Abstract
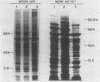
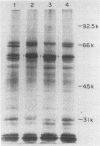
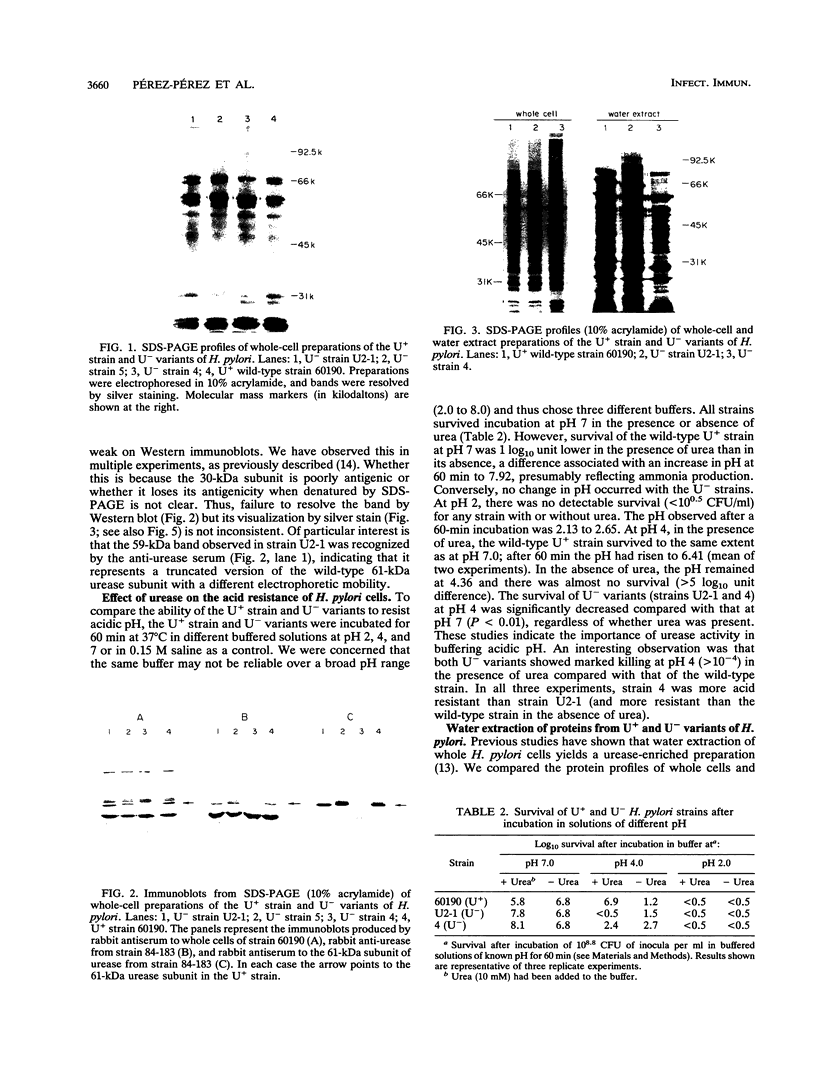
The urease of Helicobacter pylori is suspected to play a role in the pathogenesis of gastritis. Although all clinical isolates of H. pylori are urease positive (U+), we have selected and characterized several spontaneously arising urease-negative (U-) variants from wild-type strain 60190. Urease-negative variants were identified by growth in medium containing 60 mM urea and arose at a frequency of 10(-5) to 10(-6). The urease activity of the wild-type strain inhibited growth of this strain in the presence of 60 mM urea. U- variants retained the U- phenotype for more than 100 passages on medium with or without urea. The urease activities of the original U+ and derived U- cells were 9.55 to 16.7 and 0.01 to 0.17 U/mg of protein, respectively. Colonial growth and other biochemical characteristics were identical for the strains. U- variants showed three classes of whole-cell sodium dodecyl sulfate-polyacrylamide gel electrophoresis profiles: (i) identical to U+; (ii) change in the migration of the 61-kDa urease subunit; and (iii) lack of 61- and 30-kDa subunits. These differences were confirmed by immunoblotting and by protein separation using fast protein liquid chromatography. The U+ strain but not U- variants tolerated exposure to pH 4.0 for 60 min in the presence of urea. Supernatants of the U+ strain and U- variants contained vacuolating cytotoxin activity for HeLa cells in similar titers. By enzyme-linked immunosorbent assay, human serum samples recognized water extract from the U+ strain significantly better than extract from a U- variant lacking urease subunits. In conclusion, this study demonstrates that U- H. pylori variants may arise spontaneously, that urease activity enhances survival at acid pH, and that urease and cytotoxin activities are disparate phenotypes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Blaser M. J. Gastric Campylobacter-like organisms, gastritis, and peptic ulcer disease. Gastroenterology. 1987 Aug;93(2):371–383. doi: 10.1016/0016-5085(87)91028-6. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990 Apr;161(4):626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Berka R. M., Vasil M. L., Wang W. L. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect Immun. 1983 Oct;42(1):276–284. doi: 10.1128/iai.42.1.276-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Blaser M. J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992 May 25;267(15):10570–10575. [PubMed] [Google Scholar]
- Cover T. L., Dooley C. P., Blaser M. J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990 Mar;58(3):603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Puryear W., Perez-Perez G. I., Blaser M. J. Effect of urease on HeLa cell vacuolation induced by Helicobacter pylori cytotoxin. Infect Immun. 1991 Apr;59(4):1264–1270. doi: 10.1128/iai.59.4.1264-1270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent J. C., McNulty C. A., Uff J. S., Gear M. W., Wilkinson S. P. Campylobacter pylori urease: a new serological test. Lancet. 1988 Apr 30;1(8592):1002–1002. doi: 10.1016/s0140-6736(88)91827-2. [DOI] [PubMed] [Google Scholar]
- Dooley C. P., Cohen H., Fitzgibbons P. L., Bauer M., Appleman M. D., Perez-Perez G. I., Blaser M. J. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989 Dec 7;321(23):1562–1566. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- Drumm B., Perez-Perez G. I., Blaser M. J., Sherman P. M. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med. 1990 Feb 8;322(6):359–363. doi: 10.1056/NEJM199002083220603. [DOI] [PubMed] [Google Scholar]
- Dunn B. E., Campbell G. P., Perez-Perez G. I., Blaser M. J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990 Jun 5;265(16):9464–9469. [PubMed] [Google Scholar]
- Dunn B. E., Perez-Perez G. I., Blaser M. J. Two-dimensional gel electrophoresis and immunoblotting of Campylobacter pylori proteins. Infect Immun. 1989 Jun;57(6):1825–1833. doi: 10.1128/iai.57.6.1825-1833.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton K. A., Brooks C. L., Morgan D. R., Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991 Jul;59(7):2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Graham D. Y., Klein P. D. A sensitive and specific serologic test for detection of Campylobacter pylori infection. Gastroenterology. 1989 Apr;96(4):1004–1008. doi: 10.1016/0016-5085(89)91616-8. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Kirkpatrick S. S., Graham D. Y. Characterization of the Helicobacter pylori urease and purification of its subunits. Microb Pathog. 1991 Jan;10(1):15–26. doi: 10.1016/0882-4010(91)90062-f. [DOI] [PubMed] [Google Scholar]
- Forman D., Newell D. G., Fullerton F., Yarnell J. W., Stacey A. R., Wald N., Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991 Jun 1;302(6788):1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., Alm R. A., Power M. E., Logan S. M., Trust T. J. Role of two flagellin genes in Campylobacter motility. J Bacteriol. 1991 Aug;173(15):4757–4764. doi: 10.1128/jb.173.15.4757-4764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawtin P. R., Delves H. T., Newell D. G. The demonstration of nickel in the urease of Helicobacter pylori by atomic absorption spectroscopy. FEMS Microbiol Lett. 1991 Jan 1;61(1):51–54. doi: 10.1016/0378-1097(91)90012-y. [DOI] [PubMed] [Google Scholar]
- Mai U. E., Perez-Perez G. I., Allen J. B., Wahl S. M., Blaser M. J., Smith P. D. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992 Feb 1;175(2):517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai U. E., Perez-Perez G. I., Wahl L. M., Wahl S. M., Blaser M. J., Smith P. D. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J Clin Invest. 1991 Mar;87(3):894–900. doi: 10.1172/JCI115095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. J., Barrett L. J., Prakash C., McCallum R. W., Guerrant R. L. Urea protects Helicobacter (Campylobacter) pylori from the bactericidal effect of acid. Gastroenterology. 1990 Sep;99(3):697–702. doi: 10.1016/0016-5085(90)90957-3. [DOI] [PubMed] [Google Scholar]
- Marshall B. J. Campylobacter pyloridis and gastritis. J Infect Dis. 1986 Apr;153(4):650–657. doi: 10.1093/infdis/153.4.650. [DOI] [PubMed] [Google Scholar]
- Megraud F., Bonnet F., Garnier M., Lamouliatte H. Characterization of "Campylobacter pyloridis" by culture, enzymatic profile, and protein content. J Clin Microbiol. 1985 Dec;22(6):1007–1010. doi: 10.1128/jcm.22.6.1007-1010.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Hausinger R. P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989 Mar;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neithercut W. D., Greig M. A., Hossack M., McColl K. E. Suicidal destruction of Helicobacter pylori: metabolic consequence of intracellular accumulation of ammonia. J Clin Pathol. 1991 May;44(5):380–384. doi: 10.1136/jcp.44.5.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pei Z., Ellison R. T., 3rd, Lewis R. V., Blaser M. J. Purification and characterization of a family of high molecular weight surface-array proteins from Campylobacter fetus. J Biol Chem. 1988 May 5;263(13):6416–6420. [PubMed] [Google Scholar]
- Perez-Perez G. I., Dworkin B. M., Chodos J. E., Blaser M. J. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988 Jul 1;109(1):11–17. doi: 10.7326/0003-4819-109-1-11. [DOI] [PubMed] [Google Scholar]
- Perez G. I., Hopkins J. A., Blaser M. J. Antigenic heterogeneity of lipopolysaccharides from Campylobacter jejuni and Campylobacter fetus. Infect Immun. 1985 May;48(2):528–533. doi: 10.1128/iai.48.2.528-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E. D., Shon J., Tompkins L. S. Characterization of Helicobacter pylori urease mutants. Infect Immun. 1992 May;60(5):1883–1889. doi: 10.1128/iai.60.5.1883-1889.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Xu J. K., Goodwin C. S., Cooper M., Robinson J. Intracellular vacuolization caused by the urease of Helicobacter pylori. J Infect Dis. 1990 Jun;161(6):1302–1304. doi: 10.1093/infdis/161.6.1302. [DOI] [PubMed] [Google Scholar]