Abstract
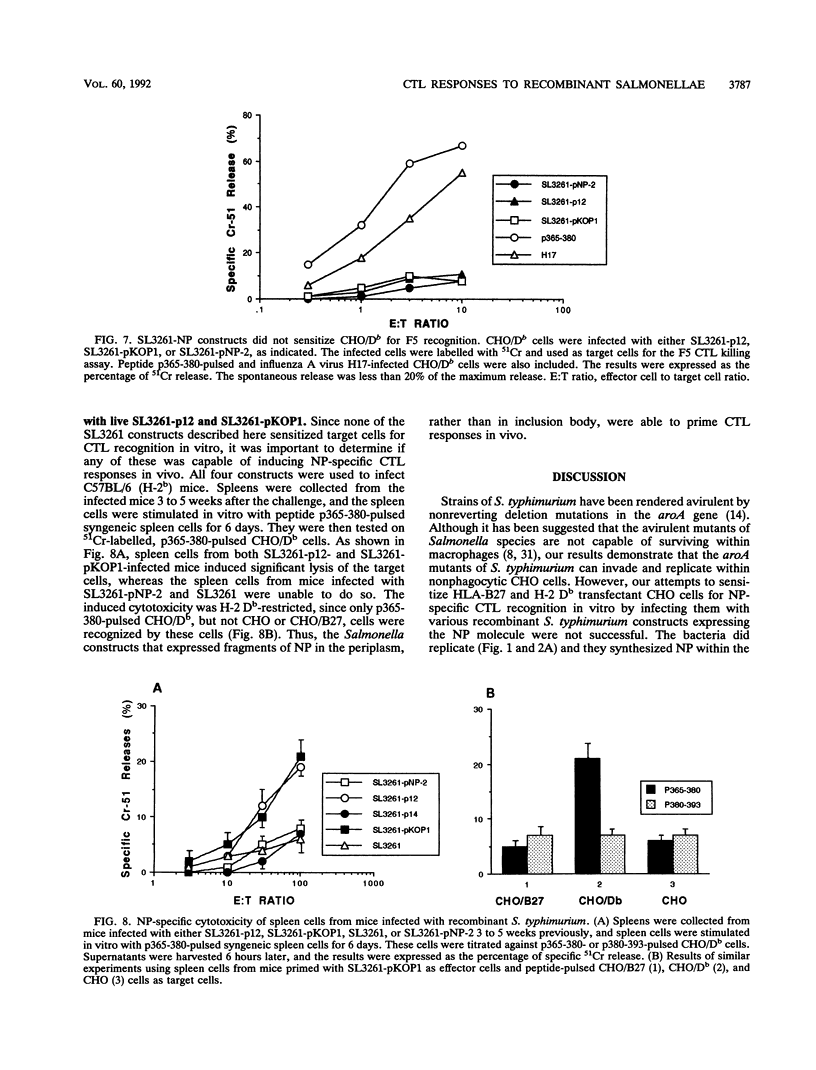
To address the question of whether Salmonella-infected nonphagocytic cells could serve as target cells for recognition by antigen-specific, major histocompatibility complex class I-restricted cytotoxic T lymphocytes (CTL), four recombinant Salmonella typhimurium constructs that expressed full-length, or fragments of, influenza A virus nucleoprotein (NP) were made. The bacteria were shown to infect Chinese hamster ovary (CHO) cells. Appropriate major histocompatibility complex restriction molecules, HLA-B27 and H-2 Db, were transfected into CHO cells, which were then infected with recombinant S. typhimurium and used as targets for NP-specific CTL. The cells in which NP was expressed by intracellularly replicating bacteria were not lysed by NP-specific CTL, although they were killed when appropriate influenza A virus or peptides were used. Thus, S.typhimurium bacteria within nonphagocytic cells were resistant to CTL recognition. In contrast to these results, mice infected with recombinant S.typhimurium that expressed fragments of NP in the periplasm were primed for NP-specific CTL responses. The results indicate that CTL responses specific to Salmonella antigens can be generated, but the bacteria may be safe from the CTL attack once they have entered the nonphagocytic cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal A., Kumar S., Jaffe R., Hone D., Gross M., Sadoff J. Oral Salmonella: malaria circumsporozoite recombinants induce specific CD8+ cytotoxic T cells. J Exp Med. 1990 Oct 1;172(4):1083–1090. doi: 10.1084/jem.172.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. A. Immunocompetent cells in resistance to bacterial infections. Bacteriol Rev. 1976 Jun;40(2):284–313. doi: 10.1128/br.40.2.284-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Campbell S. G. Immunity to intracellular bacteria. Vet Immunol Immunopathol. 1982 Jan;3(1-2):5–66. doi: 10.1016/0165-2427(82)90031-9. [DOI] [PubMed] [Google Scholar]
- Eisenstein T. K., Killar L. M., Sultzer B. M. Immunity to infection with Salmonella typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J Infect Dis. 1984 Sep;150(3):425–435. doi: 10.1093/infdis/150.3.425. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Salmonella as an intracellular parasite. Mol Microbiol. 1989 Dec;3(12):1833–1841. doi: 10.1111/j.1365-2958.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Flynn J. L., Weiss W. R., Norris K. A., Seifert H. S., Kumar S., So M. Generation of a cytotoxic T-lymphocyte response using a Salmonella antigen-delivery system. Mol Microbiol. 1990 Dec;4(12):2111–2118. doi: 10.1111/j.1365-2958.1990.tb00572.x. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Unanue E. R., Slot J. W., Schwartz A. L., Geuze H. J. Functional and ultrastructural evidence for intracellular formation of major histocompatibility complex class II-peptide complexes during antigen processing. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5553–5557. doi: 10.1073/pnas.87.14.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hsu H. S., Mayo D. R. Interactions between macrophages of guinea pigs and salmonellae. 3. Bactericidal action and cytophilic antibodies of macrophages of infected guinea pigs. Infect Immun. 1973 Aug;8(2):165–172. doi: 10.1128/iai.8.2.165-172.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. S. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev. 1989 Dec;53(4):390–409. doi: 10.1128/mr.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet S., Nixon D. F., Rothbard J. B., Townsend A., Ellis S. A., McMichael A. J. Structural homologies between two HLA B27-restricted peptides suggest residues important for interaction with HLA B27. Int Immunol. 1990;2(4):311–316. doi: 10.1093/intimm/2.4.311. [DOI] [PubMed] [Google Scholar]
- Jones I. M., Brownlee G. G. Differential expression of influenza N protein and neuraminidase antigenic determinants in Escherichia coli. Gene. 1985;35(3):333–342. doi: 10.1016/0378-1119(85)90012-5. [DOI] [PubMed] [Google Scholar]
- Killar L. M., Eisenstein T. K. Delayed-type hypersensitivity and immunity to Salmonella typhimurium. Infect Immun. 1986 May;52(2):504–508. doi: 10.1128/iai.52.2.504-508.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. R., Wang X. M., Hsu H. S., Mumaw V. R., Nakoneczna I. Electron microscopic studies on the location of bacterial proliferation in the liver in murine salmonellosis. Br J Exp Pathol. 1987 Aug;68(4):539–550. [PMC free article] [PubMed] [Google Scholar]
- Lipscombe M., Charles I. G., Roberts M., Dougan G., Tite J., Fairweather N. F. Intranasal immunization using the B subunit of the Escherichia coli heat-labile toxin fused to an epitope of the Bordetella pertussis P.69 antigen. Mol Microbiol. 1991 Jun;5(6):1385–1392. doi: 10.1111/j.1365-2958.1991.tb00785.x. [DOI] [PubMed] [Google Scholar]
- Marecki N. M., Hsu H. S., Mayo D. R. Cellular and humoral aspects of host resistance in murine salmonellosis. Br J Exp Pathol. 1975 Jun;56(3):231–243. [PMC free article] [PubMed] [Google Scholar]
- Mayo D. R., Hsu H. S., Lim F. Interactions between salmonellae and macrophages of guinea pigs. IV. Relationship between migration inhibition and antibacterial action of macrophages. Infect Immun. 1977 Oct;18(1):52–59. doi: 10.1128/iai.18.1.52-59.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Monaco J. J. A molecular model of MHC class-I-restricted antigen processing. Immunol Today. 1992 May;13(5):173–179. doi: 10.1016/0167-5699(92)90122-N. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakoneczna I., Hsu H. S. Histopathological study of protective immunity against murine salmonellosis induced by killed vaccine. Infect Immun. 1983 Jan;39(1):423–430. doi: 10.1128/iai.39.1.423-430.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakoneczna I., Hsu H. S. The comparative histopathology of primary and secondary lesions in murine salmonellosis. Br J Exp Pathol. 1980 Feb;61(1):76–84. [PMC free article] [PubMed] [Google Scholar]
- Neefjes J. J., Ploegh H. L. Intracellular transport of MHC class II molecules. Immunol Today. 1992 May;13(5):179–184. doi: 10.1016/0167-5699(92)90123-O. [DOI] [PubMed] [Google Scholar]
- O'Callaghan D., Maskell D., Liew F. Y., Easmon C. S., Dougan G. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun. 1988 Feb;56(2):419–423. doi: 10.1128/iai.56.2.419-423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tite J. P., Russell S. M., Dougan G., O'Callaghan D., Jones I., Brownlee G., Liew F. Y. Antiviral immunity induced by recombinant nucleoprotein of influenza A virus. I. Characteristics and cross-reactivity of T cell responses. J Immunol. 1988 Dec 1;141(11):3980–3987. [PubMed] [Google Scholar]
- Townsend A. R., McMichael A. J., Carter N. P., Huddleston J. A., Brownlee G. G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984 Nov;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Kuon W., Dörner C., Lang M., Riethmüller G. Organization, sequence and expression of the HLA-B27 gene: a molecular approach to analyze HLA and disease associations. Immunobiology. 1985 Dec;170(5):367–380. doi: 10.1016/S0171-2985(85)80061-9. [DOI] [PubMed] [Google Scholar]