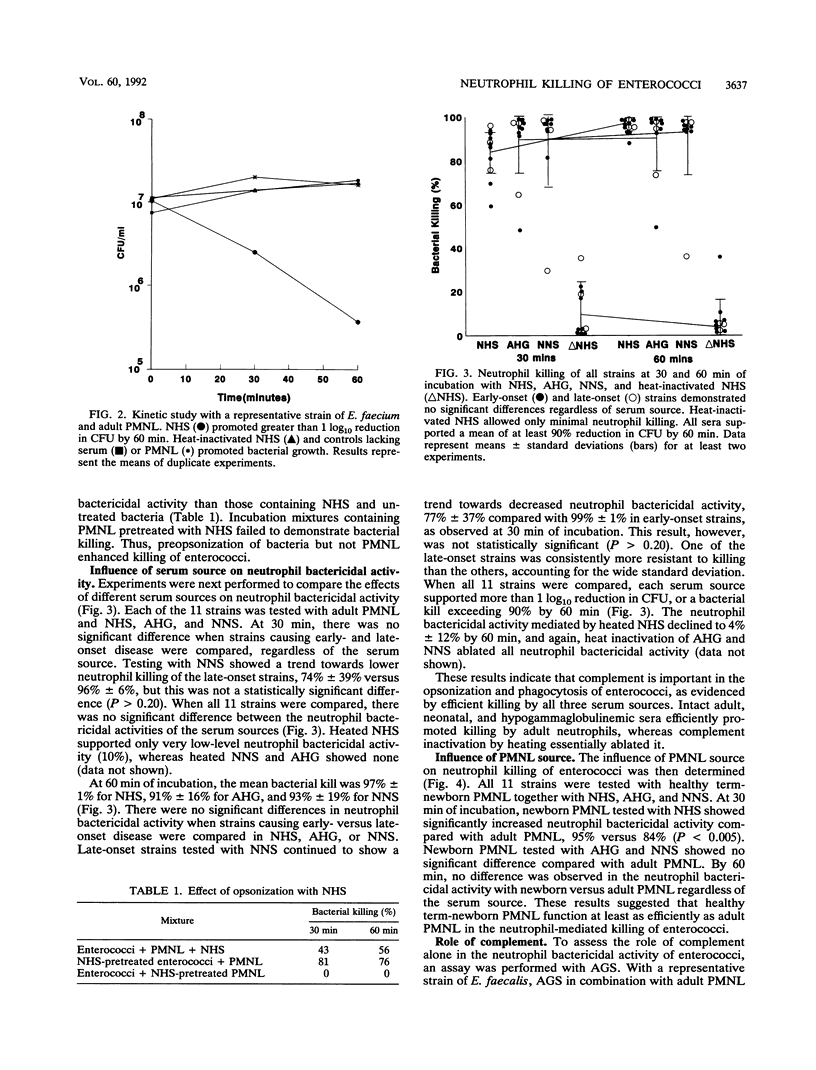
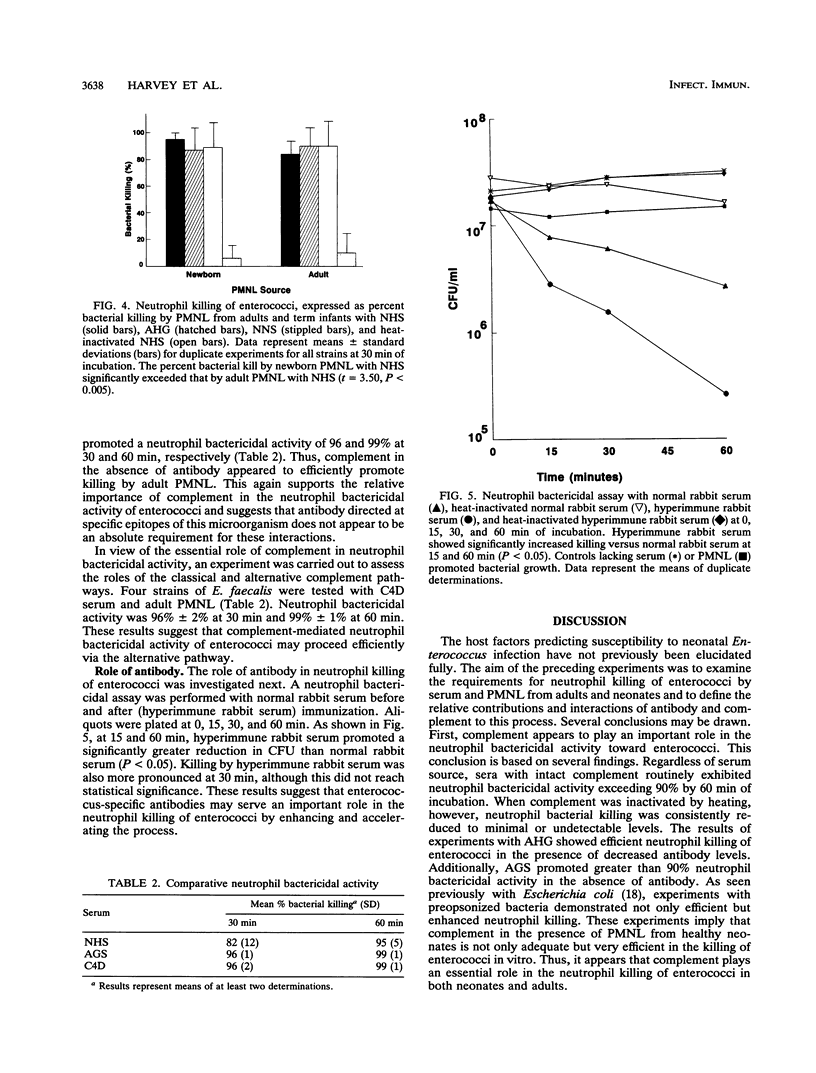
Enterococci have become a frequent causative agent in neonatal sepsis. The relative contributions of antibody and complement and their interactions in the neutrophil-mediated bacterial killing of 11 Enterococcus strains from neonates were investigated. Polymorphonuclear leukocytes (PMNL) from adult and term newborn infants were tested with normal human serum, adult hypogammaglobulinemic serum, and normal newborn serum in a neutrophil bactericidal assay. Neutrophil bactericidal activity for enterococci was not influenced by the serum source but was essentially ablated after heat inactivation of complement in all sera. No differences were observed in the killing capacity of healthy newborn versus adult PMNL regardless of serum source. Representative Enterococcus strains were then tested with agammaglobulinemic serum or C4-deficient serum, resulting in neutrophil bactericidal activities consistently exceeding 90%. A neutrophil bactericidal assay performed with normal rabbit serum and hyperimmune rabbit serum against enterococci showed that antibodies to enterococci enhanced neutrophil-mediated killing of this organism. Thus, neutrophil killing of enterococci appears to be mediated primarily by complement, with antibody playing a less essential but potentially important role. PMNL from adult and healthy term infants functioned with equal efficiency in the neutrophil killing of enterococci.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akriotis V., Biggar W. D. The effects of hypothermia on neutrophil function in vitro. J Leukoc Biol. 1985 Jan;37(1):51–61. doi: 10.1002/jlb.37.1.51. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Hughes B. J., Smith C. W. Abnormal mobility of neonatal polymorphonuclear leukocytes. Relationship to impaired redistribution of surface adhesion sites by chemotactic factor or colchicine. J Clin Invest. 1981 Oct;68(4):863–874. doi: 10.1172/JCI110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Pickering L. K., Feigin R. D. Leukocyte function in normal and infected neonates. J Pediatr. 1974 Sep;85(3):420–425. doi: 10.1016/s0022-3476(74)80134-4. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Edwards M. S., Webb B. J., Kasper D. L. Antibody-independent classical pathway-mediated opsonophagocytosis of type Ia, group B streptococcus. J Clin Invest. 1982 Feb;69(2):394–404. doi: 10.1172/JCI110463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Webb B. J., Kasper D. L., Edwards M. S. The role of complement and antibody in opsonophagocytosis of type II group B streptococci. J Infect Dis. 1986 Jul;154(1):47–54. doi: 10.1093/infdis/154.1.47. [DOI] [PubMed] [Google Scholar]
- Buchino J. J., Ciambarella E., Light I. Systemic group D streptococcal infection in newborn infants. Am J Dis Child. 1979 Mar;133(3):270–273. doi: 10.1001/archpedi.1979.02130030046007. [DOI] [PubMed] [Google Scholar]
- Chirico G., Marconi M., De Amici M., Gasparoni A., Mingrat G., Chiara A., Rondini G., Ugazio A. G. Deficiency of neutrophil bactericidal activity in term and preterm infants. A longitudinal study. Biol Neonate. 1985;47(3):125–129. doi: 10.1159/000242102. [DOI] [PubMed] [Google Scholar]
- Coudron P. E., Mayhall C. G., Facklam R. R., Spadora A. C., Lamb V. A., Lybrand M. R., Dalton H. P. Streptococcus faecium outbreak in a neonatal intensive care unit. J Clin Microbiol. 1984 Dec;20(6):1044–1048. doi: 10.1128/jcm.20.6.1044-1048.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson S. R., Baker C. J. Enterococcal sepsis in neonates: features by age at onset and occurrence of focal infection. Pediatrics. 1990 Feb;85(2):165–171. [PubMed] [Google Scholar]
- Edwards M. S., Baker C. J., Kasper D. L. Opsonic specificity of human antibody to the type III polysaccharide of group B Streptococcus. J Infect Dis. 1979 Dec;140(6):1004–1008. doi: 10.1093/infdis/140.6.1004. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Altenburger K. M., Atkinson A. W., Jr, Curry R. H. Complement in the newborn infant. Pediatrics. 1979 Nov;64(5 Pt 2 Suppl):781–786. [PubMed] [Google Scholar]
- Krause P. J., Herson V. C., Boutin-Lebowitz J., Eisenfeld L., Block C., LoBello T., Maderazo E. G. Polymorphonuclear leukocyte adherence and chemotaxis in stressed and healthy neonates. Pediatr Res. 1986 Apr;20(4):296–300. doi: 10.1203/00006450-198604000-00004. [DOI] [PubMed] [Google Scholar]
- Krause P. J., Maderazo E. G., Scroggs M. Abnormalities of neutrophil adherence in newborns. Pediatrics. 1982 Feb;69(2):184–187. [PubMed] [Google Scholar]
- Luginbuhl L. M., Rotbart H. A., Facklam R. R., Roe M. H., Elliot J. A. Neonatal enterococcal sepsis: case-control study and description of an outbreak. Pediatr Infect Dis J. 1987 Nov;6(11):1022–1026. [PubMed] [Google Scholar]
- McCARTY M., LANCEFIELD R. C. Variation in the group-specific carbohydrate of group A streptococci. I. Immunochemical studies on the carbohydrates of variant strains. J Exp Med. 1955 Jul 1;102(1):11–28. doi: 10.1084/jem.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Shinefield H. R. Changes in the pattern of neonatal septicemia and meningitis. Am J Dis Child. 1966 Jul;112(1):33–39. doi: 10.1001/archpedi.1966.02090100069006. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990 Sep;28(9):2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E. The life and times of the Enterococcus. Clin Microbiol Rev. 1990 Jan;3(1):46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Torres C. Effects of medium and inoculum variations on screening for high-level aminoglycoside resistance in Enterococcus faecalis. J Clin Microbiol. 1988 Feb;26(2):250–256. doi: 10.1128/jcm.26.2.250-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vel W. A., Namavar F., Verweij A. M., Pubben A. N., MacLaren D. M. Killing capacity of human polymorphonuclear leukocytes in aerobic and anaerobic conditions. J Med Microbiol. 1984 Oct;18(2):173–180. doi: 10.1099/00222615-18-2-173. [DOI] [PubMed] [Google Scholar]


