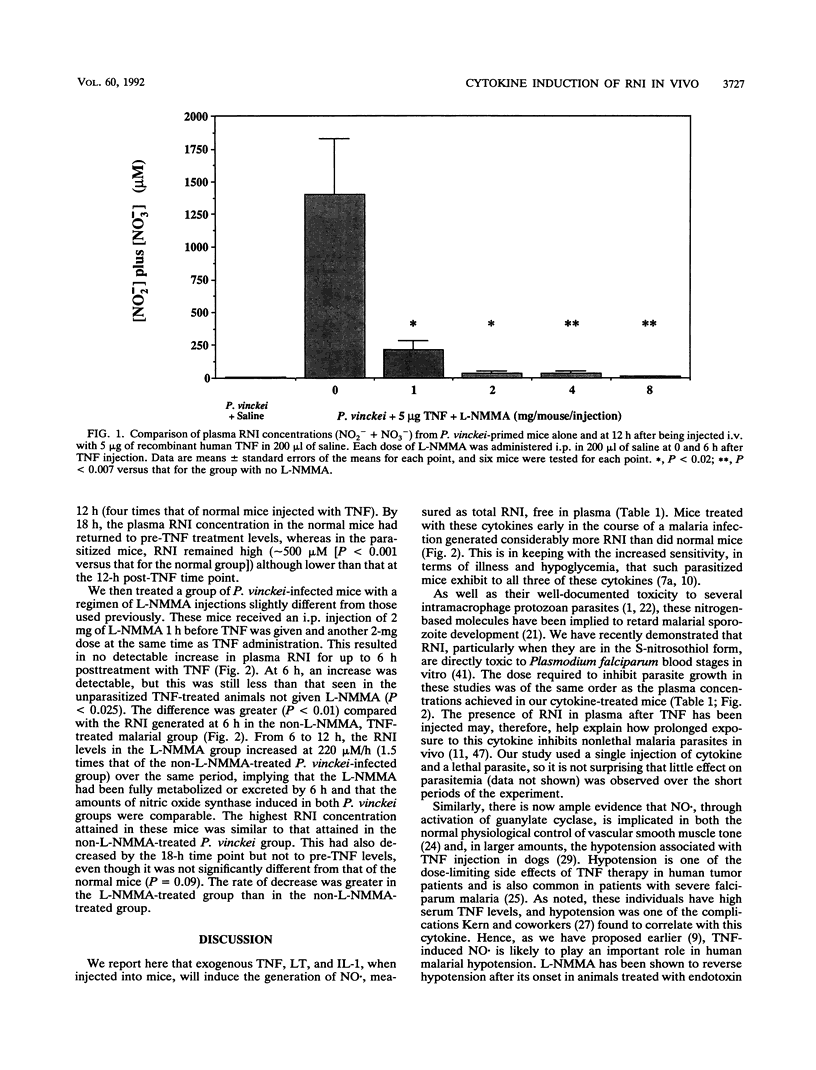
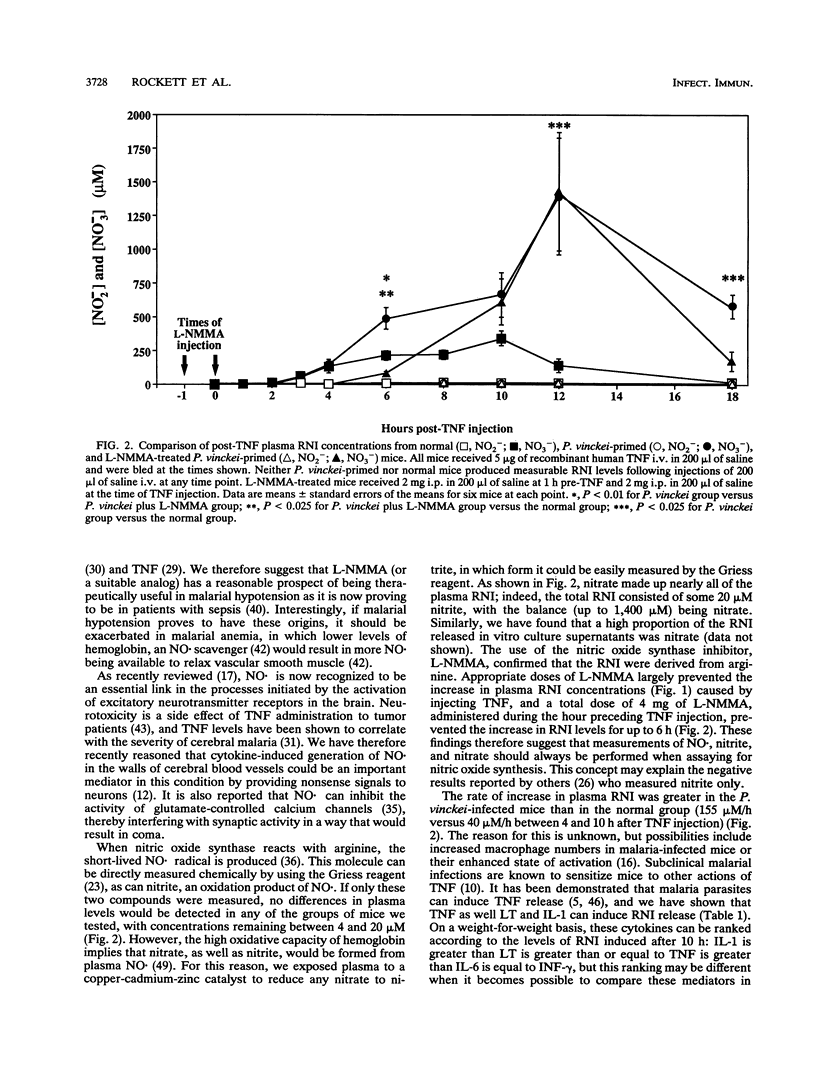
Abstract
Tumor necrosis factor and related cytokines are thought to be implicated in cell-mediated immunity and pathophysiology in malaria, but their mechanism of action has not been ascertained. Tumor necrosis factor has been reported to generate nitric oxide in vitro, so we have measured levels of this molecule and its products in the plasma of mice after they have received an injection of tumor necrosis factor, lymphotoxin, interleukin-1, gamma interferon, or interleukin-6, all of which have been reported to be increased in malaria. Total reactive nitrogen intermediate levels in plasma were assayed spectrophotometrically after exposing plasma to a copper-cadmium-zinc catalyst to convert nitrate to nitrite and then to Griess reagent. Tumor necrosis factor, lymphotoxin, and interleukin-1 all induced reactive nitrogen intermediates in vivo, with interleukin-1 showing the most activity. Tumor necrosis factor was then examined more closely. It induced more reactive nitrogen intermediates in malaria-infected mice than in normal mice, and appreciably more was in the form of nitrate than was in the form of nitrite. NG-methyl-L-arginine inhibited the in vivo generation of reactive nitrogen intermediates by tumor necrosis factor in a dose-dependent manner, implying that these molecules were arginine derived. These results are consistent with the possibility that tumor necrosis factor, lymphotoxin, and interleukin-1 may contribute to host pathology and parasite suppression through generation of nitric oxide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Aggarwal B. B., Henzel W. J., Moffat B., Kohr W. J., Harkins R. N. Primary structure of human lymphotoxin derived from 1788 lymphoblastoid cell line. J Biol Chem. 1985 Feb 25;260(4):2334–2344. [PubMed] [Google Scholar]
- Aggarwal B. B., Moffat B., Harkins R. N. Human lymphotoxin. Production by a lymphoblastoid cell line, purification, and initial characterization. J Biol Chem. 1984 Jan 10;259(1):686–691. [PubMed] [Google Scholar]
- Amezcua J. L., Palmer R. M., de Souza B. M., Moncada S. Nitric oxide synthesized from L-arginine regulates vascular tone in the coronary circulation of the rabbit. Br J Pharmacol. 1989 Aug;97(4):1119–1124. doi: 10.1111/j.1476-5381.1989.tb12569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Malarial parasites induce TNF production by macrophages. Immunology. 1988 Jun;64(2):227–231. [PMC free article] [PubMed] [Google Scholar]
- Butcher G. A., Garland T., Ajdukiewicz A. B., Clark I. A. Serum tumor necrosis factor associated with malaria in patients in the Solomon Islands. Trans R Soc Trop Med Hyg. 1990 Sep-Oct;84(5):658–661. doi: 10.1016/0035-9203(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Chaudhri G. Tumour necrosis factor may contribute to the anaemia of malaria by causing dyserythropoiesis and erythrophagocytosis. Br J Haematol. 1988 Sep;70(1):99–103. doi: 10.1111/j.1365-2141.1988.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A., Hunt N. H. Possible roles of tumor necrosis factor in the pathology of malaria. Am J Pathol. 1987 Oct;129(1):192–199. [PMC free article] [PubMed] [Google Scholar]
- Clark I. A. Does endotoxin cause both the disease and parasite death in acute malaria and babesiosis? Lancet. 1978 Jul 8;2(8080):75–77. doi: 10.1016/s0140-6736(78)91386-7. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H., Butcher G. A., Cowden W. B. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987 Nov 15;139(10):3493–3496. [PubMed] [Google Scholar]
- Clark I. A., Rockett K. A., Cowden W. B. Proposed link between cytokines, nitric oxide and human cerebral malaria. Parasitol Today. 1991 Aug;7(8):205–207. doi: 10.1016/0169-4758(91)90142-b. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Dockrell H. M., Alavi A., Playfair J. H. Changes in oxidative burst capacity during murine malaria and the effect of vaccination. Clin Exp Immunol. 1986 Oct;66(1):37–43. [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Aggarwal B. B., Benton C. V., Bringman T. S., Henzel W. J., Jarrett J. A., Leung D. W., Moffat B., Ng P., Svedersky L. P. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature. 1984 Dec 20;312(5996):721–724. doi: 10.1038/312721a0. [DOI] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Green S. J., Mellouk S., Hoffman S. L., Meltzer M. S., Nacy C. A. Cellular mechanisms of nonspecific immunity to intracellular infection: cytokine-induced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes. Immunol Lett. 1990 Aug;25(1-3):15–19. doi: 10.1016/0165-2478(90)90083-3. [DOI] [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Ignarro L. J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989 Jan;3(1):31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., Erb P., Aebischer T., De Libero G., Balzer M., Groscurth P., Keller H. U. Expression of cellular effector functions and production of reactive nitrogen intermediates: a comparative study including T lymphocytes, T-like cells, neutrophil granulocytes, and mononuclear phagocytes. Cell Immunol. 1990 Dec;131(2):398–403. doi: 10.1016/0008-8749(90)90264-r. [DOI] [PubMed] [Google Scholar]
- Kern P., Hemmer C. J., Van Damme J., Gruss H. J., Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989 Aug;87(2):139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Belloni P. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J Natl Cancer Inst. 1990 May 2;82(9):772–776. doi: 10.1093/jnci/82.9.772. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Jubran A., Gross S. S., Griffith O. W., Levi R., Adams J., Lodato R. F. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D., Hill A. V., Sambou I., Twumasi P., Castracane J., Manogue K. R., Cerami A., Brewster D. R., Greenwood B. M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990 Nov 17;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Millott S. Tumour necrosis factor (TNF-alpha) in leishmaniasis. II. TNF-alpha-induced macrophage leishmanicidal activity is mediated by nitric oxide from L-arginine. Immunology. 1990 Dec;71(4):556–559. [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- Liew F. Y., Parkinson C., Millott S., Severn A., Carrier M. Tumour necrosis factor (TNF alpha) in leishmaniasis. I. TNF alpha mediates host protection against cutaneous leishmaniasis. Immunology. 1990 Apr;69(4):570–573. [PMC free article] [PubMed] [Google Scholar]
- Manzoni O., Prezeau L., Marin P., Desagher S., Deshager S., Bockaert J., Fagni L. Nitric oxide-induced blockade of NMDA receptors. Neuron. 1992 Apr;8(4):653–662. doi: 10.1016/0896-6273(92)90087-t. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Marsden P. A., Ballermann B. J. Tumor necrosis factor alpha activates soluble guanylate cyclase in bovine glomerular mesangial cells via an L-arginine-dependent mechanism. J Exp Med. 1990 Dec 1;172(6):1843–1852. doi: 10.1084/jem.172.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Patthy A., Bajusz S., Patthy L. Preparation and characterization of Ng-mono-, di- and trimethylated arginines. Acta Biochim Biophys Acad Sci Hung. 1977;12(3):191–196. [PubMed] [Google Scholar]
- Petros A., Bennett D., Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet. 1991 Dec 21;338(8782-8783):1557–1558. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- Rockett K. A., Awburn M. M., Cowden W. B., Clark I. A. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991 Sep;59(9):3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrel P. M., Lindsay D. C., Poole-Wilson P. A., Collins P. Hypothesis: inhibition of endothelium-derived relaxing factor by haemoglobin in the pathogenesis of pre-eclampsia. Lancet. 1990 Oct 27;336(8722):1030–1032. doi: 10.1016/0140-6736(90)92490-9. [DOI] [PubMed] [Google Scholar]
- Spriggs D. R., Sherman M. L., Michie H., Arthur K. A., Imamura K., Wilmore D., Frei E., 3rd, Kufe D. W. Recombinant human tumor necrosis factor administered as a 24-hour intravenous infusion. A phase I and pharmacologic study. J Natl Cancer Inst. 1988 Sep 7;80(13):1039–1044. doi: 10.1093/jnci/80.13.1039. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Bate C. A., Kwiatkowski D., Jakobsen P. H., Playfair J. H. Two soluble antigens of Plasmodium falciparum induce tumor necrosis factor release from macrophages. Infect Immun. 1990 Sep;58(9):2923–2928. doi: 10.1128/iai.58.9.2923-2928.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Tavernier J., Fiers W., Playfair J. H. Recombinant tumour necrosis factor inhibits malaria parasites in vivo but not in vitro. Clin Exp Immunol. 1987 Jan;67(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Wallace W. J., Caughey W. S. Mechanism for the autoxidation of hemoglobin by phenols, nitrite and "oxidant" drugs. Peroxide formation by one electron donation to bound dioxygen. Biochem Biophys Res Commun. 1975 Feb 3;62(3):561–567. doi: 10.1016/0006-291x(75)90435-0. [DOI] [PubMed] [Google Scholar]


