Abstract
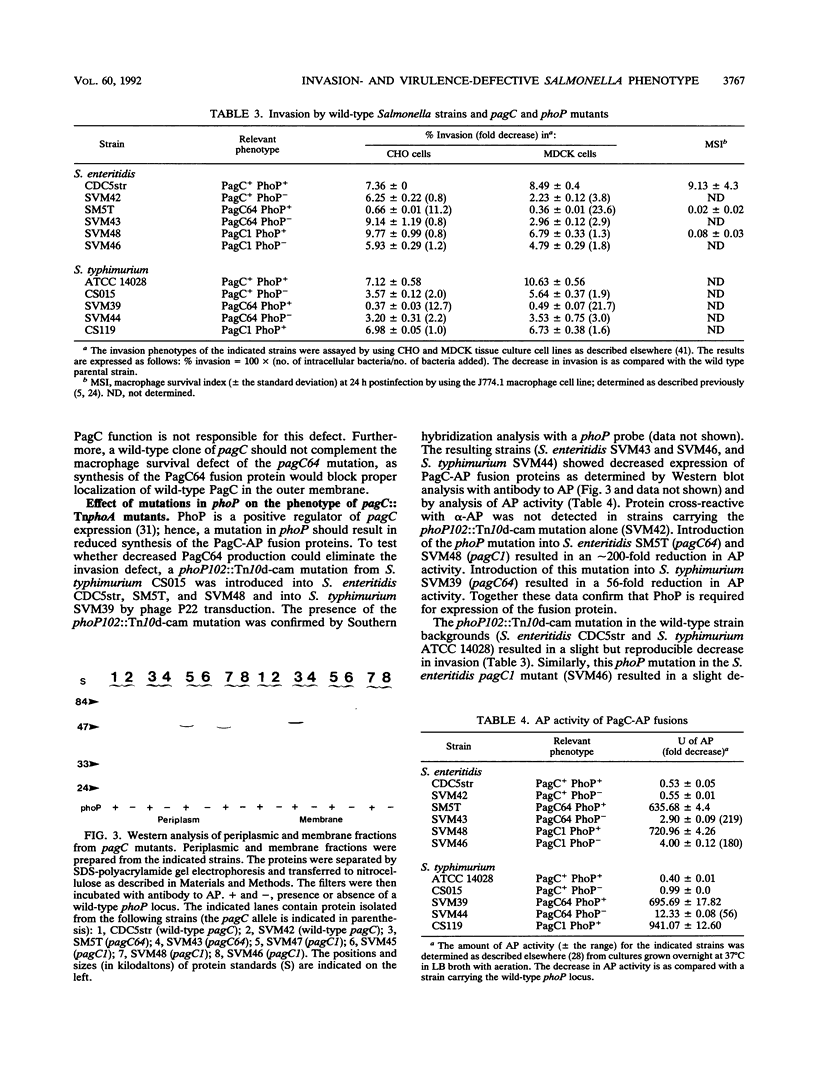
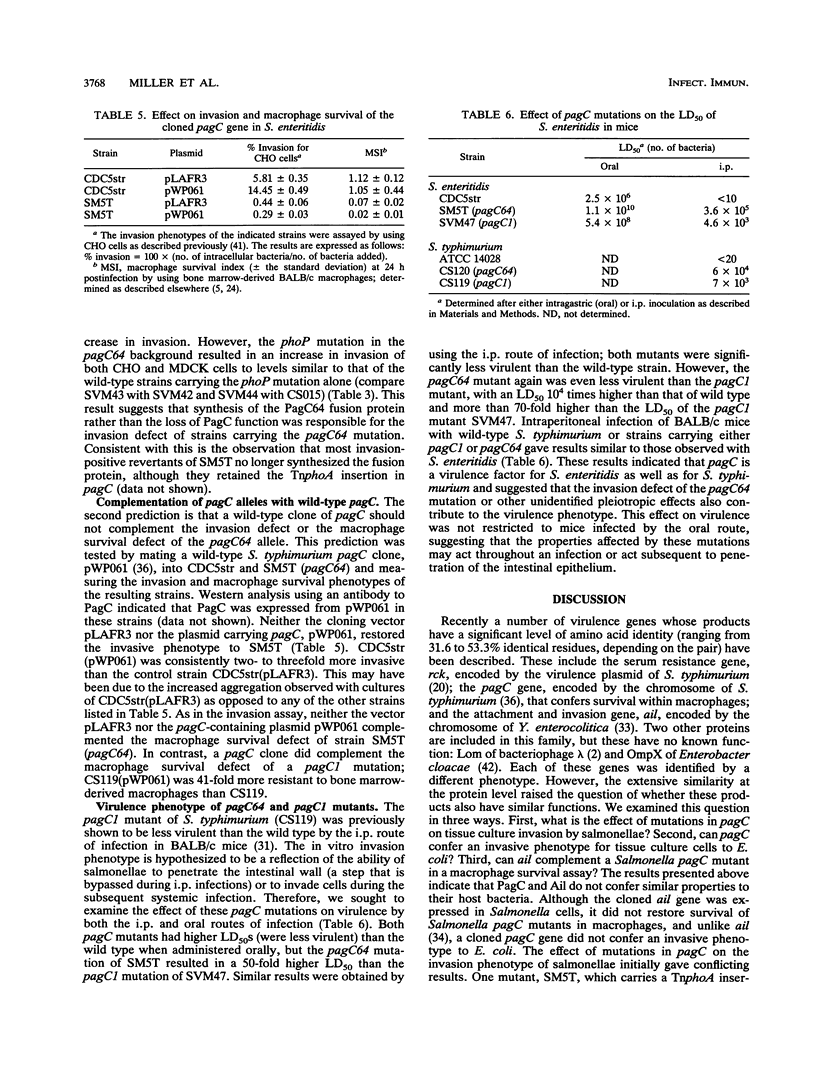
Two phenotypes believed to contribute to the pathogenesis of Salmonella infections are macrophage survival and invasion of epithelial cells. It was recently observed that the Salmonella macrophage survival factor PagC has significant amino acid similarity to the Yersinia invasion factor Ail. This observation raised the possibilities that macrophage survival is in part determined by the pathway of entry and that PagC confers an entry mechanism that does not trigger the microbicidal activities of the macrophage. Thus, we sought to investigate the role of PagC in invasion by examining (i) the invasion phenotype of pagC mutants and (ii) the invasion phenotype of Escherichia coli carrying pagC. A previously identified invasion-defective TnphoA insertion mutant of Salmonella enteritidis was found to have TnphoA inserted into the signal sequence-encoding region of pagC; the pagC allele from this mutant, SM5T, was designated pagC64. In contrast, Salmonella typhimurium carrying the pagC1 allele (a TnphoA insertion mutation, downstream of the region encoding the signal sequence) was not defective for invasion. Further analysis of these two pagC alleles suggested that the invasion-defective phenotype associated with pagC64 is not due to the loss of PagC function but rather is due to the synthesis of a hybrid PagC-alkaline phosphatase protein that is aberrantly localized, most likely to the inner membrane, and thus may prevent proper localization or function of a factor(s) required for efficient invasion. The observation that pagC did not confer an invasive phenotype to E. coli further suggests that PagC is not an invasion factor. A cloned pagC gene complemented the macrophage survival defect of S. typhimurium pagC1 mutants, but the cloned ail gene did not. Together these results suggest that the structural similarity between PagC and Ail may not extend to a similarity in function. Interestingly, S. enteritidis carrying the pagC64 allele that results in both an invasion defect and a macrophage survival defect was less virulent for mice infected intragastrically or intraperitoneally than was S. enteritidis carrying the pagC1 allele that results only in a macrophage survival defect.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barondess J. J., Beckwith J. A bacterial virulence determinant encoded by lysogenic coliphage lambda. Nature. 1990 Aug 30;346(6287):871–874. doi: 10.1038/346871a0. [DOI] [PubMed] [Google Scholar]
- Beer K. B., Miller V. L. Amino acid substitutions in naturally occurring variants of ail result in altered invasion activity. J Bacteriol. 1992 Feb;174(4):1360–1369. doi: 10.1128/jb.174.4.1360-1369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989 Jan;57(1):1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Tauxe R. V. Drug-resistant Salmonella in the United States: an epidemiologic perspective. Science. 1986 Nov 21;234(4779):964–969. doi: 10.1126/science.3535069. [DOI] [PubMed] [Google Scholar]
- Elsinghorst E. A., Baron L. S., Kopecko D. J. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5173–5177. doi: 10.1073/pnas.86.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Gumbiner B., Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J Cell Biol. 1988 Jul;107(1):221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Starnbach M. N., Francis C. L., Stocker B. A., Chatfield S., Dougan G., Falkow S. Identification and characterization of TnphoA mutants of Salmonella that are unable to pass through a polarized MDCK epithelial cell monolayer. Mol Microbiol. 1988 Nov;2(6):757–766. doi: 10.1111/j.1365-2958.1988.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Groisman E. A., Chiao E., Lipps C. J., Heffron F. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7077–7081. doi: 10.1073/pnas.86.18.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heffernan E. J., Harwood J., Fierer J., Guiney D. The Salmonella typhimurium virulence plasmid complement resistance gene rck is homologous to a family of virulence-related outer membrane protein genes, including pagC and ail. J Bacteriol. 1992 Jan;174(1):84–91. doi: 10.1128/jb.174.1.84-91.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. A., Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. A., Jones B. D., Falkow S. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1847–1851. doi: 10.1073/pnas.89.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner C. R., Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983 Dec;131(6):3006–3013. [PubMed] [Google Scholar]
- Manoil C., Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986 Sep 26;233(4771):1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983 Nov;35(1):253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Inouye H., Oliver D., Beckwith J. Mutations that alter the signal sequence of alkaline phosphatase in Escherichia coli. J Bacteriol. 1983 Apr;154(1):366–374. doi: 10.1128/jb.154.1.366-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Mekalanos J. J. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990 May;172(5):2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Bliska J. B., Falkow S. Nucleotide sequence of the Yersinia enterocolitica ail gene and characterization of the Ail protein product. J Bacteriol. 1990 Feb;172(2):1062–1069. doi: 10.1128/jb.172.2.1062-1069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Farmer J. J., 3rd, Hill W. E., Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989 Jan;57(1):121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen W. S., Miller S. I. A Salmonella typhimurium virulence protein is similar to a Yersinia enterocolitica invasion protein and a bacteriophage lambda outer membrane protein. J Bacteriol. 1991 Jan;173(1):86–93. doi: 10.1128/jb.173.1.86-93.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stone B. J., Garcia C. M., Badger J. L., Hassett T., Smith R. I., Miller V. L. Identification of novel loci affecting entry of Salmonella enteritidis into eukaryotic cells. J Bacteriol. 1992 Jun;174(12):3945–3952. doi: 10.1128/jb.174.12.3945-3952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel J., van Bussel M. J., Tommassen J., van de Klundert J. A. Molecular characterization of an Enterobacter cloacae outer membrane protein (OmpX). J Bacteriol. 1991 Jan;173(1):156–160. doi: 10.1128/jb.173.1.156-160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]