Abstract
Curcumin, the yellow pigment of the spice turmeric, has emerged as a promising anticancer agent due to its antiproliferative and antiangiogenic properties. However, the molecular mechanism of action of this compound remains a subject of debate. In addition, curcumin’s low bioavailability and efficacy profile in vivo further hinders its clinical development. This study focuses on the mechanism of action of EF24, a novel curcumin analog with greater than curcumin biological activity and bioavailability, but no increased toxicity. Treatment of MDA-MB231 breast and PC3 prostate cancer cells with EF24 or curcumin led to inhibition of HIF-1α protein levels and, consequently, inhibition of HIF transcriptional activity. This drug-induced HIF inhibition occurred in a VHL-dependent but proteasome-independent manner. We found that, while curcumin inhibited HIF-1α gene transcription, EF24 exerted its activity by inhibiting HIF-1α posttranscriptionally. This result suggested that the two compounds are structurally similar but mechanistically distinct. Another cellular effect that further differentiated the two compounds was the ability of EF24, but not curcumin, to induce microtubule stabilization in cells. EF24 had no stabilizing effect on tubulin polymerization in an in vitro assay using purified bovine brain tubulin, suggesting that the EF24-induced cytoskeletal disruption in cells may be the result of upstream signaling events rather than EF24 direct binding to tubulin. In summary, our study identifies EF24 as a novel curcumin-related compound possessing a distinct mechanism of action, which we believe contributes to the potent anticancer activity of this agent and can be further exploited to investigate the therapeutic potential of EF24.
Keywords: curcumin, structural analogs, HIF, microtubules, VHL
Introduction
Curcumin, (diferuloylmethane, structure in Fig. 1), is a naturally occurring polyphenolic compound isolated from tumeric, the spice extracted from the root of the East Indian Curcuma longa plant. Curcumin is the principal yellow component of all curry powders and pastes and has been widely used in Eastern traditional medicine to treat liver disease, rheumatoid arthritis, and insect bites. Recently, curcumin has attracted great attention as a possible novel anticancer agent due to its demonstrated antitumor activity in animal models and its minimal toxicity to normal tissues.1,2 At the molecular level, curcumin is reported to exert its anticancer activity by inhibiting several different proteins/pathways, such as nuclear factor kappaB (NFκB),1,3,4 protein kinase C (PKC), and mitogen-activated protein kinase (MAPK), and by activating protein-1 (AP-1) pathways.5-8 Nevertheless, the precise mechanism of action of curcumin in cancer cells is not clear.
Figure 1.
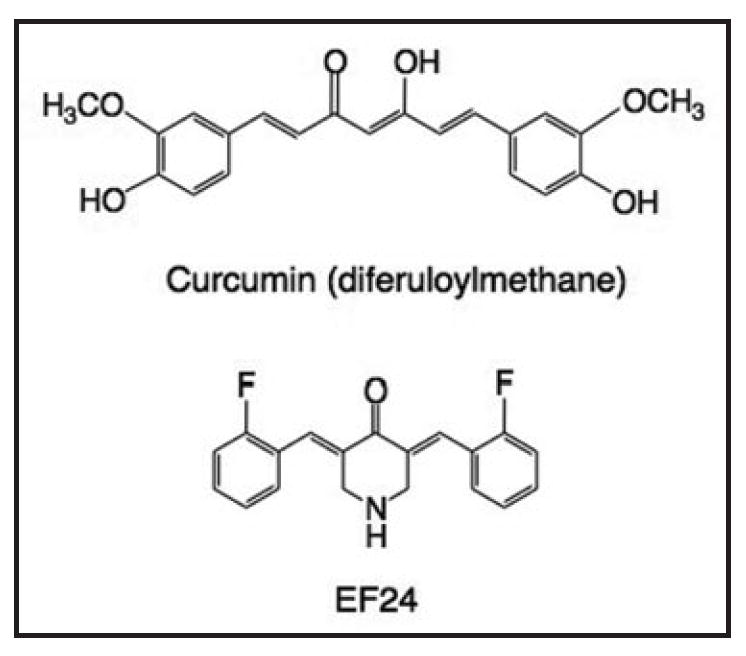
Chemical structures of curcumin and EF24.
The in vivo anticancer and antiangiogenic activity of curcumin, together with its low toxicity, seem to make this small molecule an ideal chemotherapeutic agent. Reports from phase I clinical studies have shown no treatment-related toxicity even at 8 g/day, but the highest peak plasma concentration achieved was only 1.8 μM,9 a subtherapeutic concentration. The poor absorption and bioavailability of curcumin in humans thus probably limits its clinical efficacy. The need for curcumin-like compounds with improved bioavailability characteristics has led to the chemical synthesis of a series of analogs, using curcumin as the lead structure. One such compound is the fluorinated substance EF24 (structure in Fig. 1), shown to be one of the most active synthetic curcumin analogs. Importantly, in animal models EF24 has demonstrated a superior pharmacokinetic and activity profile relative to curcumin while remaining well tolerated.10,11 In addition, the compound inhibits vascular endothelial growth factor-induced angiogenesis in rabbit and mice models and causes significant reduction in tumor size in human breast cancer xenografts in athymic nude mice.12
In this study, we set out to elucidate aspects of the molecular mechanism of action of EF24. We focused on the reported antiangiogenic activities of curcumin, partially mediated by inhibition of the hypoxia-inducible factor (HIF-1) pathway13,14 and determined whether EF24 had an effect on the HIF pathway.
Our results show that EF24 inhibited the oxygen-regulated alpha subunit of HIF-1, a mechanism previously reported for curcumin.13,14 However, EF24 is not only significantly more active than curcumin, but it also inhibits HIF-1 via a totally distinct mechanism. We found that curcumin inhibited HIF-1α transcription only at the highest concentration examined (50–100 μM), whereas EF24 downregulated HIF-1α in a dose-dependent manner at low, sublethal concentrations. We further found that EF24 blocked HIF-1α at a posttranscriptional level and in a manner dependent on the von Hippel Lindau (VHL) protein. Importantly, we also established that, unlike curcumin, EF24 disrupted the microtubule cytoskeleton, a mechanism likely contributing to the promising anticancer activity of this drug, in addition to its ability to inhibit HIF. Our results demonstrate that, while curcumin and EF24 have structural similarities, they are not mechanistically identical. Further investigation of the therapeutic potential of EF24 is warranted.
Results
Curcumin and EF24 downregulated HIF-1α protein levels and HIF-1 transcriptional activity in a dose-dependent manner
Recently, curcumin has been reported to inhibit HIF-1.13,14 However, it is not clear how it exerts its anti-HIF activity. One group showed that curcumin affects only the HIF-1β (ARNT) subunit,14 while another reported that curcumin inhibited HIF-1α.13 We treated PC3 human prostate cancer (Fig. 2A) and MDA-MB-231 breast cancer cells (Fig. 2B and C) with curcumin or EF24 and measured HIF-1α and HIF-1β protein levels. Under normal oxygenated conditions, HIF-1α protein is barely detectable due to its rapid proteasomal degradation. Hypoxia stabilizes HIF-1α protein by preventing its association with the VHL protein and subsequent targeting to the proteasome for degradation. Treatment with either EF24 or curcumin resulted in a dose-dependent downregulation of HIF-1α levels in both cell lines. In PC3 cells, treatment with as little as 1 μM EF24 resulted in almost a 90% reduction in HIF-1α protein, while 20–50 μM curcumin was required to produce a similar effect (Fig. 2A). We found that both compounds reduced HIF-1β levels (Fig. 2C), but higher concentrations than those required to reduce HIF-1α levels were needed. For example, 20 μM curcumin almost completely inhibited production of HIF-1α protein but had little, if any, effect on the HIF-1β level. These results suggested that HIF-1α is more susceptible to the effects of EF24 and curcumin than HIF-1β, and, therefore, we limited our further studies to the mechanism by which EF24 and curcumin affected the α subunit of the HIF complex.
Figure 2.

EF24 and curcumin downregulate HIF-1α and HIF-1β levels and impair the transcriptional activity of HIF. PC-3 (A) and MDA-MB231 (B and C) cells were treated with increasing concentrations of EF24 or curcumin (Curc) for 16 h and then subjected to hypoxia or remained in normoxia (N) for an additional 4 h. Whole cell extracts were analyzed by SDS-PAGE and immunoblotted with anti-HIF-1α (A and B), anti-HIF-1β (C) and actin antibodies. Treatment with the proteasome inhibitor MG132 at 10 μM for 4 h was also included as a control for HIF-1α protein stabilization resulting from inhibition of protein degradation, similar to the effects of hypoxia. (D) 1A9 cells transiently transfected with the HRE-luc construct pBI-GL V6L and treated to the indicated drug concentrations for 16 hr under normoxia or hypoxia. The cells were then harvested and analyzed for luciferase activity. Luciferase values were normalized to total cell protein content.
Upon hypoxia-induced stabilization, HIF-1α translocates to the nucleus, dimerizes with HIF-1β, and activates genes containing hypoxia response elements (HREs). To determine whether EF24 or curcumin had any effect on HIF’s transcriptional activity, we transiently transfected 1A9 human ovarian cancer cells with a reporter plasmid, which contains six tandem copies of the VEGF HREs and drives the expression of luciferase16 (Fig. 2D). As expected, hypoxia induced HIF’s transcriptional activity, as evidenced by the increase in the luciferase reporter activity. Treatment with either EF24 or curcumin resulted in a dose-dependent reduction of the transcriptional activity of HIF-1 consistent with the drug-induced reduction of HIF-1α protein levels (Fig. 2A and B). 2ME2, a small molecule that inhibits HIF-1α formation, was used as a positive control.20
Curcumin and EF24 inhibited HIF formation by a VHL protein-dependent but proteasombe independent mechanism
To further investigate the mechanism by which EF24 treatment decreases HIF-1α protein levels and its transcriptional activity, we examined the drug’s ability to target HIF-1α for proteosomal degradation. Thus, we treated PC-3 cells with 25 μM EF24 for 4 h in the presence or absence of the proteasome inhibitor MG132 (Fig. 3A). As expected, treatment with MG132 resulted in increased HIF-1α protein levels due to inhibition of HIF-1α degradation. Interestingly, MG132 had no effect on the EF24-mediated reduction in HIF-1α levels, even though it prevented HIF-1α degradation when used alone. Similar to the results with EF24, MG132 did not prevent the curcumin mediated reduction in HIF-1α levels.
Figure 3.
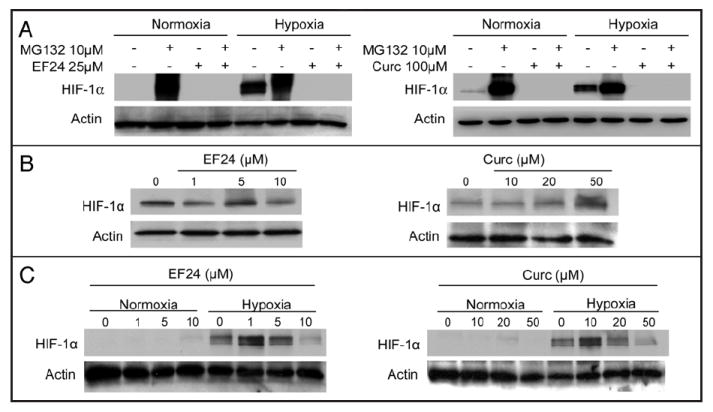
Curcumin and EF24 inhibit HIF-1α in a VHL protein-dependent but proteasome independent manner. (A) PC-3 cells were treated with 25 μM EF24 or 100 μM curcumin in the presence and absence of 10 μM MG-132 for 4 h. RCC2 renal cells with loss of VHL protein function (B) or RCC2-VHL renal cells with reconstituted wild-type VHL (C) were treated with the indicated drug concentrations for 16 h. In (B), only cells treated under normoxic conditions are shown. Equal amounts of protein from each cell lysate were resolved by SDS-PAGE, transferred and immunoblotted with antibodies against HIF-1α and actin
Since enhanced protein degradation did not appear to be the mechanism of action of EF24 or curcumin, we examined next the effects of these compounds in the context of VHL loss of function. It is well established that the VHL protein is a negative regulator of HIF-1α by targeting it to the proteasome for degradation under normoxic conditions.21 VHL loss of function mutations occur in over 50% of human renal cancers, leading to constitutively high levels of HIF-1α protein and activation of HIF-target genes. To examine the role of the VHL protein on the anti-HIF activities of EF24 and curcumin, we used an isogenic pair of renal cancer cell lines, RCC2 cells harboring high basal HIF levels due to VHL gene inactivation and RCC2-VHL cells in which the wild-type VHL gene was stably reintroduced. No decrease in HIF-1α protein was observed in the RCC2 cells following treatment with either EF24 or curcumin (Fig. 3B). However, with wild-type VHL gene expression in the RCC2-VHL cells, the ability of EF24 and curcumin to inhibit HIF-1α protein levels was restored (Fig. 3C). These results suggest that both EF24 and curcumin inhibit HIF-1α in a VHL protein-dependent manner that likely does not require proteasome activity.
Curcumin, but not EF24, inhibited HIF-1α transcription
To further explore the mechanism by which curcumin and EF24 reduced HIF-1α levels, we examined their effects on HIF-1α transcription, as assessed by northern blotting. Results in Figure 4A show that EF24 treatment of PC-3 cells had no effect on HIF-1α mRNA expression, while treatment with curcumin significantly downregulated HIF-1α mRNA in a dose-dependent manner. Actin mRNA expression is shown as a loading control. To increase the sensitivity of our assay, we also assessed HIF-1α expression by quantitative real-time RT-PCR in untreated and drug-treated PC3 cells. HIF-1α expression was normalized to that of GAPDH, used here as an internal loading control (Fig. 4B). Curcumin significantly downregulated HIF-1α gene expression, while EF24 treatment had no effect, in agreement with the northern blot analysis (Fig. 4A). Treatment with 2ME2 is included here as a negative control for lack of transcriptional inhibition of HIF-1α, as we have previously shown that 2ME2 inhibits HIF-1α protein at the level of translation20 (Fig. 4B). Similar results were obtained in MDA-MB-231 breast cancer cells (data not shown), confirming that curcumin, but not EF24, inhibits HIF-1α at the level of transcription.
Figure 4.
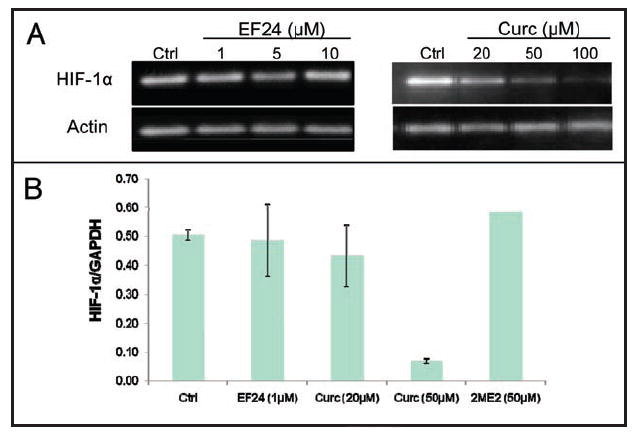
Curcumin, but not EF24, inhibited HIF-1α gene expression. Cultured PC-3 cells were treated with the indicated concentrations of EF24 or curcumin (Curc) for 16 h. Total mRNA was extracted as described in Materials and Methods. (A) HIF-1α mRNA expression of drug-treated cells was analyzed by northern blotting. (B) Relative mRNA expression of HIF-1α was determined by quantitative RT-PCR normalized to GAPDH expression.
EF24, but not curcumin, disrupts normal mitosis, interphase microtubule organization and stabilizes cellular microtubules
Our results thus far show that curcumin and EF24, even though structurally related, exert their anti-HIF activities via distinct molecular mechanisms. Examination of both compounds’ chemical structures revealed a structural resemblance to chalcones, a class of microtubule depolymerizing antimitotic agents that bind to the colchicine site.22,23 We have previously shown that microtubule-targeting drugs that bind to the colchicine site can exert antiangiogenic activity by inhibiting HIF-1α and HIF transcriptomes (e.g., VEGF) downstream of microtubule disruption.20,24 In addition, we have shown that microtubule-targeting drugs inhibit HIF protein post-transcriptionally, similar to the effects of EF24 described above.20 Thus, we hypothesized that EF24 might elicit its anti-HIF activity as a result of microtubule disruption. To evaluate potential cytoskeletal effects of EF24, we treated MCF-7 breast cancer (5A) or PC-3 prostate cancer cells (5B) with EF24 and curcumin and analyzed the cells’ cytoskeletons by confocal microscopy. Treatment with EF24 resulted in aberrant mitotic figures (Fig. 5A, arrows), indicative of microtubule disruption. The drug’s effects on interphase microtubules appeared to be stabilizing, as shown by the appearance of microtubule bundles (Fig. 5A and B). Compared with untreated control PC-3 cells, which maintain an intricate and organized network of microtubules, EF24 treatment caused microtubules to cluster (bundle) around the nucleus in a ring-like structure. No major effect on the actin cytoskeleton was observed. Treatment with cytochalasin D (CD) is also shown as a control for actin depolymerization (Fig. 5C).
Figure 5.
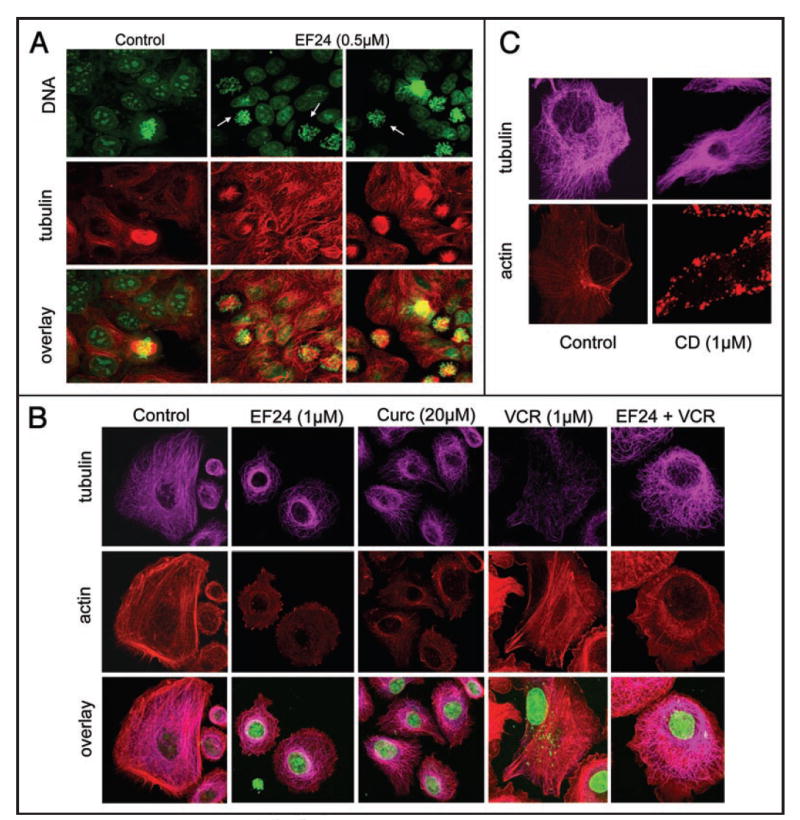
EF24 induced mitotic arrest and stabilized interphase microtubules. (A) MCF-7 breast cancer cells or (B) PC-3 prostate cancer cells were left untreated or treated with the indicated drug concentrations for 16 h. Cells were fixed and processed for immunofluorescence. Confocal laser scanning microscopy was used to analyze the images. Tubulin (A): red; (B and C): magenta), actin (red). DNA was counterstained with Sytox green. Arrows in (A) point to aberrant mitotic figures.
In contrast to EF24, curcumin had no detectable effect on the organization of either the microtubule or the actin cytoskeleton. To further probe the microtubule-stabilizing properties of EF24, we treated cells concomitantly with EF24 and the microtubule-depolymerizing drug vincristine (VCR). Vincristine alone completely depolymerized interphase microtubules (Fig. 5B). The presence of as little as 1 μM EF24 protected microtubules from the depolymerizing effects of VCR, as evidenced by the extensive microtubule network that PC-3 cells retained. Collectively, these results suggest that EF24 has a significant stabilizing effect on the microtubule cytoskeleton, which appears to be distinct from the classic effect of taxanes and other well-characterized microtubule-stabilizing drugs.
To quantitatively assess the microtubule-stabilizing effects of EF24, we conducted a cell-based tubulin polymerization assay25 (Fig. 6A). Treated and untreated PC-3 cells were lysed and fractionated into the soluble and polymer forms of tubulin. Lanes labeled “P” contained the polymerized form of tubulin (pelleted microtubule polymers), while those labeled “S” contained the soluble (α/β-tubulin dimers). Our results show that treatment with EF24 caused a dose-dependent increase in tubulin polymerization, as evidenced by the shift of tubulin from the soluble (S) to the polymerized fraction (P). To rule out non-specific sedimentation of tubulin, we reprobed the same blot with an antibody specific for acetylated tubulin. Tubulin acetylation is a posttranslational modification that occurs on the microtubule polymer, and its presence denotes stable, long-lived microtubule polymers.26 Similar to the results with total tubulin, EF24 treatment caused an incremental increase in tubulin acetylation, further attesting to the microtubule-stabilizing activity of EF24 (Fig. 6A). Curcumin, on the other hand, had no effect on tubulin polymerization nor on the proportion of acetylated tubulin, consistent with the immunofluroescence results presented in Figure 5B.
Figure 6.
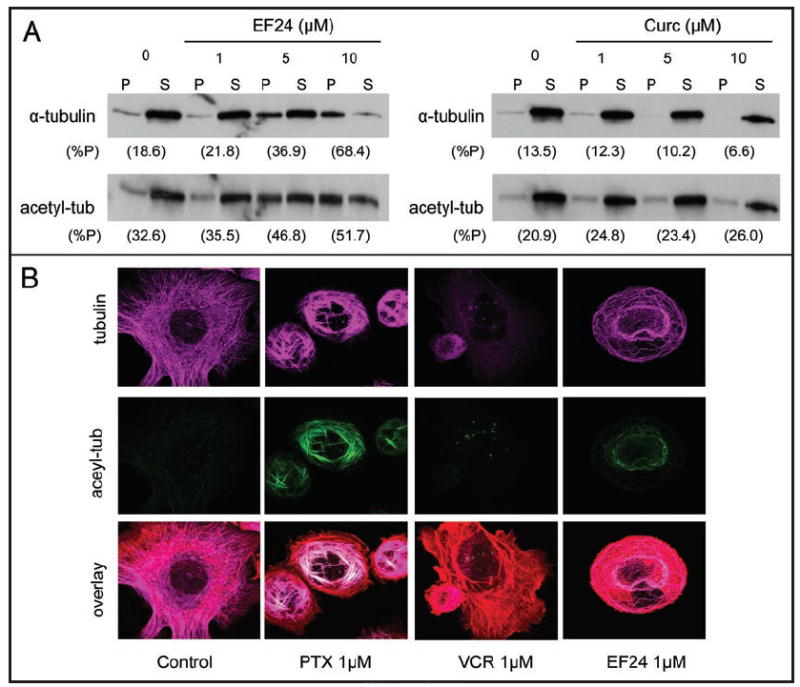
EF24, but not curcumin, induced microtubule stabilization. (A) Relative levels of polymerized (P) and solublized (S) tubulin from untreated or 16 h drug-treated PC-3 cells were analyzed by western blotting. The relative amount of tubulin in the pellet is represented by (%P). (B) Effects of drug treatment were analyzed by immunofluorescence using confocal microscopy. Tubulin (purple), acetyl-α-tubulin (green), actin (red).
Immunofluorescence staining for total and acetylated tubulin following drug treatment with either paclitaxel (PTX), VCR or EF24 is shown in Figure 6B. As expected, treatment with paclitaxel produced characteristic needle-like bundles of microtubules and relatively heavy microtubule acetylation that co-localized with the bundles. On the other hand, treatment with the microtubule-depolymerizing vincristine did not enhance tubulin acetylation. EF24 treatment resulted once again in an increase in acetylated microtubules and microtubule polymerization as compared with untreated cells. The characteristic EF24-induced microtubule perinuclear ring was composed of acetylated microtubules, indicating that this ring is composed of stable microtubules. In summary, we have demonstrated by two independent assays that EF24 confers a stabilizing effect on microtubules in a dose-dependent manner, albeit distinct from that induced by paclitaxel.
EF24 remains active in taxol- and epothilone-resistant cells with acquired β-tubulin mutations
The clinical success of the microtubule-targeting drugs used in clinical oncology, such as the taxanes and the vinca alkaloids, is hampered by the development of drug-resistance—a serious treatment obstacle accounting for the majority of cancer related deaths. We have previously shown that drug-resistance to various microtubule-targeting drugs occurs following acquired tubulin mutations, which impair drug-tubulin interactions.25,27 The microtubule-stabilizing effects induced by EF24 suggest that this drug may also bind directly to tubulin or microtubules. To examine whether EF24 binds to tubulin at the same site as other microtubule-stabilizing drugs, such as the taxanes and the epothilones, we tested the activity of EF24 in cells resistant to these drugs due to distinct mutations in the predominantly expressed β-tubulin gene M40.
Results from 72-h cytotoxicity experiments revealed that EF24 retains activity against both cell lines, suggesting that either the effects of EF24 on the microtubule cytoskeleton are indirect or that EF24 may bind to tubulin at a location distinct from the taxane site.
EF24 demonstrates weak microtubule-depolymerizing activity in vitro
To investigate whether the activity of EF24 on cellular microtubules resulted from direct binding of the compound to tubulin, we examined the effects of EF24 on tubulin polymerization in vitro. Assembly of purified tubulin was induced by glutamate in the presence of GTP (Fig. 7). As control compounds, we also examined paclitaxel (data not shown), a powerful inducer of assembly, and combretastatin A-4, a powerful inhibitor of assembly (Fig. 7, curve 6). As shown in Figure 7, the latter compound nearly eliminated the assembly of 10 μM tubulin when present at the substoichiometric concentration of 2 μM (compare curve 6 with curve 1). We also examined curcumin, but found minimal effects on the assembly reaction at concentrations up to 80 μM (curve 2). EF24, however, displayed weak, progressive partial inhibition of tubulin assembly, affecting most notably the onset of the reaction (nucleation phase) and its rate (elongation phase). Examination of the reaction rates in several experiments showed that a 50% reduction in the reaction rate occurred at about 40 μM EF24. Higher concentrations of both EF24 and curcumin produced optical distortions that were difficult to evaluate, and at 200 μM heavy precipitation of both compounds took place.
Figure 7.
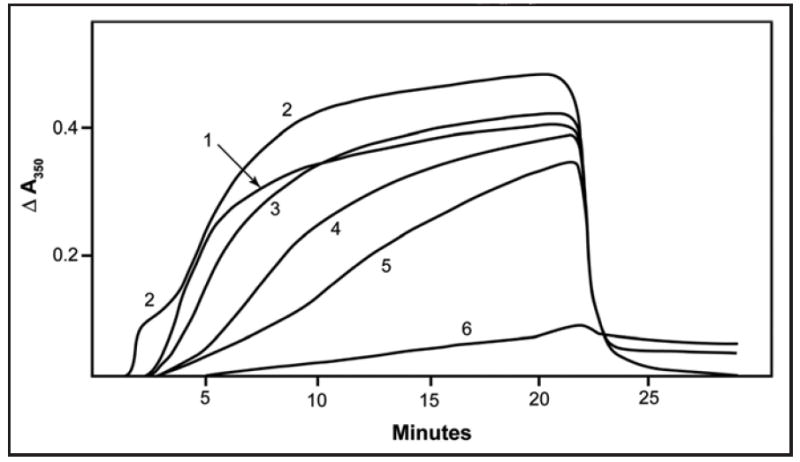
Weak inhibition of tubulin assembly by EF-24. Assembly of purified tubulin (10 μM) was monitored turbidimetrically in the absence (curve 1) or presence of the following compounds: 80 μM curcumin (curve 2), 20 μM EF24 (curve 3), 40 μM EF24 (curve 4), 80 μM EF24 (curve 5) or 2.0 μM combretastatin A-4 (curve 6). The cuvettes were held at 0°C for 1 min, jumped to 30°C at the 1 min time point, held at 30°C until the 21 min time point, at which time they were jumped back to 0°C, and the reaction was followed for an additional 8 min. The 0 to 30°C jump takes less than 20 s, and the 30 to 0°C jump takes about 1 min.
Discussion
In this study we demonstrated that the synthetic curcumin congener EF24 is an effective antiproliferative agent that exerts its activity by stabilizing cellular microtubules and by inhibiting intracellular levels of the pro-angiogenic transcription factor HIF. The hypoxia inducible transcription factor HIF-1 is an important mediator of tumor angiogenesis and survival, and inhibition of HIF levels or HIF action is an attractive therapeutic strategy for cancer chemotherapy.28 Here we show that both curcumin and even more potent EF24 inhibit intracellular levels of both HIF-1α and HIF-1β proteins This is turn results in the subsequent dowregulation of HIF’s transcriptional activity in various human epithelial cancer cell lines (Fig. 2). Our results demonstrated that inhibition of the HIF-1α protein level occurred at a lower drug concentration than that required for the inhibition of the HIF-1β protein. Thus, the oxygen-regulated α subunit of HIF was more sensitive to the effects of these compounds. Nevertheless, inhibition of the levels of both HIF subunits is a rather unique characteristic for the known HIF inhibitors, most of which have been reported to affect primarily HIF-1α either directly or indirectly.29 However, since the transcriptional activation of HIF target genes requires interaction between both the α and β HIF subunits, a drug that achieves inhibition of both should have superior anti-HIF activity in vivo. Our data are in agreement with recent reports showing that curcumin exerted anti-HIF activity by inhibiting HIF-1β14 or HIF-1α levels.13 Contrary to our results, Choi et al.,14 reported that curcumin had no effect on HIF-1α levels This discrepancy may be due to the original source of curcumin used in each of the studies, since we found it necessary to repurify curcumin. Our original commercial curcumin sample contained a mixture of three different curcuminoids, each of which may have distinct effects on cellular levels of the HIF-1 subunits.
EF24 and curcumin inhibited HIF protein levels by two distinct mechanisms (Figs. 4-6). Curcumin, on the one hand, inhibited HIF-1α at the level of transcription, as evidenced by the dose-dependent decrease of HIF-1α mRNA expression (Fig. 4). In contrast, EF24 exerted its anti-HIF activity post-transcriptionally. We have previously shown that the microtubule inhibitors (i.e., 2ME2, paclitaxel) downregulated HIF-1α downstream of microtubule disruption, regardless of drug structure, tubulin-binding site, and tubulin-stabilizing or -destabilizing activity.20,24 In addition, we have shown that the anti-HIF effects of these inhibitors depend on their relative ability to disrupt microtubules, as evidenced by the loss of the drug-induced HIF inhibition in paclitaxel- or epothilone-resistant cells harboring β-tubulin mutations that impair drug-binding to tubulin.20,24 Importantly, we showed that the tubulin inhibitors affected HIF-1α post-transcriptionally, similar to the effects of EF24. In the case of the tubulin inhibitors we found that HIF-downregulation occurred via inhibition of its translation.
This led us to examine the effects of EF24 and curcumin on cellular microtubules. We found that EF24, but not curcumin, affects both interphase and spindle microtubules in a variety of human epithelial cancer cell lines. We demonstrated that EF24 caused some cells to arrest in mitotis and stabilized interphase microtubules. The latter effect was documented by an increase in microtubule polymer mass and in tubulin acetylation in EF24-treated cells (Fig. 6). Increased acetylation of α-tubulin is a well-established marker for formation of stabilized microtubules.26 Additional evidence for formation of stabilized microtubules in EF24-treated cells were the bundled microtubules, particularly those surrounding the nucleus, as well as the EF24-mediated protection of microtubules from VCR-induced depolymerization (Figs. 5 and 6).
We wished to obtain evidence for the binding site for EF24 on tubulin by examining the compound’s activity in a panel of cells harboring distinct mutations in a β-tubulin gene that confer resistance to either paclitaxel, epothilone A (Table 1), or 2ME2 (Vβ236I, data not shown). EF24 retained its activity against all mutant lines examined, suggesting that EF24 either binds to tubulin at a different site or by a different mechanism not affected by the mutations examined. Alternatively, it is possible that EF24 does not interact directly with tubulin, despite its cellular effects on microtubules. The latter hypothesis is supported by the inability of EF24 to stabilize microtubules in vitro (Fig. 7). Unexpectedly, in these in vitro experiments EF24 showed a weak inhibitory effect on microtubule assembly, most notably on the nucleation phase of the reaction. This inhibitory effect required super-stoichiometric concentrations of EF24, in sharp contrast to the potent substoichiometric inhibition that occurred with combretastatin A-4. Although assembly studies with bovine brain tubulin typically agrees with the mechanism of action of antitubulin drugs observed in cells, we cannot exclude that the different isotypes and/or post-translational modifications in cultured cells as compared with bovine brain tubulin could have caused the different effects we observed. Nor are we able to exclude a possible metabolic conversion of EF24 within the cells to a compound with a different mechanism of action.
Table 1.
EF24 and curcumin both retain cytotoxic activity against a panel of b-tubulin mutants specific for the taxane and the epothilone binding site
| Drug | Celllines | ||||
|---|---|---|---|---|---|
| 1A9 IC50 | PTX10 (β270) | A8(β274) | |||
| IC50 | RR | IC50 | RR | ||
| EF24 | 0.21 | 0.31 | 1.5 | 0.29 | 1.4 |
| Curcumin | 15.5 | 14.8 | 0.95 | 11.5 | 0.74 |
| PTX | 3.8 | 63 | 17 | 17.5 | 4.6 |
(A) Cytotoxicites of EF24, curcumin, and paclitaxel (PTX) against 1A9 ovarian cancer cells and cell lines that have mutations in β-tubulin (1A9/PTX10 - F270V, 1A9/A8 - A274T) selected for resistance with paclitaxel and epothilone A, respectively. PTX (nM) is used as a control. The cytotoxic potential of the compounds were accessed in a 72 hour growth inhibition SRB assay as described. IC50 values derived from the experiment are given for each compound in μM. RR = relative resistance; equaled to the IC50 in resistant cell line divided by IC50 in 1A9 parental wildtype cells.
We were unable to reproduce the apparent inhibition of tubulin assembly by curcumin reported by Gupta et al.30 Possible causes for our different results could be the use of different reaction conditions, different tubulin preparations, the methodology used to monitor turbidity development, or, perhaps most likely, by other curcuminoids in the material used by Gupta et al.32
Taken together, the cellular and in vitro effects of EF24 on the microtubule cytoskeleton suggest that this compound may affect cellular microtubules indirectly by modulating pathways that influence microtubule formation and/or stability. Nevertheless, the EF24-induced microtubule stabilization in cells may result in the downstream inhibition of HIF-1α and β levels, similar to the effects of the known microtubule inhibitors. Further studies are necessary to address the causal relationship between EF24-induced microtubule disruption and reduction in HIF protein levels.
Experiments with the proteasome inhibitor MG-132 with EF24 have increased our understanding of the mechanism by which EF24 reduces intracellular levels of HIF (Fig. 3A). Our data showed that the presence of MG-132 did not rescue HIF-1α levels from the reduction caused by EF24, suggesting that EF24 is affecting HIF-1α at a step prior to protein degradation. On the other hand, EF24 treatment of RCC2 renal cells expressing an inactive VHL protein, had no effect on HIF-1α levels (Fig. 3B and C). Renal cancers are notorious for their loss of functional VHL protein, a condition that hinders the machinery necessary for HIF degradation and lead to accumulation of HIF-1α, even under normoxic conditions. Indeed, when VHL was reconstituted in the RCC2 renal cell line, and HIF was targeted for proteasomal degradation, the anti-HIF activity of EF24 was restored. This result implies that functional VHL protein is most likely required for the EF24-mediated downregulation of HIF-1α. Importantly, a recent study showed that VHL protein binds to and stabilizes cellular microtubules.31 This finding could explain the requirement for functional VHL protein for EF24 to exert its anti-HIF activity. It is possible that EF24 stabilizes microtubules in a VHL-dependent manner, and this results in downstream reduction of HIF-1α levels. Furthermore, this scenario could rationalize the apparent discrepancy between cellular microtubule stabilization and weak in vitro inhibition of microtubule assembly by EF24. The weak interaction of EF24 with tubulin would then play no role in the cellular effects of the compound.
In summary, our study has confirmed EF24 as a promising anticancer and antiangiogenic compound with a mechanism of action that is distinct than that of its parent compound, curcumin. Our results reveal that EF24 most likely affects the microtubule cytoskeleton indirectly and causes a sharp reduction HIF-1α levels by a mechanism dependent on the VHL protein and not dependent on proteasomal degradation of HIF-1α. In addition, EF24 also causes, but to a lesser extent, a reduction in HIF-1β protein levels. Further studies elucidating the relationship between EF24, VHL protein, cellular microtubules and the HIF pathway will advance our molecular understanding of the mechanism of action of EF24 and should identify tumor types likely to be sensitive to EF24 and related compounds, based on their genetic make-up. Finally, this knowledge may be useful towards the development of combination therapies using EF24 with other microtubule- and HIF inhibitors with distinct, non-overlapping mechanisms of action.
Experimental Procedures
Chemicals and antibodies
Curcumin, purchased from Sigma-Aldrich (St. Louis, MO), was found to be a mixture of three different curcuminoids. Curcumin was purified by column chromatography over silica gel to remove the minor metabolites (20%) and provide pure curcumin as pictured in Figure 1. EF24 was synthesized as previously described.10 Stock solutions (0.01 mM) were made in dimethyl sulfoxide (DMSO) and stored in aliquots at 4°C. The compounds were diluted in cell culture medium immediately prior to each experiment. Vincristine was from Eli Lilly (Indianapolis, Indiana), paclitaxel from Sigma-Aldrich, MG-132 (Z-Leu-Leu-Leu-aldehyde) from Alexis Biochemicals (San Diego, California), and the rhodamine phalloidin used in the immunofluorescence studies was from Molecular Probes (Eugene, OR). In addition, the following primary antibodies were used: rat anti-α-tubulin (Chemicon International, Temecula, CA), mouse anti-HIF-1α (BD Biosciences, San Diego, CA), mouse anti-HIF-1β (Santa Cruz Biotechnology, Santa Cruz, CA), human anti-actin antibody (Santa Cruz Biotechnology). Secondary antibodies were horseradish peroxidase-conjugated (Amersham, Piscataway, NJ), Alex Fluor 488 goat anti-mouse (Molecular Probes), and Alex Fluor 568 goat anti-rat (Molecular Probes). RPMI 1640 medium was from Sigma-Aldrich and fetal bovine serum (FBS) was from CellGro (Manassas, VA).
Cell lines
Human breast cancer MDA-MB-231 and human prostate cancer PC3 cells were grown in RPMI-1640, 10% FBS; 1A9 human ovarian carcinoma cells and paclitaxel-, epothilones-resistant cell lines derived from the 1A9 line (1A9/PTX10, 1A9/A8, respectively) were cultured in RPMI-1640, 10% FBS. Human breast cancer MCF-7 cells with green fluorescent protein-(GFP)-tubulin were cultured in RPMI-1640, 10% FBS with G418 sulfate solution.
Immunoblot analysis
Proteins (40–60 μg/lane) from whole cell extracts were resolved by 7.5% SDS-PAGE, electrotransferred to Immobilon-P membranes, and incubated with the indicated primary antibodies, followed by horseradish peroxidase-conjugated secondary antiserum. Immunoreactivity was visualized by enhanced chemiluminescence reagent (Amersham Biosciences). For sequential blotting with additional antibodies, the membranes were stripped of antibodies using a 2 M NaOH solution, and the same blots were reprobed with different primary antibodies.
HIF reporter gene assay
1A9 cells were transfected with 1 μg/well of a luciferase reporter plasmid (pBI-GL V6L) containing six HREs elements from the VEGF promoter as previously described.15,16 Enzymatic activity was measured by a chemiluminescent assay and normalized to the total protein in the cellular extracts.
Isolation and analysis of RNA
Total RNA was isolated using the RNase Easy Mini Kit (Qiagen, Inc., Valencia, CA), and northern blotting was performed with probes specific for human HIF-1α and β-actin (Ambion, Inc., Austin, TX) as previously described.17
Immunofluorescence and confocal microscopy
Exponentially growing cells were plated on 12 mm glass coverslips (Fisher Scientific, Pittsburgh, PA) in 24-well plates, and the cells were allowed to attach overnight. The following day, the cells were treated with the indicated drugs for 16 h and subjected to hypoxia for an additional 4 h. Cells were then washed and fixed onto the coverslips with PHEMO buffer (PIPES 0.068 M, HEPES 0.025 M, EGTA 0.015 M, MgCl2 0.003 M, 10% DMSO, pH 6.8) containing 3.7% formaldehyde, 0.05% glutaraldehyde, and 0.05% Triton-X-100 for 10 min at room temperature. Coverslips with fixed cells were blocked in 10% goat serum/PBS for 10 min and processed for immunofluorescence with rat anti-α-tubulin. Secondary antibodies Alexa Fluor 488 and Alexa Fluor 615 goat anti-rat antibody were added next, for visualization of primary antibody. Sytox green was used for DNA staining. In order to visualize actin fibers, rhodamine phallodin was used. Stained coverslips were mounted onto glass slides and examined using a Zeiss LSM510 point-scanning confocal microscope.
Tubulin in vitro polymerization assay
The assembly of purified bovine brain tubulin, prepared as described previously,18 was evaluated as described in detail elsewhere.19 Briefly, 10 μM tubulin was preincubated for 15 min at 30°C in 0.8 M monosodium glutamate (2 M stock solution adjusted to pH 6.6 with HCl) and 4% (v/v) DMSO and compounds as indicated. Samples were chilled on ice, and GTP (0.4 mM) was added. (All concentrations are expressed in terms of the final reaction volume.) The samples were transferred to 0°C cuvettes in a temperature-controlled Beckman DU7400 spectrophotometer. Assembly was followed turbidimetrically, with apparent absorbance monitored at 350 nm.
Quantitative RT-PCR
To quantify mRNA levels, we used a highly sensitive, quantitative RT-PCR method. Total mRNA was isolated from cultured cells using TRIZOL (Invitrogen, Carlsbad, CA, and the concentrations were verified. All real-time RT-PCR reactions were performed in duplicate in a 20 μl mixture containing 1× IQ SYBR Green supermix (BioRad, Hercules, CA), 0.2 μM of each primer and 2 μl of cDNA templates. The primers for HIF-1α were 5’-TGGTGACATGATTTACATTTCTGA-3’ and 5’-AAGGCCATTTCTGTGTGTAAGC-3’, and the primers for glyceradehyde 3-phosphate dehydrogenase (GAPDH) were 5’-GGAGTCAACGGATTTGGTCG-3’ and 5’-CTTGATTTTGGAGGGATCTCG-3’. Real-time quantification was performed using the BIO-RAD iCycler iQ system (BioRad) under the following cycling conditions: (step 1) 94°C (2 min); (step 2) 50 cycles of 94°C (15 s), 60°C (60 s). The fluorescence threshold value was calculated using the iCycle iQ system software. The standard curves for HIF-1α and GAPDH were generated, and the concentrations of the unknown samples were calculated by setting their crossing point to the standard curve. The relative expression level of HIF-1α was normalized by comparative reference with the GAPDH gene value.
Acknowledgments
We would like to give our thanks to Aurora O’Brate and Vladimir Belozerov for technical assistance and to Kathleen Kite-Powell for editorial assistance. The work was supported in part by the NIH grants CA100202, CA114335, and CA116676 to P.G.; VHL (to JPS); and 5P01CA116676-020002 to W.Z. (W.Z. is an American Cancer Society Research Scholar and a Georgia Cancer Coalition Distinguished Scholar).
Abbreviation
- NFκB
nuclear factor kappaB
- PKC
protein kinase
- MAPK
mitogen-activated protein kinase
- AP-1
activating protein-1
- VHL
von hippel lindau
References
- 1.Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM, Lutgendorf SK, Aggarwal BB, Sood AK. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–30. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–98. [PubMed] [Google Scholar]
- 3.Singh S, Aggarwal BB. Activation of transcription factor NFkappaB is suppressed by curcumin (diferuloylmethane) J Biol Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. corrected. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their downregulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–62. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- 5.Gururaj AE, Belakavadi M, Venkatesh DA, Marme D, Salimath BP. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem Biophys Res Commun. 2002;297:934–42. doi: 10.1016/s0006-291x(02)02306-9. [DOI] [PubMed] [Google Scholar]
- 6.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20:7597–609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 7.Squires MS, Hudson EA, Howells L, Sale S, Houghton CE, Jones JL, Fox LH, Dickens M, Prigent SA, Manson MM. Relevance of mitogen activated protein kinase (MAPK) and phosphotidylinositol-3-kinase/protein kinase B (PI3K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochem Pharmacol. 2003;65:361–76. doi: 10.1016/s0006-2952(02)01517-4. [DOI] [PubMed] [Google Scholar]
- 8.Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Lett. 1994;341:19–22. doi: 10.1016/0014-5793(94)80232-7. [DOI] [PubMed] [Google Scholar]
- 9.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–900. [PubMed] [Google Scholar]
- 10.Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, Hollingshead MG, Kaur G, Sausville EA, Rickles FR, Snyder JP, Liotta DC, Shoji M. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem. 2004;12:3871–83. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, Sun A, Snyder JP, Liotta DC, Jones DP, Shoji M. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–75. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Shoji M, Sun A, Kisiel W, Lu YJ, Shim H, McCarey BE, Nichols C, Parker ET, Pohl J, Mosley CA, Alizadeh AR, Liotta DC, Snyder JP. Targeting tissue factor-expressing tumor angiogenesis and tumors with EF24 conjugated to factor VIIa. J Drug Target. 2008;16:185–97. doi: 10.1080/10611860801890093. [DOI] [PubMed] [Google Scholar]
- 13.Bae MK, Kim SH, Jeong JW, Lee YM, Kim HS, Kim SR, Yun I, Bae SK, Kim KW. Curcumin inhibits hypoxia-induced angiogenesis via downregulation of HIF-1. Oncol Rep. 2006;15:1557–62. [PubMed] [Google Scholar]
- 14.Choi H, Chun YS, Kim SW, Kim MS, Park JW. Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: A mechanism of tumor growth inhibition. Mol Pharmacol. 2006;70:1664–71. doi: 10.1124/mol.106.025817. [DOI] [PubMed] [Google Scholar]
- 15.Mabjeesh NJ, Post DE, Willard MT, Kaur B, Van Meir EG, Simons JW, Zhong H. Geldanamycin induces degradation of hypoxia-inducible factor 1alpha protein via the proteosome pathway in prostate cancer cells. Cancer Res. 2002;62:2478–82. [PubMed] [Google Scholar]
- 16.Post DE, Van Meir EG. eration of bidirectional hypoxia/HIF-responsive expression vectors to target gene expression to hypoxic cells. Gene Ther. 2001;8:1801–7. doi: 10.1038/sj.gt.3301605. [DOI] [PubMed] [Google Scholar]
- 17.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamel E, Lin CM. Separation of active tubulin and microtubule-associated proteins by ultracentrifugation and isolation of a component causing the formation of microtubule bundles. Biochemistry. 1984;23:4173–84. doi: 10.1021/bi00313a026. [DOI] [PubMed] [Google Scholar]
- 19.Hamel E. Evaluation of antimitotic agents by quantitative comparisons of their effects on the polymerization of purified tubulin. Cell Biochem Biophys. 2003;38:1–22. doi: 10.1385/CBB:38:1:1. [DOI] [PubMed] [Google Scholar]
- 20.Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–75. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 21.Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence NJ, McGown AT, Ducki S, Hadfield JA. The interaction of chalcones with tubulin. Anticancer Drug Des. 2000;15:135–41. [PubMed] [Google Scholar]
- 23.Kim do Y, Kim KH, Kim ND, Lee KY, Han CK, Yoon JH, Moon SK, Lee SS, Seong BL. Design and biological evaluation of novel tubulin inhibitors as antimitotic agents using a pharmacophore binding model with tubulin. J Med Chem. 2006;49:5664–70. doi: 10.1021/jm050761i. [DOI] [PubMed] [Google Scholar]
- 24.Escuin D, Kline ER, Giannakakou P. Both microtubule-stabilizing and microtubule-destabilizing drugs inhibit hypoxia-inducible factor-1alpha accumulation and activity by disrupting microtubule function. Cancer Res. 2005;65:9021–8. doi: 10.1158/0008-5472.CAN-04-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannakakou P, Sackett DL, Kang YK, Zhan Z, Buters JT, Fojo T, Poruchynsky MS. Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem. 1997;272:17118–25. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 26.Piperno G, LeDizet M, Chang XJ. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol. 1987;104:289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giannakakou P, Gussio R, Nogales E, Downing KH, Zaharevitz D, Bollbuck B, Poy G, Sackett D, Nicolaou KC, Fojo T. A common pharmacophore for epothilone and taxanes: molecular basis for drug resistance conferred by tubulin mutations in human cancer cells. Proc Natl Acad Sci USA. 2000;97:2904–9. doi: 10.1073/pnas.040546297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 29.Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today. 2007;12:853–9. doi: 10.1016/j.drudis.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Gupta KK, Bharne SS, Rathinasamy K, Naik NR, Panda D. Dietary antioxidant curcumin inhibits microtubule assembly through tubulin binding. Febs J. 2006;273:5320–32. doi: 10.1111/j.1742-4658.2006.05525.x. [DOI] [PubMed] [Google Scholar]
- 31.Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat Cell Biol. 2003;5:64–70. doi: 10.1038/ncb899. [DOI] [PubMed] [Google Scholar]


