Abstract
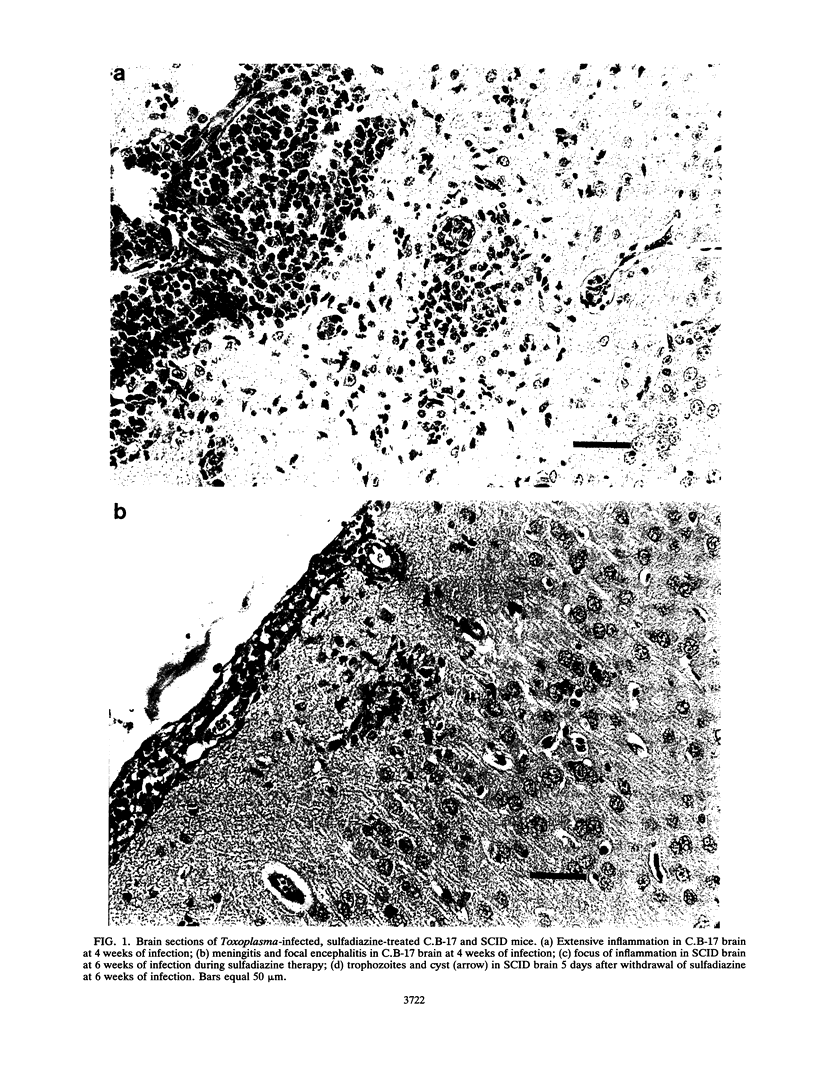
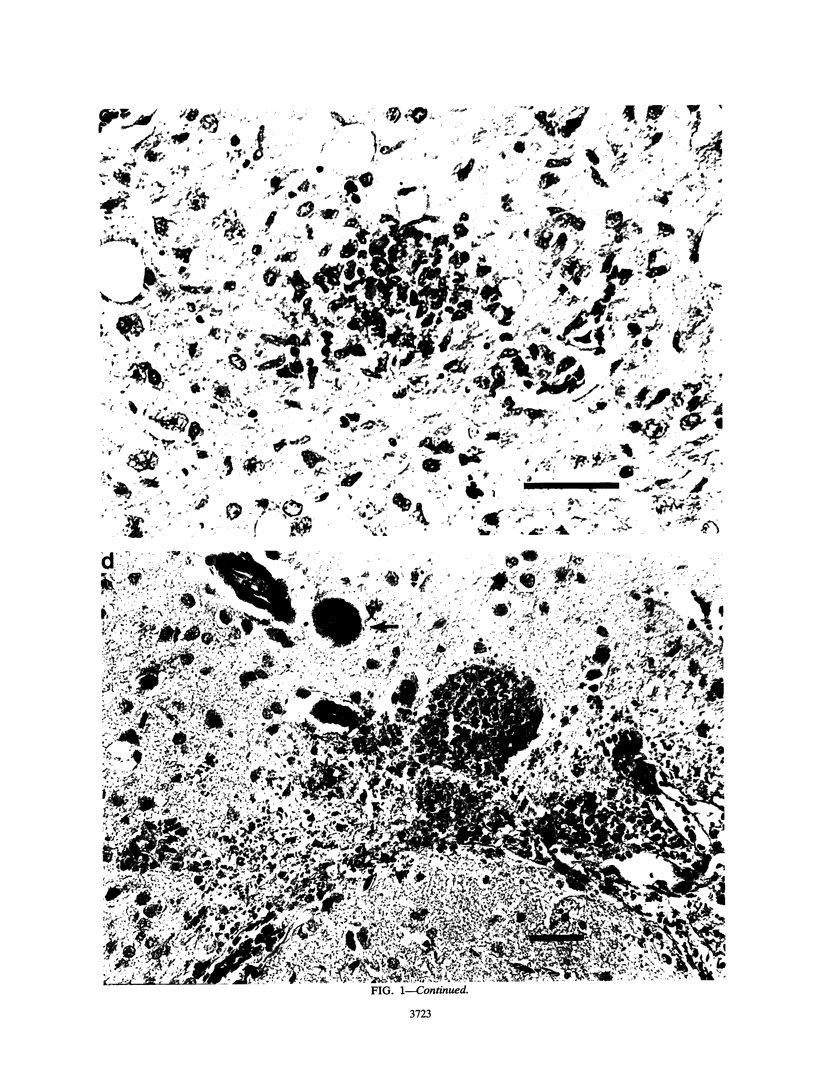
Lymphodeficient scid/scid (SCID) mice died from acute infection with a strain of Toxoplasma gondii that causes chronic infection with mild symptoms in immunocompetent non-SCID mice. However, most SCID mice reconstituted with spleen cells from immunocompetent mice 1 month prior to T. gondii infection survived in good health after a transient period during which they appeared ill. Unreconstituted SCID mice given sulfadiazine in their drinking water from day 10 of Toxoplasma infection onward survived the acute phase of infection and lived for many weeks without overt symptoms. Histological examination revealed Toxoplasma cysts in their brains. However, if sulfadiazine was withdrawn from the drinking water of these chronically infected SCID mice, the mice died within 1 week with large numbers of trophozoites throughout their brains. These findings establish SCID mice as a potentially useful resource with which to study various aspects of immunological control of T. gondii infection during either its acute or chronic phase. Furthermore, the ability to produce chronic infections with avirulent T. gondii in SCID mice and to cause acute relapsing infections at will suggests that SCID mice may be helpful in evaluating potential therapies for acute and chronic T. gondii infections in immunocompromised patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G. Depletion of L3T4+ (CD4+) T lymphocytes prevents development of resistance to Toxoplasma gondii in mice. Infect Immun. 1991 May;59(5):1614–1619. doi: 10.1128/iai.59.5.1614-1619.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J., Bosma M. J., Bosma G. C., Unanue E. R. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell-independent mechanism. J Immunol. 1986 Jul 1;137(1):4–9. [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Conley F. K., Jenkins K. A. Immunohistological study of the anatomic relationship of toxoplasma antigens to the inflammatory response in the brains of mice chronically infected with Toxoplasma gondii. Infect Immun. 1981 Mar;31(3):1184–1192. doi: 10.1128/iai.31.3.1184-1192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouin F., Garin Y. J. Toxoplasma gondii: blood and tissue kinetics during acute and chronic infections in mice. Exp Parasitol. 1991 Nov;73(4):460–468. doi: 10.1016/0014-4894(91)90070-d. [DOI] [PubMed] [Google Scholar]
- Ferguson D. J., Graham D. I., Hutchison W. M. Pathological changes in the brains of mice infected with Toxoplasma gondii: a histological, immunocytochemical and ultrastructural study. Int J Exp Pathol. 1991 Aug;72(4):463–474. [PMC free article] [PubMed] [Google Scholar]
- Ferguson D. J., Hutchison W. M. An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol Res. 1987;73(6):483–491. doi: 10.1007/BF00535321. [DOI] [PubMed] [Google Scholar]
- Harmsen A. G., Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med. 1990 Sep 1;172(3):937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofflin J. M., Conley F. K., Remington J. S. Murine model of intracerebral toxoplasmosis. J Infect Dis. 1987 Mar;155(3):550–557. doi: 10.1093/infdis/155.3.550. [DOI] [PubMed] [Google Scholar]
- Israelski D. M., Araujo F. G., Conley F. K., Suzuki Y., Sharma S., Remington J. S. Treatment with anti-L3T4 (CD4) monoclonal antibody reduces the inflammatory response in toxoplasmic encephalitis. J Immunol. 1989 Feb 1;142(3):954–958. [PubMed] [Google Scholar]
- Johnson L. L. A protective role for endogenous tumor necrosis factor in Toxoplasma gondii infection. Infect Immun. 1992 May;60(5):1979–1983. doi: 10.1128/iai.60.5.1979-1983.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Bienz K. A., Erb P. In vitro cultivation of Toxoplasma gondii cysts in astrocytes in the presence of gamma interferon. Infect Immun. 1986 Jan;51(1):147–156. doi: 10.1128/iai.51.1.147-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittas S., Kittas C., Paizi-Biza P., Henry L. A histological and immunohistochemical study of the changes induced in the brains of white mice by infection with Toxoplasma gondii. Br J Exp Pathol. 1984 Feb;65(1):67–74. [PMC free article] [PubMed] [Google Scholar]
- Lindberg R. E., Frenkel J. K. Toxoplasmosis in nude mice. J Parasitol. 1977 Apr;63(2):219–221. [PubMed] [Google Scholar]
- McLeod R., Estes R. G., Mack D. G., Cohen H. Immune response of mice to ingested Toxoplasma gondii: a model of toxoplasma infection acquired by ingestion. J Infect Dis. 1984 Feb;149(2):234–244. doi: 10.1093/infdis/149.2.234. [DOI] [PubMed] [Google Scholar]
- Mills J. Pneumocystis carinii and Toxoplasma gondii infections in patients with AIDS. Rev Infect Dis. 1986 Nov-Dec;8(6):1001–1011. doi: 10.1093/clinids/8.6.1001. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Manabe T., Maekawa Y., Oka M., Himeno K. Role of L3T4+ and Lyt-2+ T cell subsets in protective immune responses of mice against infection with a low or high virulent strain of Toxoplasma gondii. Microbiol Immunol. 1991;35(3):215–222. doi: 10.1111/j.1348-0421.1991.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Parker S. J., Roberts C. W., Alexander J. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin Exp Immunol. 1991 May;84(2):207–212. doi: 10.1111/j.1365-2249.1991.tb08150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopenian D. C., Anderson P. S. Adoptive immunity in immune-deficient scid/scid mice. I. Differential requirements of naive and primed lymphocytes for CD4+ T cells during rejection of minor histocompatibility antigen-disparate skin grafts. Transplantation. 1988 Dec;46(6):899–904. [PubMed] [Google Scholar]
- Roths J. B., Marshall J. D., Allen R. D., Carlson G. A., Sidman C. L. Spontaneous Pneumocystis carinii pneumonia in immunodeficient mutant scid mice. Natural history and pathobiology. Am J Pathol. 1990 May;136(5):1173–1186. [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Schaefer M., Hurd M., Surh C. D., Ron Y. Mature murine B and T cells transferred to SCID mice can survive indefinitely and many maintain a virgin phenotype. J Exp Med. 1991 Sep 1;174(3):717–728. doi: 10.1084/jem.174.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl W., Matsubayashi H., Akao S. Modification of subclinical toxoplasmosis in mice by cortisone, 6-mercaptopurine and splenectomy. Am J Trop Med Hyg. 1966 Nov;15(6):869–874. doi: 10.4269/ajtmh.1966.15.869. [DOI] [PubMed] [Google Scholar]
- Strannegård O., Lycke E. Effect of antithymocyte serum on experimental toxoplasmosis in mice. Infect Immun. 1972 May;5(5):769–774. doi: 10.1128/iai.5.5.769-774.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer T. L., Waldor M. K., Steinman L., Conley F. K. Depletion of T-4+ lymphocytes with monoclonal antibody reactivates toxoplasmosis in the central nervous system: a model of superinfection in AIDS. J Immunol. 1987 Jun 1;138(11):3737–3741. [PubMed] [Google Scholar]