Abstract
The differential effects of insulin sensitivity and adiposity on androgen concentrations in women with polycystic ovary syndrome (PCOS) are unclear. To address this issue, we divided 43 overweight women into 4 groups based on both their clinical classification (PCOS or Normal), and whether they were insulin resistant (IR) or insulin sensitive (IS) by their steady-state plasma glucose (SSPG) concentrations. Total testosterone concentrations were significantly increased as a function of both clinical classification (PCOS vs. Normal, p < 0.0001) and SSPG concentration (IR vs., IS, p = 0.002). Mean testosterone concentrations were higher in PCOS-IR compared to either PCOS-IS, Normal-IR or Normal-IS women (p < 0.005). Additionally, there was a statistically significant interaction (p=0.03) between clinical classification (PCOS vs. Normal) and insulin sensitivity (IR vs. IS) for testosterone concentrations. In contrast, androstenedione concentrations were higher in women with PCOS (p=0.001), irrespective of whether they were IR or IS (p=0.31), and no interaction between clinical classification and insulin sensitivity was discerned (p=0.34). These results indicate that both PCOS and insulin resistance independently contributed to increased total testosterone concentrations within a group of overweight/obese women. These findings are consistent with the hypothesis that the ovaries of women with PCOS are hypersensitive to the ability of insulin to increase testosterone production, and the more insulin resistant the patient, the higher the testosterone concentration. In contrast, androstendione concentrations seem to be independent of differences in insulin resistance. Our findings emphasize the need to increase understanding of the factors that modulate ovarian androgen secretion.
Keywords: PCOS, Insulin resistance, Testosterone, Obesity
Introduction
Women with polycystic ovary syndrome (PCOS) are characterized by increased prevalence of insulin resistance (1–5) as well as obesity (6). Even though excess adiposity increases the likelihood of an individual becoming insulin resistant (7–10), not all obese women are insulin resistant (8–10), nor do all insulin resistant women develop PCOS (11). The complex relationship among obesity, insulin resistance, and the hyperandrogenic state in PCOS is still unclear. For example, differences in the ability of compensatory hyperinsulinemia in insulin resistant women to produce a hyperandrogenic state has been suggested to play a central role in determining who will, or will not, develop PCOS (13). Alternatively, others have emphasized that obesity is responsible for both insulin resistance and hyperandrogenism (14).
The goal of this study was to test that neither insulin resistance nor obesity, by themselves, lead to the development of PCOS. To address this issue, we recruited subjects who were similar for body mass index (BMI) in both healthy and PCOS women, and we measured insulin sensitivity directly by insulin-mediated glucose disposal (IMGU) rather than using surrogate measurements of insulin sensitivity such as HOMA-IR or QUICKI.
We created 4 groups that were similar for BMI, different insulin sensitivity and different disease status. Average plasma concentrations of total testosterone, free-testosterone, and androstenedione were compared among these 4 groups. This sampling design helped us to distinguish any associations of clinical diagnosis of PCOS or insulin resistance with androgen metabolism while minimizing confounding effect of obesity.
Materials and Methods
Subjects
The study sample consisted of 43 women, selected from a larger number of individuals who had volunteered in response to local newspaper advertisements describing two clinical research studies; one aimed at defining the relationship between obesity, insulin resistance, and cardiovascular disease, while the other was focused on the metabolic impact of an insulin sensitizing drug on insulin resistant women with PCOS. The women with a normal menstrual history were selected from a group of 57 obese individuals that had volunteered for a weight loss study, whereas the women with PCOS were selected from 42 subjects volunteering for our study evaluating the impact of rosiglitazone in women with PCOS. In both instances Stanford University Medical Center’s Institutional Review Board approved the experimental protocol, and informed consent was obtained before subjects were screened at the Medical Center’s General Clinical Research Center (GCRC). In order to qualify for enrollment, volunteers had to be in otherwise good health, with a BMI ≥ 25.0 kg/m2, a fasting plasma glucose concentration <126 mg/dL, and normal laboratory results on a routine blood and chemical screening panel. PCOS was defined according to the 1990 National Institute of Child Health and Human Development (NICHD) consensus criteria (15) as having oligomenorrhoea or amenorrhoea (eight or fewer menses per year, or ≥45 mean days between bleeding episodes), clinical (Ferryman-Gallway score ≥8) and biochemical evidence of hyperandrogenism, with normal serum thyroid-stimulating hormone and prolactin concentrations as determined in the Stanford Hospital Clinical Laboratory.
Study Procedures
Volunteers meeting the above general entry criteria were then admitted to the GCRC, where IMGU was quantified by a modification (16) of the insulin suppression test (IST) as described and validated by our research group (17, 18). Briefly, after an overnight fast and before infusion, baseline blood samples were taken and then subjects were infused for 180 minutes with octreotide (0.27µg/m2·min), insulin (32 mIU/m2·min), and glucose (267 mg/m2·min). Blood was drawn at 10-minute intervals during the final 30 minutes of the infusion to measure plasma glucose and insulin concentrations, and the mean of these repeated measurements was taken as an estimate of average steady-state plasma glucose (SSPG) concentrations within each individual; a higher SSPG concentration indicated a more insulin-resistant individual.
The result of the IST was used to define an individual as being either IR (SSPG concentration ≥ 180 mg/dL) or IS (SSPG concentration ≤ 100 mg/dL). These values represent the upper and lower tertiles of SSPG concentrations that were measured in 490 healthy volunteers in a previous study (19). Furthermore, results from two prospective studies have shown that dividing a group of apparently healthy individuals into the third with the highest SSPG concentrations (IR) and lowest SSPG concentrations (IS) define individuals at being either at increased (IR) or decreased (IS) risk to develop adverse consequences related to a loss of insulin sensitivity (20, 21).
Volunteers who met the criteria for being either IR or IS were then considered eligible to be included in this analysis, and plasma obtained from the overnight fasting sample from the IST used to measure concentrations of glucose, insulin and androgenic hormones . Based upon the results of the IST, we were able to enroll 23 (IR=11 and IS=12) women with PCOS and 20 women (IR=12 and IS=8) with a normal menstrual history (Normal-IS, Normal-IR). The ages of the study population ranged from 24–40 years, and their BMI values from 25.5 to 33.7 kg/m2
Plasma glucose and insulin concentrations were quantified as described previously (19–21), and determinations of testosterone (CV=2.8–6.8%), free-testosterone (CV=0.22–7.5 %), and androstenedione (CV=6.6–10.3 %) were made using ELISA kits (Diagnostic Systems Laboratories Inc. Texas)
Statistical Methods
Data on each experimental variable are summarized as the mean ± 1 standard deviation (SD). Group means for baseline characteristics (Table 1) were compared using t-tests. These comparisons were made without any adjustment for multiple comparisons to conserve power for assessing how well the case-control design was achieved. In contrast, a more conservative approach was taken in regard to Type I error for analysis of the androgen concentrations to minimize the possibility of false positive findings on study outcomes. Specifically, overall tests of differences in means among groups were evaluated using two-way analysis of variance (ANOVA). he two-way ANOVA model consisted of main effects for disease (PCOS versus Normal) and insulin sensitivity (IR versus IS) as well as their interaction. Where a significant interaction was detected, we tested “simple effects” (22) by comparing means between each pair of groups and we maintained an overall Type I error rate of 5% across the multiple simple effect comparisons within each outcome using sequential Bonferroni adjustment (23). Data for androgen concentrations met assumptions for ANOVA, being approximately normally distributed and homoscedastic. Statistical analyses were performed in Statview version 5 (SAS Institute, Inc., Cary, NC).
Table 1.
Demographic and Clinical Characteristics (mean ± SD)
| PCOS | Normal | |||
|---|---|---|---|---|
| IR (n=11) |
IS (n=12) |
IR (n=12) |
IS (n=8) |
|
| SSPG (mg/dL) | 232±25a | 80±18b | 224±33a | 76±21b |
| Age (years) | 32±5ab | 29±6a | 35±3c | 34±5bc |
| BMI (kg/m2) | 28.7±2.3abc | 28.4±2.3ad | 30.3±2.3bde | 30.8±1.8ce |
| Fasting Glucose (mg/dL) | 98±8ab | 92±8ac | 99±10bc | 85±3d |
| Fasting Insulin (mIU/L) | 18±9a | 7±5b | 16±7a | 7±3b |
Within each row of this table, means with different superscripts differ significantly at p < 0.05. PCOS: polycystic ovary syndrome, Normal: women with normal ovulation, IR: insulin resistance, IS: insulin sensitive, SSPG: steady state plasma glucose, BMI: body mass index.
Results
Demographic and Clinical Characteristics
By selection, SSPG concentrations were approximately three-fold higher in the PCOS-IR and Normal -IR as compared to the PCOS-IS and Normal-IS groups (Table 1). SSPG concentrations were higher to the same degree in the PCOS-IR and Normal-IR groups, and SSPG concentrations in the PCOS-IS and Normal- IS groups were also identical. The PCOS-IS subjects were younger, and the BMI were lower in both PCOS groups. Finally, consistent with the differences in SSPG concentrations, fasting plasma glucose and insulin concentration were significantly higher in the IR groups.
Androgen concentrations
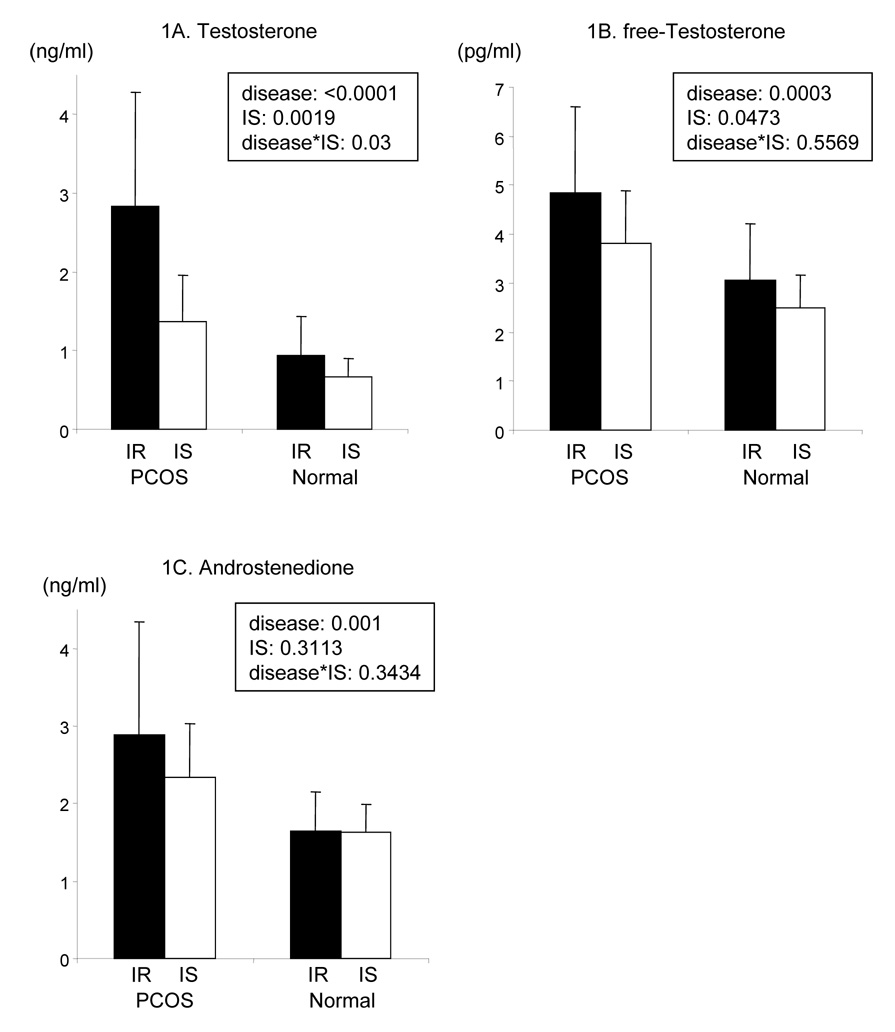
The data in Fig. 1A demonstrate that total testosterone concentrations were significantly increased (2.06 ± 1.3 vs. 083 ± 0.42 ng/mL, p>0.001) when the values in the two PCOS groups (PCOS-IR + PCOS-IS) were compared to the two Normal groups (Normal IR + Normal IS). Total testosterone concentrations were also higher in the IR individuals, irrespective of clinical diagnosis (p< 0.002). In addition, the interaction between disease state (PCOS vs. Normal) and insulin sensitivity (IR vs. IS) was statistically significant (p = 0.03). This interaction arose because the difference between IR and IS was much stronger in PCOS (difference in means = 1.46 ng/ml, standard error = 0.35 ng/ml) than in Normal (difference in means = 0.28 ng/ml, standard error = 0.39 ng/ml). Analysis of simple effects reveals that mean total testosterone concentrations were higher in PCOS-IR than in any other group: PCOS-IR vs. Normal-IR (difference in means = 1.88 ng/ml, standard error = 0.35 ng/ml, p = 0.0004), PCOS-IR vs PCOS-IS (difference in means = 1.46 ng/ml, standard error = 0.35 ng/ml, p = 0.0042), and PCOS-IR vs Normal-IS (difference in means = 2.16 ng/ml, standard error = 0.39 ng/ml, p = 0.0007). In addition, mean testosterone concentrations between Normal groups were not significantly different.
Figure 1. Androgen concentrations.
1A: plasma testosterone concentrations, 1B: plasma free-testosterone concentrations, 1C: plasma androstenedione concentrations. IR: insulin resistant women, IS: insulin sensitive women: PCOS: polycystic ovary syndrome, Normal: normally ovulating women, PCOS-IR (n=11), PCOS-IS (n=12), Normal-IR (n=12), Normal-IS (n=8), disease: comparison between PCOS and normally ovulating women, IS: comparison between insulin sensitive and insulin resistant women, disease*IS: interaction between disease state (PCOS vs. Normal) and insulin sensitivity. The statistical significance was calculated by two-way ANOVA.
The results in Fig. 1B indicate that free-testosterone concentrations were also significantly higher (p<0.005) in the two groups with PCOS as compared to the combined IR-Normal and IS- Normal groups (4.3 ± 1.5 vs. 2.8 ± 1.0). Total testosterone and free-testosterone concentrations were also increased as a function of being IR, (p<0.05). However, in contrast to total testosterone measurements, there was no statistically significant interaction between disease state and insulin sensitivity (p = 0.56).
In contrast to the changes in total testosterone and free-testosterone concentration, the results in Fig. 1C demonstrate that androstenedione concentrations were modulated only by disease status. Thus, androstenedione concentrations were significantly higher in those with PCOS (p = 0.001), irrespective of whether they were IR or IS (p=0.31), and no interaction between disease state and insulin sensitivity was discerned (p = 0.34).
Discussion
The most important pathophysiological finding in this study was the identification of significant interaction between disease state (PCOS vs. Normal) and insulin sensitivity (IR vs. IS) in the modulation of total plasma testosterone concentration. This finding is consistent with the fact that the highest total testosterone concentrations were seen in the PCOS-IR group, and the lowest levels in Normal-IS individuals. The significant interaction between the presence of PCOS and insulin resistance is consistent with the notion of ovarian hypersensitivity to insulin stimulation of testosterone production in women with PCOS as recently proposed by Baillargeon and Nestler (25). Their suggested formulation provides an explanation for the fact that total and free testosterone concentrations were increased in the PCOS-IS patients in this study, as well as the demonstration by Baillargeon and Carpentier (26) that androgen concentrations fall when lean, normoinsulinemic, insulin sensitive individuals with PCOS are given diazoxide; an agent that decreases insulin secretion. Implicit in these notions is that insulin stimulates ovarian androgen secretion, and that this can be shown to occur in vitro using ovarian androgen tissue from women with PCOS (27–30). Our findings clearly do not provide additional information regarding direct ovarian responses to insulin or androgen signaling, but the notion of ovarian hypersensitivity to insulin stimulation in women with PCOS provides a reasonable explanation for why the present study finds interaction between insulin resistance/hyperinsulinemia and the presence of PCOS in modulation of plasma testosterone concentrations.
The results in Fig. 1B indicate that free-testosterone concentrations were also higher, both as a function of disease state (PCOS vs. Normal) and insulin sensitivity (IR vs. IS), but in this instance we could not discern a significant degree of interaction between disease state and insulin sensitivity. Thus, although these results are similar to the total testosterone findings in terms of the significant effects on androgen concentrations of disease state and insulin sensitivity, they differ in the lack of evidence of significant interaction. The reason for this latter discrepancy is not clear, but might result from something as simple as our use of a free-testosterone assay that does not have the precision required to discern interaction between disease and insulin sensitivity (31).
It should be noted that total and free-testosterone concentrations were higher in PCOS-IS women than in either of the Normal groups, and that despite being considered to be overweight/obese, with a mean BMI of 28.4 kg/m2, these patients remained quite insulin sensitive (mean SSPG concentration=80 mg/dL). Thus, in contrast to some published reports (11, 14), the results of this study demonstrate that obese women with PCOS can also be insulin sensitive, and, as a corollary, that normal insulin sensitivity in PCOS is not confined to nonobese women. As such, our results reinforce previous findings that obese women, in general, can be insulin sensitive, with low levels of insulin (8–10),
In contrast to the impact of differences in insulin sensitivity on testosterone and free-testosterone concentrations, androstenedione concentrations, as shown in Fig. 1C, only varied whether or not subjects had PCOS. Thus, androstenedione concentrations were significantly higher in PCOS-IR and PCOS-IS than in Normal-IR and Normal-IS (p=0.001), but were comparable within each disease group. These data are consistent with in vitro studies showing that LH-stimulated androstenedione secretion from cultured theca cells obtained from women with PCOS is enhanced as compared to cells from normal individuals (27, 30). However, it is not clear if insulin stimulation of androstenedione is greater in ovaries obtained from women with PCOS (30, 32). Similarly, there is disparity between our finding that androstenedione concentrations did not differ between PCOS-IR and PCOS-IS women, despite the marked difference in insulin resistance and insulin concentration, and the observation by Baillargeon and Carpentier that androstenedione concentrations fell in insulin sensitive women with PCOS in association with diazoxide inhibition of insulin secretion (26). The explanation for this disparity concerning the relationship between insulin and androstenedione concentrations is not readily apparent. In fact, the per cent change in androstenedione concentrations following diazoxide in the 9 patients studied by Baillargeon and Carpentier (26) was comparable to the difference we saw between PCOS-IR (n=11) to PCOS-IS (n=12) women. The fact that our comparison was cross-sectional and theirs was before and after the intervention in the same subject, may explain why they perceived a statistically significant difference and we did not. In any event, this difference should not obscure the fact that we both came to the conclusion that there is an interaction between the metabolic effects of insulin on ovarian androgen secretion and the presence of PCOS.
A major strength of the results of this study is that they are based on the use of a specific method to quantify insulin-mediated glucose uptake (IMGU), rather than such surrogate estimates as fasting plasma insulin concentration, plasma glucose × plasma insulin concentration, HOMA-IR, QUICKI, etc. (20, 31, 32). Although these surrogate estimates of insulin action are correlated to specific measures of IMGU, the relationships are relatively modest in magnitude, with correlation coefficients of ~0.6 (31–33); thereby accounting for no more that 36% of the actual variability in IMGU in the population studied. In contrast, the IST used in this study, and the hyperinsulinemic, euglycemic clamp technique, often referred to as the “gold standard” for quantifying IMGU are highly correlated (r >0.9) in normal women and patients with type 2 diabetes, both normal and obese (18). Thus, the method used to separate the subjects in this study on the basis of their degree of insulin sensitivity is quite precise; an advantage that is further accentuated by enrolling only the most insulin resistant and sensitive individuals, excluding those intermediate in terms of IMGU.
On the other hand, quantities of subjects in each of the four groups are relatively small, which may limit the ability to generalize from our results. However, our precise division of participants as to degree of insulin sensitivity, while also carefully selecting for a comparable degree of obesity in all four groups, increases the chances that our results can be confirmed in larger studies. Despite these shortcomings, we believe that the results presented provide useful information concerning the relationship between obesity, insulin resistance, and PCOS.
In conclusion, our results suggest that obesity, insulin resistance, and compensatory hyperinsulinemia are neither necessary nor sufficient for the development of PCOS. Thus, women who are extremely insulin resistant/hyperinsulinemic can have a normal menstrual history. Furthermore, the diagnosis of PCOS in the presence of normal insulin sensitivity is not confined to nonobese women, and some patients with PCOS, despite being overweight/obese, remain very insulin sensitive with low plasma insulin concentrations (PCOS-IS). The simplest interpretation of these data is that the ovaries of women with PCOS are hypersensitive to the ability of insulin to increase testosterone production, and the more insulin resistant /hyperinsulinemic an individual, the higher will be the total testosterone and free-testosterone concentrations. The ovaries of women with PCOS also seem to be hyper-responsive to LH-stimulation of androstenedione secretion, but this abnormality seems to be independent of differences in insulin resistance/hyperinsulinemia. These results emphasize the need to increase understanding of the factors that modulate ovarian androgen secretion.
Acknowledgments
Supported in part by National Institutes of Health Grant RR-000700
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 2.Holte J. Disturbances in insulin secretion and sensitivity in women with the polycystic ovary syndrome. Bailliere’s Clin Endocrinol Metab. 1996;10:221–247. doi: 10.1016/s0950-351x(96)80085-1. [DOI] [PubMed] [Google Scholar]
- 3.Dunaif A. Insulin resistance and the polycystic ovary syndrome; mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 4.Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:2694–2698. doi: 10.1210/jcem.83.8.5054. [DOI] [PubMed] [Google Scholar]
- 5.Godarzi MO, Korenman SG. The importance of insulin resistance in polycystic ovary syndrome. Fertil Steril. 2003;80:255–258. doi: 10.1016/s0015-0282(03)00734-9. [DOI] [PubMed] [Google Scholar]
- 6.Salehi M, Bravo-Vera R, Sheikh A, Gouller A, Poretsky L. Pathogenesis of polycystic ovary syndrome: what is the role of obesity? Metabolism. 2004;53:358–376. doi: 10.1016/j.metabol.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Olefsky J, Reaven GM, Farquhar JW. Effects of weight reduction on obesity. doi: 10.1172/JCI107560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Studies of lipid and carbohydrate metabolism in normal and hyperlipoproteinemic subjects. J Clin Invest. 1974;53:64–76. doi: 10.1172/JCI107560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reaven GM. Obesity, insulin resistance, and cardiovascular disease. Recent Progress. Horm Res. 2004;59:207–223. doi: 10.1210/rp.59.1.207. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–809. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin T, Allison G, Abbasi F, Lamendola C, Reaven G. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism. 2004;53:495–499. doi: 10.1016/j.metabol.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Acien P, Quereda F, Matallin P, Villarroya E, Lopez-Fernandez JA, Acien M, Mauri M, Alfayate R. Insulin, androgens, and obesity in women with and without polycystic ovary syndrome: a heterogeneous group of disorders. Fertil Steril. 1999;72:32–40. doi: 10.1016/s0015-0282(99)00184-3. [DOI] [PubMed] [Google Scholar]
- 13.Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980;50:113–116. doi: 10.1210/jcem-50-1-113. [DOI] [PubMed] [Google Scholar]
- 14.Nestler JE. Insulin regulation of human ovarian androgens. Hum Reprod 12 Suppl. 1997;1:53–62. doi: 10.1093/humrep/12.suppl_1.53. [DOI] [PubMed] [Google Scholar]
- 15.Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2002;26:883–896. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- 16.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: a rational approach. In: Dunaif A, Haseltine F, Merriam GR, editors. Polycystic Ovary Syndrome. Cambridge, MA: Blackwell Scientific; 1992. pp. 3773–3784. [Google Scholar]
- 17.Pei D, Jones CN, Bhargava R, Chen YD, Reaven GM. Evaluation of octreotide to assess insulin-mediated glucose disposal by the insulin suppression test. Diabetologia. 1994;37:843–845. doi: 10.1007/BF00404344. [DOI] [PubMed] [Google Scholar]
- 18.Shen SW, Reaven GM, Farquhar JW. Comparison of impedance to insulin-mediated glucose uptake in normal subjects and in subjects with latent diabetes. J Clin Invest. 1970;49:2151–2160. doi: 10.1172/JCI106433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenfield MS, Doberne L, Kraemer F, Tobey TA, Reaven GM. Assessment of insulin resistance with the insulin suppression test and the euglycemic clamp. Diabetes. 1981;30:387–392. doi: 10.2337/diab.30.5.387. [DOI] [PubMed] [Google Scholar]
- 20.Yeni-Komshian H, Carantoni M, Abbasi F, Reaven GM. Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in 490 healthy nondiabetic volunteers. Diabetes Care. 2000;23:171–175. doi: 10.2337/diacare.23.2.171. [DOI] [PubMed] [Google Scholar]
- 21.Yip J, Facchini FS, Reaven GM. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. J Clin Endocrinol Metab. 1998;83:2773–2776. doi: 10.1210/jcem.83.8.5005. [DOI] [PubMed] [Google Scholar]
- 22.Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86:3574–3578. doi: 10.1210/jcem.86.8.7763. Cochran WG, Cox GM. Experimental designs John Wiley & Sons, Inc., New York, NY. 1950. [DOI] [PubMed] [Google Scholar]
- 23.Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 24.Bethesda, MD: National Institutes of Health, United States Department of Health and Human Services; National Institutes of Health Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: the Evidence Report. 1998
- 25.Baillargeon J-P, Nestler JE. Polycystic ovary syndrome: A syndrome of ovarian hypersensitivity to insulin. J Clin Endocrinol Metab. 2005;91:22–24. doi: 10.1210/jc.2005-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baillargeon J-P, Carpentier A. Role of insulin in the hyperandrogenemia of lean women with polycystic ovary syndrome and normal insulin sensitivity. Fertil Steril. 2007;88:886–893. doi: 10.1016/j.fertnstert.2006.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab. 1994;79:1158–1165. doi: 10.1210/jcem.79.4.7962289. [DOI] [PubMed] [Google Scholar]
- 28.Willis D, Mason H, Gilling-Smith C, Franks S. Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J Clin Endocrinol Metab. 1996;81:302–309. doi: 10.1210/jcem.81.1.8550768. [DOI] [PubMed] [Google Scholar]
- 29.Franks S, Gharani N, Gilling-Smith C. Polycystic ovary syndrome: evidence for a primary disorder of ovarian steroidogenesis. J Steroid Biochem Mol Biol. 1999;69:269–272. doi: 10.1016/s0960-0760(99)00044-8. [DOI] [PubMed] [Google Scholar]
- 30.Franks S, Gilling-Smith C, Watson H, Willis D. Insulin action in the normal and polycystic ovary. Endocrinol Metab Clinics North Am. 1999;28:361–378. doi: 10.1016/s0889-8529(05)70074-8. [DOI] [PubMed] [Google Scholar]
- 31.Miller KK, Rosner w, Lee H, et al. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocrinol Metab. 2004;89:525–533. doi: 10.1210/jc.2003-030680. [DOI] [PubMed] [Google Scholar]
- 32.Nestler JE, Jakubowicz DJ, de Vargas AF, et al. Insulin stimulatesd testosterone biosynthesis by human theca cells from women with polycystic ovary syndrome by activatin ints own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998;83:2001–2205. doi: 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- 33.Abbasi F, Reaven GM. Evaluation of the quantitative insulin sensitivity check index as an estimate of insulin sensitivity in humans. Metabolism. 2002;51:235–237. doi: 10.1053/meta.2002.28970. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Abbasi F, Reaven GM. Impact of degree of obesity on surrogate estimates of insulin resistance. Diabetes Care. 2004;27:1998–2002. doi: 10.2337/diacare.27.8.1998. [DOI] [PubMed] [Google Scholar]