Abstract
LYRIC/AEG-1 is a unique protein that has been shown to promote tumor cell migration and invasion through activation of the transcription factor NF-κB. We performed yeast two-hybrid screening to detect LYRIC/AEG-1 associated proteins, and identified BCCIP, a CDKN1A and BRCA2-associated protein involved in cell cycle regulation and DNA repair. Here we demonstrate association between LYRIC/AEG-1 and BCCIP in mammalian cells, and define the region of interaction. Co-expression of the two proteins resulted in decreased levels of BCCIPα, an effect partially abrogated by proteasome inhibition. A truncated LYRIC/AEG-1 construct lacking the interaction region did not alter BCCIPα protein levels. Coincidentally, it was observed that overexpression of BCCIPα in DU145 prostate tumor cells induced an apparent neuroendocrine differentiation. In summary, our data suggest LYRIC/AEG-1 is a negative regulator of BCCIPα, promoting proteasomal degradation either through direct interaction, or potentially through an indirect mechanism involving downstream effects of the NF-κB signaling pathway.
Introduction
LYRIC, also known as AEG-1 [1] and metadherin [2], is a highly conserved protein of unknown function. Elevated expression has been reported in breast and prostate tumors, melanoma and glioblastoma [1–4], and evidence suggests that LYRIC/AEG-1 actively contributes to malignant progression. LYRIC/AEG-1 overexpression activates both NF-kappaB and Akt signaling pathways [5, 6], and the protein acts synergistically with Ha-Ras to promote anchorage-independent growth [1, 7]. In prostate tumor cell lines, LYRIC/AEG-1 knockdown resulted in reduced viability and invasiveness [3]. Overexpression of LYRIC/AEG-1 in non-tumorigenic cells is not sufficient to induce transformation [1, 7], but studies to date suggest that LYRIC/AEG-1 is an important factor promoting progression or metastasis of a variety of tumors.
In our ongoing characterization of LYRIC/AEG-1, we performed a yeast two-hybrid screen to identify associated proteins. We here present evidence that LYRIC/AEG-1 interacts with BCCIP (TOK-1), a BRCA2-and CDKN1A (p21Cip1/Waf-1)-associated protein with two major isoforms, BCCIPα and BCCIPβ. Co-expression of LYRIC/AEG-1 with BCCIPα in prostate tumor cells resulted in decreased BCCIPα protein levels, relative to control, suggesting LYRIC/AEG-1 as a possible negative regulator of BCCIPα activity. BCCIPα binds to the cell cycle regulator p21, and enhances p21-mediated inhibition of Cdk2 kinase [8]. Loss of BCCIP impairs cell cycle G1/S checkpoint activation following DNA damage [9], and in conjunction with BRCA2, BCCIP plays a role in homologous recombination repair of DNA damage [10], and contributes to maintenance of chromosome stability [11].
Reduced BCCIPα expression has been observed in breast cancer and glioma cell lines, and exogenous expression of the protein causes growth delay [12], thus BCCIPα has properties of a tumor suppressor. Roversi et al. [13] identified BCCIP as a positional candidate gene lost during glioma progression. In contrast, LYRIC/AEG-1 expression increases in malignant glioma, and contributes to migration and invasiveness [14]. Our data suggest that the inverse relationship between these two proteins is not merely coincidental, and we postulate that one mechanism for down regulation of BCCIPα may be increased expression of LYRIC/AEG-1, within the context of other changes contributing to tumor progression.
Materials and methods
Cell culture and transfection
Tissue culture materials were purchased from Invitrogen/Life Technologies (Carlsbad, CA). Human embryonic kidney 293T cells were maintained in Dulbecco's Modified Eagle Medium with 10% FBS (Hyclone, Logan UT) and 50 µg/ml gentamycin. DU145 human prostate tumor cells were grown in RPMI with 10% FBS and 50 µg/ml gentamycin. Transfections were carried out using Lipofectamine LTX (Invitrogen) as per the manufacturer’s protocol.
Eukaryotic expression constructs
A PCR cloning strategy was used to amplify the desired region of cDNA using primers incorporating appropriate restriction sites (Table 1). PCR reactions were routinely 35 cycles annealing at 55°C, using Platinum Taq DNA Polymerase HiFi (Invitrogen). Products were purified by agarose gel electrophoresis and GeneClean (QBiogene, Inc., Carlsbad CA), restriction digestion and ligation were conducted using standard protocols [15] and plasmids were transformed into NovaBlue competent cells (EMD biosciences, Gibbstown, NJ). Plasmid DNA was purified using Qiagen (Valencia CA) kits, and constructs were verified by sequencing at the Yale University WM Keck Facility. MacVector software (MacVector, Inc, Cary NC) was used for primer design and sequence analysis.
Table 1.
Primers used for cloning
| construct | primer sequences (forward and reverse) |
|---|---|
| pSOS-LYRIC | 5’-GGCGGATCCGCTGGGCCGCGGCTTGC-3’ |
| 5’-GGCGTCGACTCACGTTTCTCGTCTGGC-3’ | |
| HA-BCCIPα | 5’-GCGGAATTCACATGGCGTCCAGGTCTAAGC-3’ |
| 5’-GCGCTCGAGATTATGACAGAGCAATTCCAAC-3’ | |
| HA-BCCIPc | 5’-GCGGAATTCACATGGCGTCCAGGTCTAAGC-3’ |
| 5’-GCGCTCGAGTGGCTTCATTCATCCTGGCTTG-3’ | |
| Δ463 | 5’-CGCGAATTCGACGGGAGGGAAGATGGCTG-3’ |
| 5’-CGCGGATCCTTCTGTGTCCTGGGTGATAGAG-3’ | |
| Δ238 | 5’-CGCGAATTCGACGGGAGGGAAGATGGCTG-3’ |
| 5’-CACGGATCCTGCAGTTGTAAGTTGCTCGGTG-3’ | |
| NΔ280 | 5’-CACGAATTCAGTCAATGGAGGAGGCTGGAGTG-3’ |
| 5’-GTGGGATCCTTCACGTTTCCCGTCTGGC-3’ | |
| NΔ169 | 5’-CACGAATTCAAAGTCAGATGCTAAAGCAGTG-3’ |
| 5’-GTGGGATCCTTCACGTTTCCCGTCTGGC-3’ | |
| hsNΔ169 | 5’-CACGAATTCAAAGTCAGATGCTAAAGCAGTG-3’ |
| 5’-GTGGGATCCTTCACGTTTCTCGTCTGGC-3’ |
FLAG-tagged full-length rat LYRIC (RNF) was described previously [16]. FLAG-tagged truncation constructs were generated by PCR from rat liver cDNA, and cloned into pFLAG-CMV-5a or pFLAG-CMV-2, for C-terminal or NH2-terminal FLAG tag, respectively (Sigma-Aldrich, St. Louis MO). The rat FLAG constructs were on hand at the time of the yeast two-hybrid screen, and used for initial co-precipitation experiments. Transfections in DU145 cells used human LYRIC/AEG-1 constructs. Full-length human cDNA encoding LYRIC/AEG-1 was cloned into the pcDNA4/TO expression vector (Invitrogen), with no epitope tag. A control pcDNA4-lacZ construct was provided by Invitrogen. The truncated construct, hsNΔ169, was amplified by RT-PCR from DU145 RNA, and cloned into pFLAG-CMV2. RNA was isolated using an RNeasy kit (Qiagen) and cDNA was synthesized using random hexamers and Superscript III (Invitrogen).
Full-length BCCIPα and splice variant c were amplified by RT-PCR from commercially available human prostate RNA (Ambion, Austin TX), and cloned into the pCMV-HA vector (Clontech, Mountain View, CA), incorporating an HA tag at the NH2-terminus of the expressed protein. The HA-tagged control for immunofluorescence, HA-CLASP1, is a partial cDNA encoding the C-terminal 583 amino acids of the protein cloned into pCMV-HA.
Yeast two-hybrid screening
Protein interactions were identified using the CytoTrap yeast two-hybrid system (Stratagene, La Jolla CA) as per the manufacturer’s protocol. Human LYRIC/AEG-1 cDNA encoding amino acids 71–582 was amplified by PCR and cloned into the pSos vector. A commercially available (Stratagene) human prostate cDNA library cloned in the pMyr target vector was co-transformed with pSos-LYRIC into yeast strain cdc25H using an S.c. EasyComp Transformation kit (Invitrogen). Control transformations, media and growth conditions were performed as per the CytoTrap kit protocol. Colonies containing putative interacting proteins were cultured in YPD broth, plasmid DNA was isolated using a Y-DER Yeast DNA Extraction Reagent Kit (Pierce, Rockford IL) and the insert was partially sequenced and identified by BLAST search. Interactions of interest were confirmed by co-transforming pSos-LYRIC individually with candidate pMYR target plasmids.
Western blotting and immunoprecipitation (IP)
Protein extraction, IP, and Western blotting were performed as described previously [16]. Anti-FLAG agarose (Sigma) and the HA-Tag IP Application Set (Pierce) were used to IP FLAG-tagged and HA-tagged proteins, respectively. Anti-LYRIC PAb5393 was described previously [16]; anti-HA, anti-FLAG, anti-p21 and anti-β-actin were from Sigma-Aldrich; anti-BCCIP was from Novus Biologicals (Littleton, CO). Secondary antibodies were goat anti-rabbit HRP conjugate (Sigma-Aldrich) or goat anti-mouse HRP (Invitrogen). Densitometry analysis was performed using a Kodak 1D electrophoresis documentation and analysis system, with statistical comparisons by Student’s t-test.
Indirect immunofluorescence (IIF)
For IIF, cells were cultured on permanox plastic chamber slides (Nalge/Nunc-Fisher Scientific, Pittsburgh PA) and stained as previously described [16]. IIF was visualized using a Nikon Microphot FX epifluorescence microscope equipped with a SPOT camera (Diagnostic Instruments, Inc., Sterling Heights MI), and images were annotated for publication using Adobe Photoshop 7 for Apple Macintosh.
Results
BCCIP is identified as a LYRIC/AEG-1 associated protein
To gain insight as to a potential function for LYRIC/AEG-1, a yeast two-hybrid assay was performed to identify associated proteins. A cloned portion of LYRIC/AEG-1, lacking the first 70 NH2-terminal amino acids encompassing the hydrophobic domain, was used as bait to screen a commercially available human prostate library. Potentially interesting candidates were retested individually and confirmed in the yeast system. One clone, chosen for further investigation, contained cDNA encoding amino acids 3–78 of a protein originally identified by two different groups as BCCIP, a BRCA2-interacting protein [12]and TOK-1, a p21(Cip1/Waf1) binding protein [8]. Three isoforms have been described, BCCIPα, BCCIPβ and BCCIP-c, which differ in their carboxy termini. To verify interaction with LYRIC/AEG-1, full-length cDNAs for BCCIPα and the BCCIP-c isoform were cloned into a vector providing an HA epitope tag. HA-BCCIP constructs were each co-transfected with FLAG-tagged LYRIC/AEG-1 into 293T cells, and LYRIC/AEG-1 was immunoprecipitated with anti-FLAG agarose. Western blot of cell lysates with anti-FLAG and anti-HA antibodies verified that LYRIC/AEG-1 and both BCCIP isoforms were expressed (Fig. 1a lanes 1–3), and that FLAG-LYRIC/AEG-1 was successfully immunoprecipitated (Fig. 1a lanes 4–6 upper panel). Both isoforms of BCCIP co-precipitated with LYRIC/AEG-1 and were detected by Western blot with anti-HA antibody (Fig 1a lanes 4–6 lower panel), demonstrating interaction between LYRIC/AEG-1 and BCCIP in mammalian cells.
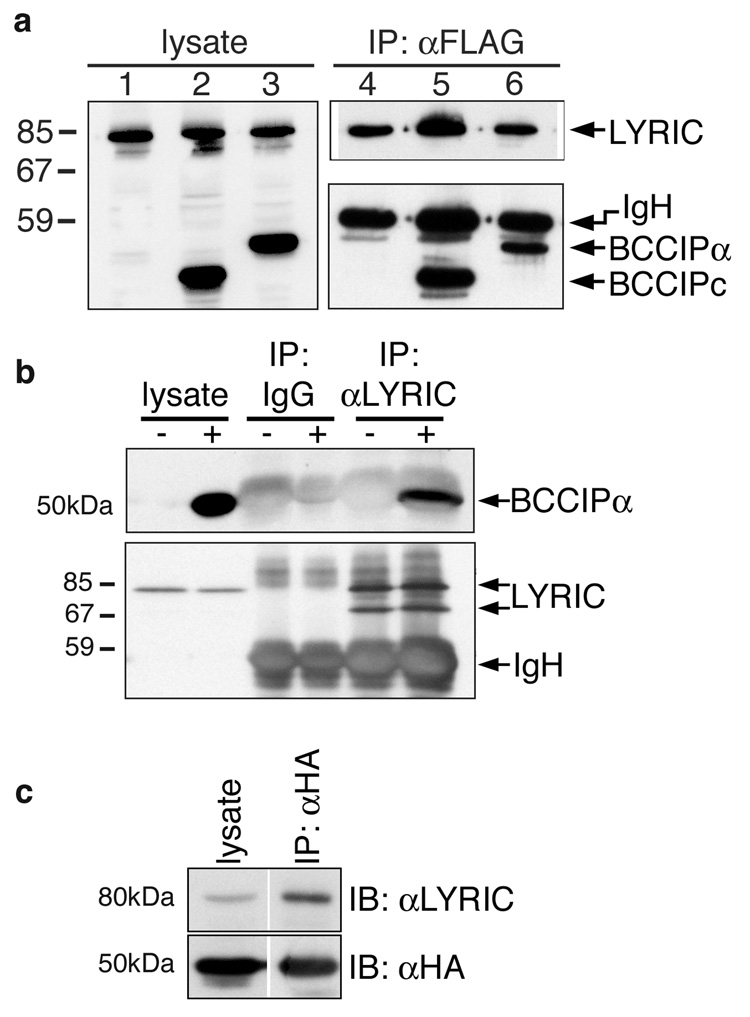
Figure 1. LYRIC/AEG-1 associates with BCCIP.
(a) 293T cells were co-transfected with plasmids encoding full-length FLAG-tagged LYRIC/AEG-1 with empty HA-vector (lanes1&4), HA-BCCIPc (lanes 2&5) or HA-BCCIPα (lanes 3&6). Expression was verified by Western blotting of cell lysates with anti-FLAG and anti-HA (lanes 1–3). FLAG-LYRIC was immunoprecipitated using anti-FLAG agarose (lanes 4–6, upper), and both isoforms of BCCIP co-precipitated (lanes 4–6, lower). (b) HA-BCCIPα (+) or empty vector (−) were transfected into 293T and endogenous LYRIC was IP’d using PAb5393. IP with pre-immune rabbit serum (IgG) was the negative control. BCCIPα co-precipitated with LYRIC (upper), and precipitation of LYRIC was confirmed by blotting with PAb5393 (lower). (c) HA-BCCIPα was transfected into 293T and IP’d with anti-HA agarose. Endogenous LYRIC co-precipitated and was detected with PAb5393.
Association between endogenous human LYRIC/AEG-1 and HA-BCCIPα was demonstrated by transfection of 293T cells with the HA-BCCIPα plasmid, and immunoprecipitation of native LYRIC/AEG-1 with polyclonal antibody (PAb) 5393. HA-tagged BCCIPα co-precipitated with endogenous LYRIC/AEG-1, while neither protein was precipitated by pre-immune rabbit serum used as a negative control (Fig. 1b). In the reverse of this experiment, HA-tagged BCCIPα transfected into 293T was precipitated with anti-HA antibody, and endogenous LYRIC/AEG-1 co-precipitated (Fig. 1c).
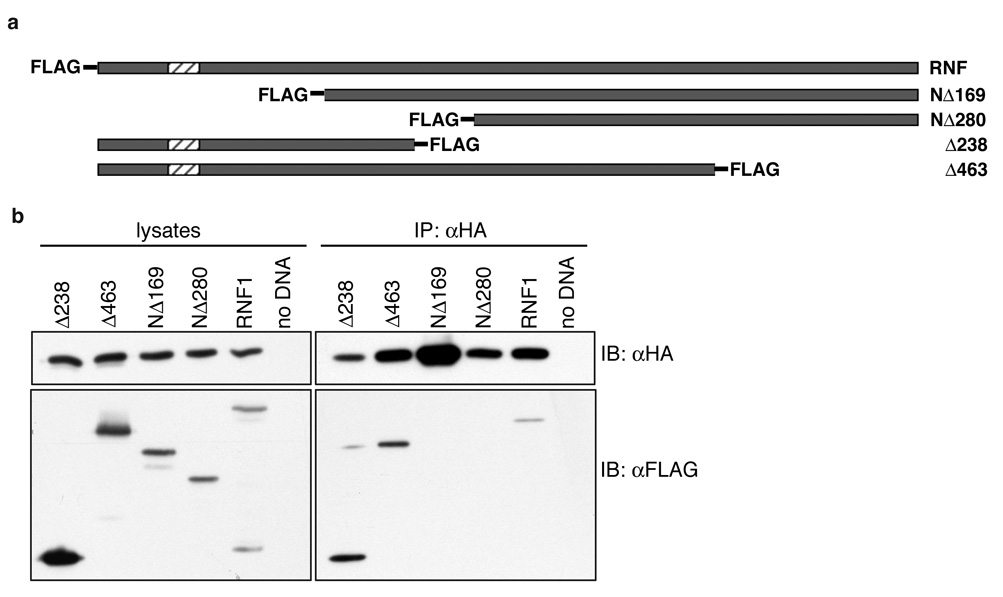
To define the region of LYRIC/AEG-1 necessary for association, a series of FLAG-tagged rat LYRIC/AEG-1 constructs with amino-or carboxy-terminal deletions were tested for interaction with HA-BCCIPα (Fig. 2a). Constructs were co-transfected with HA-BCCIPα into 293T cells, and protein expression was verified by Western blotting (Fig 2b, left panel). HA-BCCIPα was immunoprecipitated with anti-HA antibody, and LYRIC co-precipitation was detected by Western blot. Full-length LYRIC/AEG-1 (RNF1) and constructs Δ238 and. Δ463, lacking the carboxyl terminus of LYRIC/AEG-1, co-precipitated with BCCIPα (Figure 2b, right panel). In contrast, BCCIPα did not associate with FLAG-tagged constructs NΔ169 and NΔ280, lacking the amino terminus of LYRIC/AEG-1. These data, in conjunction with the two-hybrid system, suggest the minimal region of LYRIC/AEG-1 required for interaction with BCCIPα lies within amino acids 72–169.
Figure 2. NH2-terminal region of LYRIC is required for association with BCCIPα.
(a) FLAG-tagged deletion constructs, indicating the region expressed, drawn to scale with full-length LYRIC (RNF). (b) 293T cells were transfected with BCCIPα and each of the LYRIC constructs. Protein expression was verified by Western blotting of total cell lysates (left). BCCIPα was precipitated with anti-HA agarose, and only constructs Δ238 and Δ463, and full length LYRIC co-precipitated.
LYRIC/AEG-1 overexpression promotes proteasomal degradation of BCCIPα
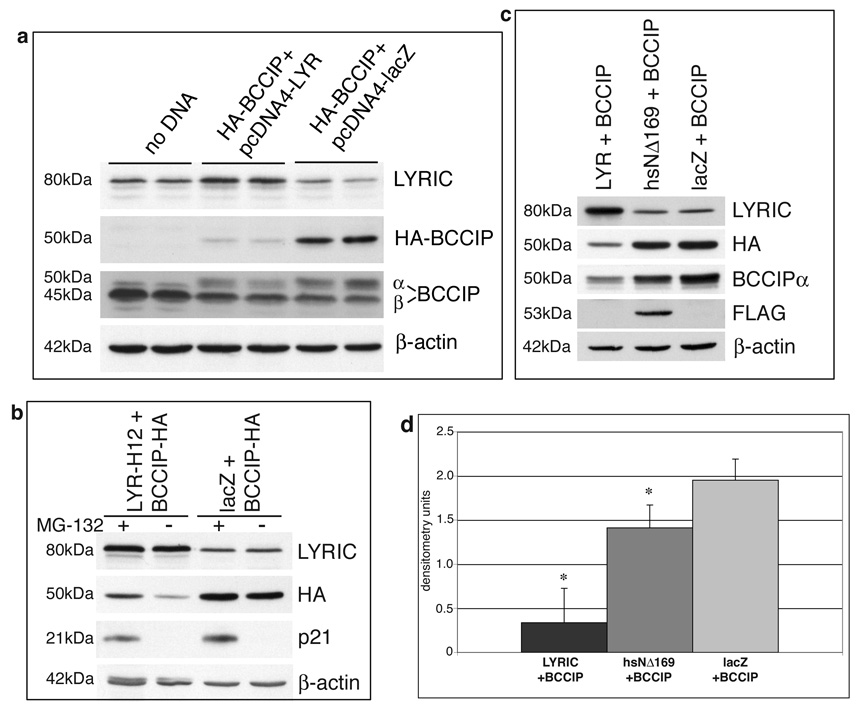
BCCIPα expression is reduced in human breast and brain cancer cells, and re-introduction of the protein into MCF7 breast cancer cells has been shown to result in growth suppression [12]. To examine protein interaction in tumor cells, HA-BCCIPα and pcDNA4-LYR-H12, a construct expressing untagged full-length human LYRIC/AEG-1, were co-transfected into human prostate tumor DU145 cells, which express very low levels of endogenous BCCIPα (Fig. 3a). Western blot analysis showed that protein levels of HA-BCCIPα were greatly reduced when LYRIC/AEG-1 was co-transfected, in contrast to robust expression of HA-BCCIPα with a control lacZ vector encoding β-galactosidase (Fig. 3a). Both the LYRIC/AEG-1 and lacZ constructs utilize the same plasmid backbone, therefore the effect on BCCIPα is presumed to be specific to the presence of LYRIC/AEG-1.
Figure 3. LYRIC overexpression reduces BCCIPα protein levels.
(a) DU145 cells were co-transfected with plasmids encoding HA-BCCIPα and LYRIC or β-galactosidase. Pairs of lanes represent two independent transfections. LYRIC overexpression resulted in decreased levels of BCCIPα protein (lanes 3&4), relative to negative control lacZ plasmid (lanes 5&6). Endogenous BCCIPβ did not appear to be affected. (b) DU145 cells were transfected with HA-BCCIPα and LYRIC or lacZ plasmids then treated with MG-132 (+) or DMSO (−)overnight. Western blot shows LYRIC overexpressed approximately 4-fold, resulting in a two-fold decrease in BCCIPα protein levels. Proteasome inhibition by MG-132 abrogated the effect. p21 was detected only with proteasome inhibition. β-actin was a loading control. (c) DU145 cells were transfected with plasmids encoding BCCIPα and full length LYRIC, deletion mutant hsNΔ169, or β-galactosidase and analyzed by Western blot. Decreased levels of HA-BCCIP were seen only with full-length LYRIC, the deletion mutant had no effect. (d) HA-BCCIP levels were determined by densitometry of the bands from Western blots as shown in panel (c). Graph depicts average and standard deviation of HA-BCCIP expression from three independent transfection experiments. The difference in HA-BCCIP protein levels in cells co-transfected with LYRIC and hsNΔ169 was statistically significant (p<0.01).
We hypothesized that LYRIC/AEG-1 overexpression promoted increased proteasomal degradation of BCCIPα. When DU145 cells were transfected and then treated overnight with MG-132, a cell-permeable proteasome inhibitor, BCCIPα protein expression was restored despite the presence of LYRIC/AEG-1, although levels were still slightly reduced relative to the control (Fig. 3b). These results suggest that BCCIPα protein turnover is regulated through proteasomal degradation, and that overexpression of LYRIC/AEG-1 promotes increased degradation of BCCIPα.
Degradation of BCCIPα requires NH2-terminal region of LYRIC/AEG-1
Given the data that LYRIC/AEG-1 and BCCIPα interact, we questioned whether protein association would be necessary to induce degradation of BCCIPα. To address this, HA-BCCIPα was co-transfected with full-length LYRIC/AEG-1, or with a FLAG-tagged human LYRIC/AEG-1 construct lacking the region previously determined (see Fig. 2) to be necessary for co-precipitation with BCCIPα. BCCIPα protein levels were decreased in DU145 cells overexpressing full length LYRIC/AEG-1, but expression of the truncated hsNΔ169 protein did not affect BCCIPα (Fig. 3c). The difference between the two was statistically significant (p<0.01) when protein bands from three independent experiments were quantitated by densitometry (Fig. 3d). These results indicate that the NH2-terminal 169 amino acid region of LYRIC/AEG-1, shown to be required for association with BCCIPα, is also necessary for modulation of BCCIPα protein levels.
Expression of BCCIPα induces apparent neuroendocrine differentiation in DU145
BCCIPα was identified as a nuclear protein [8, 12], while LYRIC/AEG-1 is found in both the cytoplasm and nucleus of cultured cells [17, and personal observation]. To determine whether overexpression of LYRIC/AEG-1 alters the cellular distribution of BCCIPα, we co-transfected the proteins into DU145 cells and analyzed them by indirect immunofluorescence. BCCIPα was distributed through the cytoplasm, and LYRIC/AEG-1 did not alter this localization (data not shown), although co-transfected cells had a reduced fluorescence intensity consistent with decreased BCCIPα protein levels.
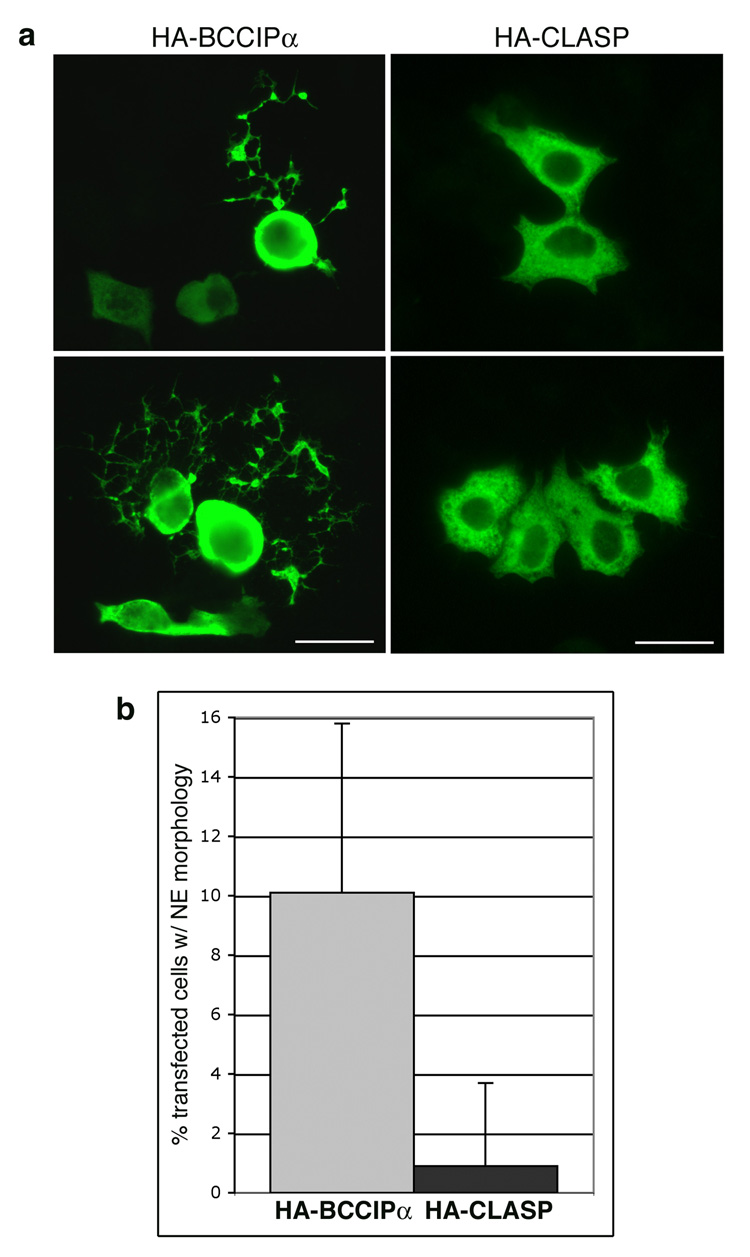
A subpopulation of cells transfected with HA-BCCIP and the control vector showed distinctive morphological changes, so the experiment was repeated using HA-BCCIP alone. For comparison, control cells were transfected with an unrelated protein, HA-CLASP. Control cells exhibited a normal polygonal phenotype, while a subpopulation of cells transfected with BCCIPα were rounded, with long, multi-branching processes extending from the body of the cell (Fig. 4a). These cells were quantitated by counting the number of transfected cells with at least one branched extension equal to or greater than the length of the cell body. Ten percent of the HA-BCCIPα transfected cells developed this morphology, which occurred in less than 1% of the control cells (Fig. 4b). The extensions resemble neurites, suggesting that the cells have undergone neuroendocrine differentiation (NED). Previous studies [18, 19] have shown that treatment of DU145 cells with EGF or IL-6 inhibits proliferation and promotes NED, and that differentiation is dependent upon tyrosine kinase activity. We transfected DU145 cells with HA-BCCIPα, allowed them to recover for six hours, then treated overnight with genistein, a protein tyrosine kinase inhibitor, or rapamycin, an inhibitor of the serine/threonine kinase mTor. Neither treatment reduced the formation of the neurite-like extensions (data not shown), thus the change in morphology induced by BCCIPα does not appear to be dependent upon mTor or protein tyrosine kinase activity.
Figure 4. Overexpression of BCCIPα induces formation of neurite-like extensions.
DU145 cells on chamber slides were transfected with HA-BCCIPα or HA-CLASP1, incubated overnight, fixed and stained with anti-HA and a fluorescent-tagged secondary antibody. (a) CLASP-1 transfected cells displayed a normal polygonal morphology, while a sub-population of cells overexpressing BCCIPα were rounded with elaborate branching extensions, suggestive of neuroendocrine (NE) differentiation. Two patterns were commonly seen, cells with a single long extension (upper panel) and cells with multiple convoluted processes (lower panel). Size bar represents 25µ. (b) To quantitate cells putatively undergoing NE differentiation, the number of rounded cells with extensions in a given microscope field was counted and expressed as a percent of the total number of transfected cells visible in the field. Graph depicts the average and standard deviation from 10 random microscope fields (approximately 200 cells for each transfection). Ten percent of BCCIPα expressing cells developed this phenotype, while less than 1% of these cells were observed in the control. The difference was statistically significant as determined by Student’s t-test (p<0.001).
Discussion
This study was undertaken to shed light on the function of LYRIC/AEG-1, a unique and highly conserved protein that has only recently been recognized as a contributor to tumor progression and metastasis. Using yeast two-hybrid and mammalian cells, we identified BCCIP as an interaction partner for LYRIC/AEG-1, and suggest a direct association between the two proteins. Relatively little is known about BCCIPα, but studies to date demonstrate roles in cell cycle regulation [8, 9, 12, 20], homologous recombination repair [10, 21] and cytokinesis [11].
LYRIC/AEG-1 and BCCIPα appear to have contrasting roles in tumor progression; BCCIPα has tumor suppressor properties, and expression is down-regulated during carcinogenesis, while LYRIC/AEG-1 overexpression contributes to tumor progression. We theorize that interaction between the two proteins results in decreased BCCIPα activity, i.e. LYRIC/AEG-1 is a negative regulator of BCCIPα. The data from this study suggest that BCCIPα protein degradation is accelerated when LYRIC/AEG-1 is overexpressed, thus providing a potential mechanism for post-translational regulation of activity. Effects on mRNA processing or stability that result in decreased BCCIP α protein levels are also possible, and have not been ruled out.
The region of LYRIC/AEG-1 necessary for interaction with BCCIPα was determined to lie within the first 169 amino acids from the NH2-terminus; however, analysis of the primary sequence offers no insight as to a specific binding motif. This same amino terminal region was also shown to be required to mediate decreased BCCIP α protein levels. One interpretation of these results is that direct interaction between the two proteins results in targeting of BCCIPα for proteasomal degradation. This could occur through displacement of a stabilizing chaperone protein, such as HSP90, or by changes in protein conformation that lead to modifications that then target BCCIPα for proteolysis. Alternatively, BCCIPα degradation is not necessarily dependent upon direct interaction, but could be a downstream consequence of other activities mediated by the NH2-terminal domain of LYRIC/AEG-1. LYRIC/AEG-1 has previously been shown to activate NF-κB [5], a powerful transcriptional regulator involved in many signaling pathways. In a recent report, Sarkar et al. [14], more clearly defined the mechanism, and determined that the first 71 amino acids of the NH2-terminal region of LYRIC/AEG-1 were crucial for NF-κB activation. These findings raise the possibility that LYRIC/AEG-1 acts through NF-κB to promote expression of a specific gene or set of genes that ultimately impact BCCIPα protein synthesis or stability. NF-κB regulates a wide variety of genes, and further investigations will be necessary to define the relevant pathways affected.
One intriguing sidenote was the observation that BCCIPα overexpression induced morphological changes consistent with neuroendocrine differentiation (NED) in DU145 cells. NED has been previously reported in DU145 and LNCaP prostate cell lines [18], and in prostate cancer NED is associated with high-grade and hormone refractory tumors [22]. The potential for BCCIPα to trigger a differentiation program raises interesting questions with regard to its role in maintaining a normal phenotype in epithelial cells. Future studies will address the inter-relationship between BCCIPα and LYRIC/AEG-1, particularly as it pertains to the loss of cellular differentiation during tumor progression.
Acknowledgements
The authors thank DongFang Yang and William Querbes for technical assistance, and Laura Bangs and Sandy DeAngelis for administrative assistance. Supported by U.S. Army grant DAMD17-02-1-0132, and NIH Grant RR-P20 RR17695.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–374. doi: 10.1016/s1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- 3.Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, Place RF, Pookot D, Majid S, Igawa M, Dahiya R. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene. 2007;26:7647–7655. doi: 10.1038/sj.onc.1210572. [DOI] [PubMed] [Google Scholar]
- 4.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 5.Emdad L, Sarkar D, Su ZZ, Randolph A, Boukerche H, Valerie K, Fisher PB. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: implications for tumor progression and metastasis. Cancer Res. 2006;66:1509–1516. doi: 10.1158/0008-5472.CAN-05-3029. [DOI] [PubMed] [Google Scholar]
- 6.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–1121. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 7.Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci U S A. 2006;103:17390–17395. doi: 10.1073/pnas.0608386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ono T, Kitaura H, Ugai H, Murata T, Yokoyama KK, Iguchi-Ariga SM, Ariga H. TOK-1, a novel p21Cip1-binding protein that cooperatively enhances p21-dependent inhibitory activity toward CDK2 kinase. J Biol Chem. 2000;275:31145–31154. doi: 10.1074/jbc.M003031200. [DOI] [PubMed] [Google Scholar]
- 9.Meng X, Lu H, Shen Z. BCCIP functions through p53 to regulate the expression of p21Waf1/Cip1. Cell Cycle. 2004;3:1457–1462. doi: 10.4161/cc.3.11.1213. [DOI] [PubMed] [Google Scholar]
- 10.Lu H, Guo X, Meng X, Liu J, Allen C, Wray J, Nickoloff JA, Shen Z. The BRCA2-interacting protein BCCIP functions in RAD51 and BRCA2 focus formation and homologous recombinational repair. Mol Cell Biol. 2005;25:1949–1957. doi: 10.1128/MCB.25.5.1949-1957.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng X, Fan J, Shen Z. Roles of BCCIP in chromosome stability and cytokinesis. Oncogene. 2007;26:6253–6260. doi: 10.1038/sj.onc.1210460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Yuan Y, Huan J, Shen Z. Inhibition of breast and brain cancer cell growth by BCCIPalpha, an evolutionarily conserved nuclear protein that interacts with BRCA2. Oncogene. 2001;20:336–345. doi: 10.1038/sj.onc.1204098. [DOI] [PubMed] [Google Scholar]
- 13.Roversi G, Pfundt R, Moroni RF, Magnani I, van Reijmersdal S, Pollo B, Straatman H, Larizza L, Schoenmakers EF. Identification of novel genomic markers related to progression to glioblastoma through genomic profiling of 25 primary glioma cell lines. Oncogene. 2006;25:1571–1583. doi: 10.1038/sj.onc.1209177. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ, Fisher PB. Molecular basis of nuclear factor-kappaB activation by astrocyte elevated gene-1. Cancer Res. 2008;68:1478–1484. doi: 10.1158/0008-5472.CAN-07-6164. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch EF, Maniatis T, editors. Molecular Cloning: A Laboratory Manual. 2 ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 16.Britt DE, Yang DF, Yang DQ, Flanagan D, Callanan H, Lim YP, Lin SH, Hixson DC. Identification of a novel protein, LYRIC, localized to tight junctions of polarized epithelial cells. Exp Cell Res. 2004;300:134–148. doi: 10.1016/j.yexcr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Emdad L, Sarkar D, Su ZZ, Lee SG, Kang DC, Bruce JN, Volsky DJ, Fisher PB. Astrocyte elevated gene-1: recent insights into a novel gene involved in tumor progression, metastasis and neurodegeneration. Pharmacol Ther. 2007;114:155–170. doi: 10.1016/j.pharmthera.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C, Huang J. Phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway is essential for neuroendocrine differentiation of prostate cancer. J Biol Chem. 2007;282:3571–3583. doi: 10.1074/jbc.M608487200. [DOI] [PubMed] [Google Scholar]
- 19.Humez S, Monet M, Legrand G, Lepage G, Delcourt P, Prevarskaya N. Epidermal growth factor-induced neuroendocrine differentiation and apoptotic resistance of androgen-independent human prostate cancer cells. Endocr Relat Cancer. 2006;13:181–195. doi: 10.1677/erc.1.01079. [DOI] [PubMed] [Google Scholar]
- 20.Meng X, Yue J, Liu Z, Shen Z. Abrogation of the transactivation activity of p53 by BCCIP down-regulation. J Biol Chem. 2007;282:1570–1576. doi: 10.1074/jbc.M607520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H, Yue J, Meng X, Nickoloff JA, Shen Z. BCCIP regulates homologous recombination by distinct domains and suppresses spontaneous DNA damage. Nucleic Acids Res. 2007;35:7160–7170. doi: 10.1093/nar/gkm732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Wu C, di Sant'Agnese PA, Yao JL, Cheng L, Na Y. Function and molecular mechanisms of neuroendocrine cells in prostate cancer. Anal Quant Cytol Histol. 2007;29:128–138. [PubMed] [Google Scholar]