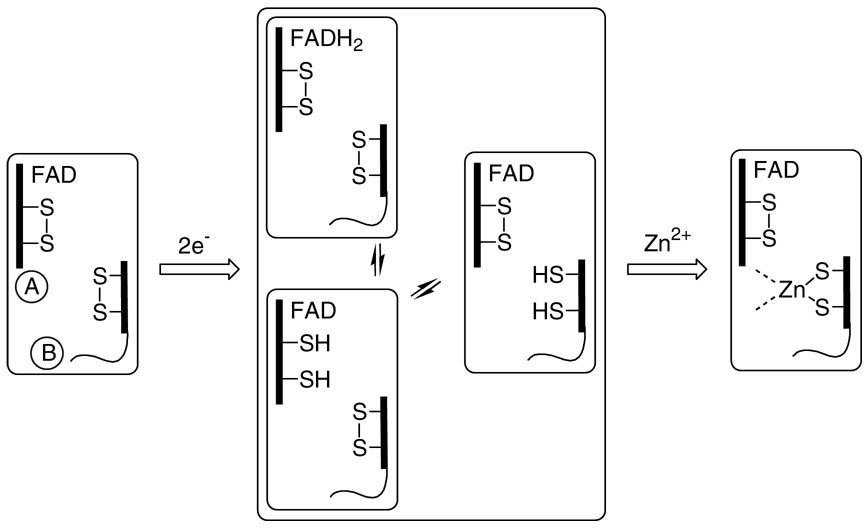
SCHEME 1.
Two electron reduction of Erv2p generates a Zn2+ binding site and modulates reductive titration of the protein. Only representative states for 2-electron reduced Erv2p are shown. A redox-active CxC disulfide at the C terminus of the B subunit reacts across the subunit interface of the homodimer with the proximal disulfide of the A subunit (see also Figure 1).