Abstract
PTEN tumor suppressor inactivation is the earliest step in endometrial carcinogenesis, occurring in morphologically unremarkable endometrial glands in half of normal women. We test the hypothesis that sex hormones positively or negatively select for these “latent precancers” by examining their emergence, persistence, and regression rates under differing hormonal conditions. Peri and postmenopausal women had an intake endometrial biopsy and underwent hormonal therapy with progestin-impregnated intrauterine device (“IUD”, n=21), cyclic oral progestins (n=28), or surveillance only (n=22), with followup biopsies. For comparison, premenopausal naturally cycling endometrial biopsies were studied as single timepoints in 87 patients, and multiple surveillance timepoints in 34. Biopsies in which any PTEN protein null glands were found by immunohistochemistry were scored as containing a latent endometrial precancer. All groups had a similar proportion of latent precancers at intake, but differed after therapy. Emergence rates were highest (21%) for the naturally cycling premenopausal group, in comparison to just 9% for untreated perimenopausal women. The IUD group had the highest rate of regression, with a 62% pre and 5% post therapy rate of latent precancers. This contrasted to non-significant changes for the oral progestin and untreated control groups. Delivery of high doses of progestins locally to the endometrium by IUD leads to ablation of pre-existing PTEN-inactivated endometrial latent precancers, and is a possible mechanism for reduction of long term endometrial cancer risk known to occur in response to this hormone.
INTRODUCTION
Endometrioid (Type I) endometrial adenocarcinoma, the most common form of endometrial cancer, is a hormonally responsive tumor that has been associated with the exposure risk factor of estrogens unopposed by progestins, and in up to 83% of cases, inactivation of the PTEN tumor suppressor gene(1). Unopposed estrogens increase the risk for this malignancy 3-10 fold, in a dose and duration dependent manner(2). Although estrogens have received much of the attention as an endometrial cancer promoting agent, progestins are equally potent risk reducing agents. A component of the progestin protection may be ascribed to its biological function as an estrogen antagonist, much of which is effected through progestin induced downregulation of the estrogen receptor itself. Estrogen risks are obviated by addition of progestins such as medroxyprogesterone acetate, which protects against development of endometrial hyperplasia(3;4), and when administered in a combined low dose oral contraceptive formulation may reduce endometrial cancer risk to 0.2-0.5 times that of the population background(5;6). The fact that these stable risk reductions occur irrespective of the presence or absence of histologically diagnosable premalignant lesions suggest that they act in part through subclinical, or previously unrecognized changes in the target tissue which occur prior to development of morphologic alterations visible by routine microscopy.
We have postulated that hormonal and mutational mechanisms are linked in the very earliest stages of endometrial carcinogenesis through the selective effects of hormones upon genetically defective compared to intact endometrial cells. Initial mutations are probably not rate limiting(7), as a high burden of somatic mutation is accrued in histologically normal endometrial tissues as random events during monthly regeneration(8), a process that generates kilos of new endometrial tissue throughout the reproductive years. These first events of endometrial carcinogenesis occur with sufficient frequency that they should be considered a part of normal endometrial biology rather than a pathologic state. We have designated these somatically mutated endometrial clones as “latent precancers” because they constitute a preclinical phase of disease detectable only with specialized biomarkers in which additional changes in the affected cells are required before they develop any histologic phenotype, or even clinically measurable increased cancer risk(8). Hormonal selection of pre-existing mutated clones is one possible mechanism of risk stratification which explains known hormonal epidemiologic and molecular genetic data in a unified model.
The PTEN tumor suppressor gene is a useful biomarker for the earliest stages of carcinogenesis, as it is mutated in up to 83% of sporadic endometrioid endometrial cancers(9), induces endometrial cancer upon inactivation in mice(10), and is known to be inactivated well in advance of development of established disease. 43% of normal premenopausal naturally cycling women have small numbers of immunohistochemically detected PTEN deficient endometrial glands which when microdissected have been shown to bear acquired mutations and deletions of the PTEN gene itself(8). Progression from this stage to carcinoma must be extremely inefficient, as the lifetime risk of endometrial cancer is only 2.61%(11).
There is evidence that latent precancers specifically defective in the PTEN gene, are linked to hormonal modification of endometrial cancer risk. PTEN-defective latent endometrial precancers maintain high levels of nuclear estrogen and progesterone receptors(8). Physiologic expression of endometrial gland PTEN protein is greatest in a mitotic, estrogen-rich environment, when its tumor suppressor functions are required to control the rate of cell division(12). Under estrogen stimulation PTEN mutant cells would thus be expected to have a selective proliferative advantage which is lost upon progestin exposure, when even genetically intact glands shut down PTEN expression. Under conditions of a normal monthly menstrual cycle the progestin exposures are insufficient to ablate latent precancers, only 17% of which disappear a year later(8). If the dose and duration of progestins are increased to therapeutic levels, PTEN mutant latent precancers undergo a 90% rate of involution, thereby resetting the carcinogenesis “clock”(13).
The current study further tests the hypothesis that ablation of pre-existing PTEN-defective endometrial latent precancers is a potential mechanism for progestin reduction of endometrial cancer risk. From our previous work, we know that immunohistochemically PTEN-null glands are likely to harbor PTEN mutations and/or deletions(8;9). Therefore, we used highly sensitive PTEN immunohistochemistry to detect changes in the prevalence of latent precancers which occur over time in the endometria of women undergoing defined hormonal therapies. Latent precancer rates were determined pre and post therapy for women undergoing low dose intermittent oral progestin therapy, and high dose local progestin delivery by an intrauterine device. Results are compared with a comparable age group undergoing routine gynecologic care in the perimenopausal period, and data from premenopausal naturally cycling women.
MATERIALS AND METHODS
Patient Selection
Women in Northern Norway (Tromso region) with successive endometrial biopsies taken under different hormonal conditions form the three main experimental groups of this study. Clinical aspects of these patients have been published elsewhere(14;15), but will be summarized here. Patients presenting with symptomatic endometrial bleeding and a diagnosis of endometrial hyperplasia had an intake endometrial biopsy followed by treatment either with a progestin impregnated intrauterine device (Group 1, IUD releasing 20 micrograms of levonorgestrel per day (Levonova device, Schering, Turku, Finland) or systemic oral progestin (Group 2, medroxyprogesterone 10 mg) administered daily for 10 days a month and repeated for 3 months. Patients were rebiopsied after receiving therapy. A comparison group (Group 3) of women with successive biopsies undergoing clinical surveillance for management of perimenopausal symptoms and signs constituted a progestin “untreated” group from the same patient population. Just under half (10 of 22 reported in results) received low dose hormonal replacement therapy as follows: norestrin 1mg/estradiol 2mg daily (6 patients), estriol 1-2mg daily (3 patients), tibolone 2.5 mg daily (1patient), no therapy (12 patients). All patient materials were compiled from existing pathologic tissues generated as part of routine patient care (“discarded materials”), except for the IUD group, which was consented in advance. The study was approved by the local ethical committee at the University of Tromso, by the Norwegian council of medical advice, the Norwegian Medicines Agency, and the Brigham and Women’s Hospital Human Studies Committee.
Two additional groups of untreated patients were assembled for comparison. These were women with incidental endometrial biopsies, or endometrial sampling performed for a variety of reasons (vaginal bleeding, uterine fibroids) where the endometrial histology was unremarkable. From Norway, pathology report and medical record review identified histologically normal proliferative endometrial biopsies from endogenously cycling premenopausal women without a recent or concurrent history of supplemental hormone use (Group 4). These were available only as single biopsies, without multiple sample points over time. Second, data from repeat biopsies of histologically normal proliferative endometrium of premenopausal endogenously cycling women in Boston, USA (Group 5) were made available for comparison from the original study that established baseline long-term persistence rates of morphologically unremarkable PTEN-null clones in normal cycling endometrium(8).
Pathology Materials
Pre and post-treatment archival paraffin embedded blocks containing endometrial biopsy tissue were available for 26 IUD treated, 30 oral progestin treated, and 28 untreated control patients and single blocks from 99 proliferative reference patients. All post-treatment biopsies were obtained while still on hormonal therapy. Tissue amount was deemed adequate if a minimum of 5 endometrial glands, or equivalent quantities of dislodged endometrial epithelium were identified. Of these, 22 were rejected because of inadequate amounts of endometrial tissue to perform immunohistochemistry, 1 control because of active progestin implant (Implanon) treatment, and 8 were rejected because of high background or other artifact during PTEN immunohistochemistry itself. This left paired biopsies from 21 IUD treated, 28 oral progestin treated, and 22 untreated control patients and single blocks from 87 proliferative reference patients for whom complete diagnostic, sampling interval, and immunohistochemical results are available and reported in the results section.
Pathologist diagnostic review using EIN (Endometrial Intraepithelial Neoplasia) criteria
Slides were diagnosed according to EIN terminology by a gynecologic pathologist (GM) using published criteria(16;17). Areas diagnosed as EIN were required to meet four criteria: 1)Area of glands exceeds area of stroma; 2)when a localizing lesion is present, epithelial cells within the architecturally crowded focus was cytologically different compared to background; 3)area meeting architectural and cytologic criteria must have a minimum size of 1mm; 4)exclusion of mimics and carcinoma. Endometria diagnosed as anovulatory had proliferative glands with focal cystic dilatation or branching, with or without associated vascular thrombi and stromal breakdown, corresponding to persistent proliferative endometria (disordered proliferative pattern) and endometrial hyperplasia without cytologic atypia. Endometrial polyps are localizing lesions that met at least two of the following three diagnostic criteria in an area confirmed to be endometrial functionalis; 1)irregular gland architecture, 2)altered stroma; 3)thick walled vessels.
PTEN immunohistochemistry
Paraffin sections of endometrial biopsy and curetting specimens were rehydrated, and underwent antigen retrieval by microwave before overnight (4′C) incubation with 1:300 murine monoclonal anti-PTEN antibody 6h2.1 (Cascade Biosciences, Winchester, MA) as previously described(8). Slides were washed, and incubated with appropriate secondary biotinylated immunoglobulin (Vectastain ABC kit, Vector Laboratories, Inc., Burlingame, CA) and signal detected by sequential addition of avidin peroxidase and 3,3′-diaminobenzidine. Slides were counterstained by methyl-green and coverslipped.
Each block underwent PTEN staining in independent duplicate immunohistochemical runs that included standard known slides as a run quality control. Staining adequacy was assessed by internal positive control staining in the slide of interest (normal endometrial stroma), a negative control of each slide in which primary antibody had been omitted, and review of run controls. PTEN status was scored visually (by GLM) as “PTEN null” if any endometrial glands devoid of PTEN protein were seen, and “PTEN normal” if all endometrial glands visualized expressed PTEN protein. Null glands were generally devoid of PTEN protein in all component cells, recognizable by reference to immediately adjacent PTEN-staining stroma.
Data Reduction
Data were entered into an excel spreadsheet which was imported into Systat (version 11, Systat Software, Inc., Point Richmond, CA) for statistical analysis.
RESULTS
Clinical and demographic characteristics of all patient groups are shown in Table 1. Histologic diagnosis of intake biopsies is shown in Table 2.
Table 1. Clinical characteristics of patient groups.
| Group # | Group Name | n | Treatment | Biopsies | Site | Age,mean (median)/range |
|---|---|---|---|---|---|---|
| 1 | IUD | 21 | progestin-impregnated IUD | pre-post | Norway | 49 (50)/33-62 |
| 2 | p-cycle | 28 | cyclic oral medroxyprogesterone | pre-post | Norway | 48 (49)/32-58 |
| 3 | Control | 22 | perimenopause surveillance | sequential | Norway | 53 (51) /37-93 |
| 4 | PE1 | 87 | normal proliferative, endogenous cycle | single | Norway | 42 (43)/29-52 |
| 5 | PE2 | 34 | normal proliferative, endogenous cycle | sequential | Boston | 41 (42)/24-52 |
Table 2. Intake endometrial histopathology of patient groups.
| Pathology Diagnosis | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|---|
| IUD | p-cycle | Control | PE1 | PE2 | Total | |
| Benign | 8 | 9 | 10 | 87 | 34 | 148 |
| EIN | 1 | 2 | 1 | 0 | 0 | 4 |
| Endometrial Polyp | 3 | 1 | 0 | 0 | 0 | 4 |
| Unopposed estrogen effect | 9 | 16 | 11 | 0 | 0 | 36 |
| Total | 21 | 28 | 22 | 87 | 34 | 192 |
Patient age was similar within the separate perimenopausal (Groups 1-3, average 48-53 years) and premenopausal (Groups 4-5, average 41-42 years) groups, but as expected, differ between them (ANOVA p<0.001). Using all 192 patients reported in this study we tested whether there was a correlation between decade of age at time of first available biopsy and the PTEN status of that biopsy. The proportion of PTEN-null glands at initial presentation did increase steadily with decade of age from a low of 32% in women less than 30 years old, to a high of 53% in women over 50, but the magnitude of the effect was not significant (Cochran’s Test of Linear Trend, p=0.522).
The prevalence of latent precancers at initial presentation (no treatment) was similar between all five groups studied (Chi-Square p=0.077). The proportion of latent precancers in followup biopsies, however, showed significant differences between the 4 available groups(Chi-Square p=0.002), suggesting a possible effect of intervention.
Sequential sampling before and after an interval of treatment provided an initial reference point (pre-treatment) to determine the overall magnitude and significance of a post-treatment effect. This was analyzed by pre and post therapy group comparison of latent precancer proportions (Table 3). “Post therapy” refers to samples obtained following a specified duration of hormonal treatment, while therapy was still being administered. The only group which showed a significant change overall in the proportion of PTEN-null endometria over time is the IUD treated patients (Fishers exact p<0.001). 62% of pre-treatment endometria contained PTEN null glands, declining to only 5% after an average of 49 days of treatment with the progestin impregnated IUD.
Table 3. Prevalence of PTEN-null endometria by groups.
| Group # | Group Name | n | Pre-Treatment %(n) | Post-Treatment % (n) | Interval, mean (median)/range | p, (Fisher exact) unpaired |
|---|---|---|---|---|---|---|
| 1 | IUD | 21 | 62 (13) | 5 (1) | 251 (164) /38-1142 | <0.001 |
| 2 | p-cycle | 28 | 68 (19) | 39 (11) | 332 (154) /38-1248 | 0.060 |
| 3 | Control | 22 | 41 (9) | 18 (4) | 442 (252) /43-1601 | 0.185 |
| 4 | PE1 | 87 | 49 (43) | nd | 0 | nd |
| 5 | PE2 | 34 | 35 (12) | 50 (17) | 401 (400)/26-1167 | 0.327 |
| Totals | 50 (96/192) | 31 (33/105) | ||||
| Chi-square | p=0.077 | p=0.002 |
The pattern of change in latent precancers in paired samples over time in individual patients provides some insights into the balance of PTEN-null gland emergence, persistence, and regression events within each treatment group (Table 4). 29-50% of patients in each group had no PTEN-null clones at any time during the study. Those patients who had a minimum of one biopsy with a latent precancer were classified as: 1)emergent, when only the second biopsy contained a latent precancer, 2)persistent, when both the first and second samples has a latent precancer (Figure 1A), and 3)regressed, when a latent precancer seen in the first biopsy was absent in the second (Figure 1B). Highest regression rates were seen in the IUD group, where 62% of all patients had a latent precancer at intake, and all of these regressed by the second biopsy (chi square p<0.001). Highest emergence rates were seen in the (untreated) groups of normal endogenously cycling proliferative endometria (Group 5, PE2), where 21% of all patients developed a new latent precancer during followup, but this was not statistically significant (chi square p=0.116). Persistence rates were highest in the endogenously cycling proliferative endometria (29%), and the cyclical low dose oral progestin perimenopausal (35.7) group (chi square p=0.006).
Table 4. Latent precancer absence, emergence, persistence, and regression in successive samples of individual patients.
Each patient was assigned to one of four groups depending on the presence or absence of immunohistochemically detected PTEN-null glands (latent precancers) in successive (first and second, as shown in Figure 1) biopsies over time: Absence (first and second biopsies without PTEN-null glands), emergence (PTEN null glands in second, but not first biopsy), persistence (PTEN-null glands in both first and second biopsy), and regression (PTEN-null glands seen in first biopsy are not seen in second). For each group (row), the percentage of patients with latent precancers having a pattern of absence, emergence, persistence, or regression is shown.
| Group # | Group Name | n | Absence % (n) | Emergence % (n) | Persistence % (n) | Regression % (n) |
|---|---|---|---|---|---|---|
| 1 | IUD | 21 | 33.3 (7) | 4.8 (1) | 0.0 (0) | 61.9 (13) |
| 2 | p-cycle | 28 | 28.6 (8) | 3.6 (1) | 35.7 (10) | 32.1 (9) |
| 3 | Control | 22 | 50.0 (11) | 9.1 (2) | 9.1 (2) | 31.8 (7) |
| 5 | PE2 | 34 | 44.1 (15) | 20.6 (7) | 29.4 (10) | 5.9 (2) |
| Total | Total | 105 | 39.0 (41) | 10.5 (11) | 21.0 (22) | 29.5 (31) |
| Pearson chi-sq | 0.383 | 0.116 | 0.006 | <0.001 |
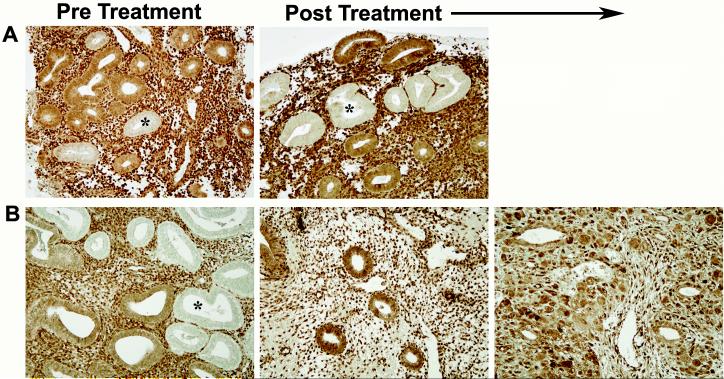
Figure 1.
Fate of latent endometrial precancers under differing hormonal conditions.
Panel A. Persistence of PTEN-null glands between initial biopsy and that taken after 69 days of interrupted low dose oral progestin therapy in a 53 year old woman. Panel B: Regression of PTEN-null glands following 491 days of treatment with a progestin impregnated IUD. Secretorily exhausted glands (center) and pronounced pseudodecidual stromal change (right), characteristic features of a high dose progestin response, are seen after therapy. Asterisk indicates an example of PTEN-null glands. PTEN immunohistochemistry with antibody 6h2.1.
DISCUSSION
Changes in endometrial latent precancer rates, as detected by PTEN immunohistochemistry, were studied in untreated controls, and women either taking cyclic low dose oral progestins or having placement of a progestin impregnated IUD. All groups initially had comparable proportions of patients whose endometria contained PTEN null-glands, but these diverged significantly after therapy. The progestin impregnated IUD group, which delivered the highest local dose of progestins for the longest period, experienced a strong trend towards regression of pre-existing latent precancers, with all latent precancers seen at intake disappearing on post-therapy followup. Involution rates were modest and statistically insignificant for the other treatment and control groups.
Progestins are capable of inducing dose dependent apoptotic cell death of neoplastic endometrial cells grown in culture, but with a rapid extinction over a period of a few days. Subsequent withdrawal is accompanied by resumption of apoptotic cell death on a scale several orders of magnitude greater than achieved in the preceding steady state(18). Dose and schedule of administration therefore interact in defining the net effect. Local delivery of progestins by placement of an impregnated intrauterine device provides a very high endometrial concentration of hormone while diminishing the complications of systemic distribution. Such devices have even been effective in treatment of established well differentiated endometrial adenocarcinoma(19), and present non-surgical alternative therapies for management of premalignant endometrial lesions.
Declines in pre-existing latent precancers were not seen in endogenously driven normal menstrual cycles, and cyclic oral administration of low dose progestins. It is entirely possible that the cancer protective effects seen in low dose oral progestin and combination contraceptive administration require a longer duration to achieve than the relatively short term followup in this study. There was a measurable, but not statistically significant difference, however, in the emergence of new latent precancers during followup between these groups, with 21% of all cycling proliferative patients developing emergent latent precancers during followup, compared to only 4-9% in the more quiescent endometria of the three perimenopausal groups (Groups 1,2,3). This is no surprise as random mutagenesis, the presumed mechanism of origin for most latent precancers, is expected to occur in proportion to the mitotic activity of the source tissue(7;20).
Despite a high rate of acquired PTEN mutation in histologically normal tissues, and a correspondingly low lifetime incidence of endometrial cancer, there are good reasons to link inactivation of PTEN to endometrial carcinogenesis. PTEN knockout mice develop endometrial carcinoma in 20% of cases.(10) PTEN mutation is the most common genetic defect in endometrioid endometrial adenocarcinomas.(21) Carry-forward of exact PTEN mutations seen in precancers to subsequent cancers in the same patient establishes lineage continuity between premalignant and malignant phases of disease(9). Loss of PTEN tumor suppressor functions including Akt-dependent enabling of apoptosis and control of cell division rates, confer proliferative and survival advantages long associated with the neoplastic phenotype(1).
We have previously used immunohistochemistry as a tool for detection of PTEN protein null endometrial glands, and know that these are due to irreversible changes in the structure of the PTEN gene itself, rather than transient alteration of protein expression from an intact gene. In our hands, loss of PTEN protein as assessed by immunohistochemistry is highly associated with presence of mutations of the PTEN gene or deletions of the 10q23 locus for PTEN(8;9;22;23). In the case of small numbers of paraffin embedded endometrial glands isolated by laser capture microdissection, we have found that all PTEN expressing glands identified by immunohistochemistry have a wild-type (normal) genotype, whereas 84% of non-expressing samples have either a mutation or loss of at least one 10q23 heterozygous marker in the region of the PTEN locus(8). A key aspect of achieving such a high concordance between genetic inactivating events and loss of protein by immunohistochemistry is use of appropriate antibodies (not all commercially available reagents meet this standard(24)).
Sampling error must be considered as a variable in monitoring of latent precancers for purposes of defining their fate in response to interventional therapies. Typically only a very small number of affected glands are present in a single biopsy, representing less than 2-3% of all present. While this is an advantage in providing abundant positively staining glands within the same specimen for interpretive comparison of loss of signal, they may be missed in a single specimen due to simple sampling error. This must account for some fraction of “emergent” precancers which were seen after therapy but missed at intake. For this reason, we have described these events as relative comparisons between therapeutic subgroups of patients which share the same sampling errors.
A potential problem in studying physically small premalignant lesions is that the biopsy itself may be destructive, removing the affected cells exclusive of a treatment effect. Data of persistence over time in normal proliferative endometrium suggests that sufficient PTEN-null glands usually remain after biopsy to be detected in future samples, as fully 83% (10 of 12) of those patients having a latent precancer at one timepoint will have a discernible latent precancer during followup(8). As was the case with sampling error, comparison between groups of differently managed patients undergoing similar tissue sampling has controlled this variable.
Lastly, consistently defined hormonal exposures, such as those likely to change endometrial cancer risk, are difficult to achieve outside the controlled setting of a clinical trial. This was a problem in a previous retrospective study that showed a 90% rate of PTEN-null endometrial precancer involution, in response to vary doses and intervals of medically administered progestins(13). The current study makes use of a systematic approach to therapy to generate more consistent groups for comparison.
This is a study exploring those biologic events which are effective during a preclinical phase and have the potential to alter the course of subsequent disease. Ultimately, long term patient outcomes, including a protracted followup interval after withdrawal of therapeutic hormones are an unmatchable gold standard for establishing therapeutic efficacy. We have shown that short term progestin administration, previously associated with long-term favorable clinical outcomes (cancer risk reduction), can increase the rate of involution of latent precancers while on therapy. This provides additional evidence that the preclinical phases of carcinogenesis have accessible short term laboratory endpoints that may be assessed to evaluate the impact of a variety of cancer prevention therapies.
Acknowledgments
This work was supported by NIH grant RO1-CA92301 (G. Mutter)
REFERENCES
- (1).Mutter GL. PTEN, a protean tumor suppressor. Am J Pathol. 2001;158:1895–8. doi: 10.1016/S0002-9440(10)64656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Parazzini F, La Vecchia C, Bocciolone L, Franceschi S. The epidemiology of endometrial cancer. Gynecol Oncol. 1991;41:1–16. doi: 10.1016/0090-8258(91)90246-2. [DOI] [PubMed] [Google Scholar]
- (3).Woodruff JD, Pickar JH. Incidence of endometrial hyperplasia in postmenopausal women taking conjugated estrogens (Premarin) with medroxyprogesterone acetate or conjugated estrogens alone. Am J Obstet Gynecol. 1994;170:1213–23. doi: 10.1016/s0002-9378(94)70129-6. [DOI] [PubMed] [Google Scholar]
- (4).Writing Group for the PEPI Trial Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275:370–5. doi: 10.1001/jama.1996.03530290040035. [DOI] [PubMed] [Google Scholar]
- (5).Stanford JL, Brinton LA, Berman ML, Mortel R, Twiggs LB, Barrett RJ, et al. Oral contraceptives and endometrial cancer: Do other risk factors modify the association. Int J Cancer. 1993;54:243–8. doi: 10.1002/ijc.2910540214. [DOI] [PubMed] [Google Scholar]
- (6).Weiderpass E, Adami HO, Baron JA, Magnusson C, Lindgren A, Persson I. Use of oral contraceptives and endometrial cancer risk (Sweden) Cancer Causes Control. 1999 Aug;10(4):277–84. doi: 10.1023/a:1008945721786. [DOI] [PubMed] [Google Scholar]
- (7).Cairns J. Mutation and cancer: the antecedents to our studies of adaptive mutation. Genetics. 1998 Apr;148(4):1433–40. doi: 10.1093/genetics/148.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Mutter GL, Ince TA, Baak JPA, Kust G, Zhou X, Eng C. Molecular identification of latent precancers in histologically normal endometrium. Cancer Res. 2001;61:4311–4. [PubMed] [Google Scholar]
- (9).Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JPA, Lees J, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–30. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- (10).Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/- mice. Cancer Res. 2000 Jul 1;60(13):3605–11. [PubMed] [Google Scholar]
- (11).Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 19752002. http://seer.cancer.gov/csr/1975_2002/2005. [Google Scholar]
- (12).Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Ziebold U, Eng C. Changes in endometrial PTEN expression throughout the human menstrual cycle. J Clin Endocrinol Metab. 2000;85:2334–8. doi: 10.1210/jcem.85.6.6652. [DOI] [PubMed] [Google Scholar]
- (13).Zheng W, Baker HE, Mutter GL. Involution of PTEN-Null Endometrial Glands with Progestin Therapy. Gynecol Oncol. 2004;92:1008–13. doi: 10.1016/j.ygyno.2003.11.026. [DOI] [PubMed] [Google Scholar]
- (14).Vereide AB, Arnes M, Straume B, Maltau JM, Orbo A. Nuclear morphometric changes and therapy monitoring in patients with endometrial hyperplasia: a study comparing effects of intrauterine levonorgestrel and systemic medroxyprogesterone. Gynecol Oncol. 2003 Dec;91(3):526–33. doi: 10.1016/j.ygyno.2003.07.002. [DOI] [PubMed] [Google Scholar]
- (15).Vereide AB, Kaino T, Sager G, Orbo A. Bcl-2, BAX, and apoptosis in endometrial hyperplasia after high dose gestagen therapy A comparison of responses in patients treated with intrauterine levonorgestrel and systemic medroxyprogesterone. Gynecol Oncol. 2005 May 6; doi: 10.1016/j.ygyno.2005.02.030. e-pub ahead of print May, 2005. [DOI] [PubMed] [Google Scholar]
- (16).Mutter GL, Duska L, Crum CP. Endometrial Intraepithelial Neoplasia. In: Crum CP, Lee K, editors. Diagnostic Gynecologic and Obstetric Pathology. 1 ed. Saunders; Philadelphia: 2005. pp. 493–518. [Google Scholar]
- (17).Silverberg SG, Mutter GL, Kurman RJ, Kubik-Huch RA, Nogales F, Tavassoli FA. Tumors of the uterine corpus: epithelial tumors and related lesions. In: Tavassoli FA, Stratton MR, editors. WHO Classification of Tumors: Pathology and Genetics of Tumors of the Breast and Female Genital Organs. 1 ed. IARC Press; Lyon, France: 2003. pp. 221–32. [Google Scholar]
- (18).Wang S, Pudney J, Song J, Schwartz PE, Zheng W. Mechanisms involved in the evolution of progestin resistence in human endometrial hyperplasia - Precursor of endometrial cancer. Gynecol Oncol. 2003;88:108–17. doi: 10.1016/s0090-8258(02)00008-2. [DOI] [PubMed] [Google Scholar]
- (19).Montz FJ, Bristow RE, Bovicelli A, Tomacruz R, Kurman RJ. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002 Apr;186(4):651–7. doi: 10.1067/mob.2002.122130. [DOI] [PubMed] [Google Scholar]
- (20).Jackson AL, Loeb LA. The mutation rate and cancer. Genetics. 1998 Apr;148(4):1483–90. doi: 10.1093/genetics/148.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Mutter GL, Ince TA. Molecular Pathogenesis of Endometrial Cancer. In: Fuller A, Seiden MV, Young R, editors. Uterine Cancer: American Cancer Society Atlas of Clinical Oncology. 1 ed. B.C.Decker; Hamilton,Ontario,Canada: 2004. pp. 10–21. [Google Scholar]
- (22).Gimm O, Perren A, Weng LP, Marsh DJ, Yeh JJ, Ziebold U, et al. Differential Nuclear and Cytoplasmic Expression of PTEN in Normal Thyroid Tissue, and Benign and Malignant Epithelial Thyroid Tumors. Am J Pathol. 2000 May;156(5):1693–700. doi: 10.1016/s0002-9440(10)65040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Perren A, Weng L, Boag A, Ziebold U, Thakore K, Dahia P, et al. Immunocytochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. Am J Pathol. 1999;155:1253–60. doi: 10.1016/S0002-9440(10)65227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Pallares J, Bussaglia E, Martinez-Guitarte JL, Dolcet X, Llobet D, Rue M, et al. Immunohistochemical analysis of PTEN in endometrial carcinoma: a tissue microarray study with a comparison of four commercial antibodies in correlation with molecular abnormalities. Mod Pathol. 2005 May;18(5):719–27. doi: 10.1038/modpathol.3800347. [DOI] [PubMed] [Google Scholar]