Abstract
Background
Pancreatic β-cell ATP-sensitive potassium (KATP) channels are critical links between nutrient metabolism and insulin secretion. In humans, reduced or absent β-cell KATP channel activity resulting from loss-of-function KATP mutations induces insulin hypersecretion. Mice with reduced KATP channel activity also demonstrate hyperinsulinism, but mice with complete loss of KATP channels (KATP knockout mice) show an unexpected insulin undersecretory phenotype. Therefore we have proposed an “inverse U” hypothesis to explain the response to enhanced excitability, in which excessive hyperexcitability drives β-cells to insulin secretory failure without cell death. Many patients with type 2 diabetes treated with antidiabetic sulfonylureas (which inhibit KATP activity and thereby enhance insulin secretion) show long-term insulin secretory failure, which we further suggest might reflect a similar progression.
Methods and Findings
To test the above hypotheses, and to mechanistically investigate the consequences of prolonged hyperexcitability in vivo, we used a novel approach of implanting mice with slow-release sulfonylurea (glibenclamide) pellets, to chronically inhibit β-cell KATP channels. Glibenclamide-implanted wild-type mice became progressively and consistently diabetic, with significantly (p < 0.05) reduced insulin secretion in response to glucose. After 1 wk of treatment, these mice were as glucose intolerant as adult KATP knockout mice, and reduction of secretory capacity in freshly isolated islets from implanted animals was as significant (p < 0.05) as those from KATP knockout animals. However, secretory capacity was fully restored in islets from sulfonylurea-treated mice within hours of drug washout and in vivo within 1 mo after glibenclamide treatment was terminated. Pancreatic immunostaining showed normal islet size and α-/β-cell distribution within the islet, and TUNEL staining showed no evidence of apoptosis.
Conclusions
These results demonstrate that chronic glibenclamide treatment in vivo causes loss of insulin secretory capacity due to β-cell hyperexcitability, but also reveal rapid reversibility of this secretory failure, arguing against β-cell apoptosis or other cell death induced by sulfonylureas. These in vivo studies may help to explain why patients with type 2 diabetes can show long-term secondary failure to secrete insulin in response to sulfonylureas, but experience restoration of insulin secretion after a drug resting period, without permanent damage to β-cells. This finding suggests that novel treatment regimens may succeed in prolonging pharmacological therapies in susceptible individuals.
In a mouse study aiming to understand why long-term treatment for type 2 diabetes with sulfonylureas eventually fails, Colin Nichols and Maria Remedi suggest that slow restoration of insulin secretion may be possible after a drug-resting period.
Editors' Summary
Background.
Diabetes is an increasingly common chronic disease characterized by high blood sugar (glucose) levels. In normal people, blood sugar levels are controlled by the hormone insulin. Insulin is released by β-cells in the pancreas when blood glucose levels rise after eating (glucose is produced by the digestion of food). In fasting people, membrane proteins called ATP-sensitive potassium (KATP) channels keep the β-cell in a “hyperpolarized” state in which they do not secrete insulin. After a meal, glucose enters the β-cell where its chemical breakdown converts ADP into ATP (the molecule that provides the energy that drives cellular processes). The increased ratio of ATP to ADP closes the KATP channels, “depolarizes” the β-cells, and allows the entry of calcium ions, which trigger insulin release. The released insulin then “instructs” insulin-responsive muscle and fat cells to take up glucose from the bloodstream. In type 2 diabetes, the commonest type of diabetes, the muscle and fat cells gradually become nonresponsive to insulin and consequently blood glucose levels rise. Over time, this hyperglycemia increases the risk of heart attacks, kidney failure, and other life-threatening complications. On average, people with diabetes die 5–10 y younger than people without diabetes.
Why Was This Study Done?
People with type 2 diabetes are often initially treated with drugs called sulfonylureas (for example, glibenclamide). Sulfonylureas help to reduce blood glucose levels by inhibiting (in effect, closing) the KATP channels, which enhances insulin secretion. Unfortunately, after patients have been treated for several years with sulfonylureas, their β-cells often stop secreting insulin and the patients then have to inject insulin to control their blood sugar levels. The mechanism by which chronic sulfonylurea treatment affects β-cell behavior is poorly understood, which means that it is hard to improve this antidiabetes treatment. Mice that have been genetically altered so that they have no KATP channels (KATP knockout mice) also rapidly lose their ability to secrete insulin, although they secrete unusually large amounts at birth. This suggests that permanent membrane depolarization (β-cell hyperexcitability) may cause insulin secretory failure. In this study, the researchers investigate whether this mechanism might be responsible for sulfonylurea-induced loss of insulin secretion.
What Did the Researchers Do and Find?
The researchers implanted slowly releasing pellets of glibenclamide into wild-type mice and then monitored their blood glucose levels and glucose tolerance (the speed of glucose removal from the blood after a glucose “meal”) for up to 128 d; the pellets released drug for 90 d. The glibenclamide-implanted mice progressively developed diabetes, lost the ability to secrete insulin in response to glucose and, after 1 wk of treatment, were as glucose intolerant as adult KATP knockout mice. Compared to freshly isolated β-cells from untreated wild-type mice, glucose-stimulated insulin secretion by β-cells isolated from glibenclamide-treated wild-type mice and from KATP knockout mice was reduced to a similar degree. However, the secretory capacity of β-cells isolated from the glibenclamide-treated wild-type mice was restored to normal within hours of drug washout and was normal in β-cells isolated from treated mice 1 mo after exhaustion of the slow-release pellets. Consistent with this result, there was no obvious β-cell death in the glibenclamide-treated mice.
What Do These Findings Mean?
Although findings from animal studies do not always reflect what happens in people, these findings suggest that insulin secretion might sometimes fail in people who take sulfonylureas for a long time, because these drugs cause β-cell hyperexcitability. The finding that the secretory failure caused by sulfonylurea treatment is reversible is important because it suggests that short-acting sulfonylureas might be re-evaluated to see whether they could delay sulfonylurea-induced failure of the insulin secretory response by providing the pancreatic β-cells with periods when they are not depolarized. This finding (and the absence of β-cells death in the glibenclamide-treated mice) also suggests that there may be a way to reverse the loss of the insulin secretory response in patients who have taken sulfonylureas for a long time. Both approaches could help patients with diabetes delay or even avoid the need for insulin injections.
Additional Information.
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.0050206.
This study is further discussed in a PLoS Medicine Perspective by Renstrom and colleagues
The MedlinePlus encyclopedia provides information for patients about diabetes (in English and Spanish)
The US National Diabetes Information Clearinghouse provides information on all aspects of diabetes (in English and Spanish)
The International Diabetes Federation also provides comprehensive information about diabetes
Wikipedia has pages on KATP channels and on sulfonylurea drugs (note: Wikipedia is a free online encyclopedia that anyone can edit; available in several languages)
Introduction
Pancreatic β-cell ATP-sensitive potassium (KATP) channels are a critical link between nutrient metabolism and insulin secretion, maintaining the blood sugars in a narrow physiological range. In fasted animals, KATP channels provide the dominant β-cell membrane conductance, maintaining the cell in a hyperpolarized state and stopping insulin secretion. Conversely, in the fed state, glucose metabolism increases the [ATP]/[ADP] ratio, closing KATP channels, causing membrane depolarization and voltage-dependent Ca2+ entry, which in turn trigger insulin secretion [1].
The KATP channel is a heteroctameric complex composed of four inwardly rectifying K+ channel subunits (Kir6.2) and four sulfonylurea (SU) receptors (SUR1) [2]. Loss-of-function mutations of β-cell KATP channel subunits (SUR1, ABCC8, OMIM accession number 600509; and Kir6.2, KCNJ11, OMIM 600937; GenBank, http://www.ncbi.nlm.nih.gov/) underlie congenital hyperinsulinism (HI) in humans [3–7], a genetic disease characterized by relative hyperinsulinemia and hypoglycemia [8]. In HI, reduced or absent KATP channel activity is expected to result in constitutive depolarization, elevated intracellular [Ca2+], and hypersecretion of insulin [9]. In order to replicate the human disease, mice lacking Kir6.2 or SUR1 and mice expressing dominant-negative mutant Kir6.2-encoding transgenes have been generated. Kir6.2- or SUR1-knockout (KO) mice show insulin hypersecretion immediately after birth, but rapidly and unexpectedly progress to glucose intolerance and insulin hyposecretion as adults [10–12]. Conversely, the β-cell–specific Kir6.2[AAA] dominant-negative mouse [13] demonstrates only a ∼70% decrease in β-cell KATP channel activity, and exhibits insulin hypersecretion with hyperinsulinemia that persist through adulthood. Heterozygous Kir6.2+/− and SUR1+/− mice, which also have reduced KATP gene dosage (∼60%), also show enhanced glucose tolerance and glucose-sensitive insulin secretion (GSIS) that is maintained throughout adulthood, without progression to secretory failure [14].
We therefore conclude that varying degrees of genetic suppression of KATP channel activity will all lead to enhanced excitability, but with different long-term consequences for insulin secretion, depending on the severity of suppression: incomplete loss of KATP channels (e.g., in Kir6.2[AAA] [13] or in heterozygous Kir6.2+/− or SUR1+/− mice [14]) causes a maintained hyperinsulinism, whereas complete loss (in Kir6.2- and SUR1-KO mice) causes transient hypersecretion that is followed by a secretory deficit and reduced glucose tolerance [10–12]. The recombinant phenotypes of many HI mutations [6,15,16] actually suggest that reduced, but not complete, absence of KATP channels [17] is likely. Henwood et al. [18] have demonstrated that some HI patients with KATP channel mutations clearly maintain some KATP channel activity, because the patients were responsive to KATP channel drugs. There are also limited reports that some HI patients, even those nonsurgically treated, can spontaneously progress to type 2 diabetes [19–21]. HI is also often present at the onset of clinically overt type 2 diabetes, which is interpreted as the endocrine pancreas trying to compensate for primary defects in insulin sensitivity in the peripheral tissues [22]. Thus the progression from relative to absolute insulin deficiency by decreasing insulin secretory capacity appears as the result of a detrimental long-term increased workload of β-cells [23].
SUs, such as tolbutamide and glibenclamide (glyburide), are widely used in patients with type 2 diabetes, because they induce insulin secretion independently of the metabolic state of the β-cell [24–26]. These antidiabetic drugs bind to the SUR1 subunit, leading to inhibition of KATP channel activity, membrane depolarization, and insulin secretion. Patients with type 2 diabetes chronically treated with SUs often progress to a failure of β-cells to secrete insulin [27–29]. The systemic or cellular mechanism underlying such failure is not well understood, although it could be linked either to the evolution of the disease or to a specific effect of the drugs [30–32]. Consistent with all these studies, the UK Prospective Diabetes Study (UKPDS) revealed that 48% of the patients with type 2 diabetes treated for 6 y with glibenclamide required additional therapy to maintain their normal blood sugars [33].
Despite the widespread use of SUs, there is also evidence that chronic SU (tolbutamide and glibenclamide) treatment may induce Ca2+-dependent β-cell apoptosis in rat islets [34] and human islets incubated with glibenclamide demonstrated a significant decrease in insulin content (24 h incubation), as well as an approximate 2-fold increase in β-cell apoptosis (4 d incubation) [35,36]. Glibenclamide-treated Min6 cells reportedly also showed a reversible reduction in insulin content and an accelerated apoptotic β-cell death [37–39], although SU (glibenclamide)-induced apoptosis was apparently specifically enhanced only by expression of the receptor SUR1, but not SUR2B, in HEK 293 cells [40].
The parallels between the long-term consequence of genetic hyperexcitability in mice and desensitization/apoptosis to prolonged SU treatment in humans lead us to hypothesize that, in vivo, SUs will induce an increase in electrical activity leading to enhanced insulin secretion in the short term, but that in the longer term they may still cause membrane hyperexcitability but paradoxically lead to insulin secretory failure, reproducing the KATP channel KO mouse phenotype. In this paper we specifically tested this hypothesis using a novel pharmacological approach by implanting slow-release (90 d) SU (glibenclamide) pellets.
Methods
Animals
Wild-type C57BL/6 mice were obtained from Jackson Laboratories (6-wk-old males, JAX mice; http://jaxmice.jax.org/). Kir6.2 KO mice (a gift from Dr. Susumo Seino) were originally generated by targeted disruption of the gene encoding Kir6.2 in the 129Sv mouse strain [10]. SUR1 KO mice (a gift from Dr. Mark Magnuson) were originally generated in the C57BL/6 mice by pronuclear microinjection of 1 ng/μl CMV-Cre expression vector (pBS185) into embryos obtained from a mating of Sur1lox+neo /w and Sur1w /w mice [11]. All experiments were performed in compliance with the relevant laws and institutional guidelines, and were approved by the Washington University Animal Studies Committee.
Acute Glibenclamide Injection in Wild-Type C57BL/6 Mice
Fed wild-type (WT) mice were acutely injected with glibenclamide (0, 1, 3, 10, and 30 μg) and tested for blood glucose levels over time. Intraperitoneal glucose–glibenclamide tolerance tests were performed in fasted (12 h) 6-wk-old WT mice by simultaneous injection of a bolus of glucose (1.5 g/kg) plus glibenclamide at the indicated doses. Glibenclamide responsivity following implantation was assessed by injection of 30 μg/ml in fed mice. Blood was assayed for glucose content using the Glucometer Elite XL (Bayer, http://www.bayer.com).
Blood Glucose and Plasma Insulin Measurements on Mice Implanted with Glibenclamide Pellets
Glibenclamide (glyburide) pellets at the concentration of 0.0001, 0.001, 0.025, 0.25, and 2.5 mg per 90-d release were obtained from Innovative Research of America (Sarasota, FL). Six-week old males were anesthetized with tribromoethanol (Avertin; 0.25 mg/g mouse body weight). The skin on the lateral side of the neck of the animal was lifted and pellets were implanted under the skin of the neck using a stainless steel precision trochar. Blood samples taken from the tail vein in fed and fasting conditions, and during glucose tolerance tests (GTTs), were assayed for glucose content as described above. Intraperitoneal GTTs were performed in WT, Kir6.2-KO, and SUR1-KO mice implanted with different glibenclamide pellet concentrations. For insulin tolerance tests (ITTs), mice were injected with 0.5 U insulin/kg following 6-h fasting. Blood was taken at different times (as indicated in figures) and assayed for glucose content. Plasma insulin was measured on glibenclamide-treated mice at 2 d and 42 d after pellet implantation using a rat insulin ELISA kit.
Islet Isolation
Mice were anesthetized with halothane (0.2 ml) in an anesthetizing chamber and killed by cervical dislocation. Pancreata were removed and injected with Hank's balanced salt solution (HBSS; Sigma-Aldrich, http://www.sigmaaldrich.com/) containing collagenase (0.3 mg/ml) (pH 7.4). Collagenase type XI was obtained from Sigma-Aldrich. Pancreata were digested for 5 min at 37 °C, hand-shaken, and washed three times in cold HBSS solution [41]. Islets were isolated by hand under a dissecting microscope and pooled islets were maintained in CMRL-1066 culture medium (GIBCO) supplemented with fetal calf serum (FCS, 10%), penicillin (100 units/ml), and streptomycin (100 μg/ml).
86Rb+ Efflux Experiments
Isolated islets were pre-incubated with 86Rb+ (rubidium chloride 1.5 mCi/ml, Amersham Biosciences) for 1 h. Loaded islets were placed in microcentrifuge tubes (30 per group) and washed twice with RPMI-1640 media (Sigma-Aldrich). 86Rb+ efflux was assayed by replacing the bathing solution with Ringer's solution with metabolic inhibitor (MI), with or without 1 μmol/l glibenclamide. MI solution contained 2.5 mg/ml oligomycin, 1 mM 2-deoxyglucose, together with 10 mM tetraethylammonium to block voltage-gated K+ channels, 10 μM nifedipine to block Ca2+ entry, and 30 mM KCl to maintain Em ∼ 0. The bathing solution was replaced with fresh solution every 5 min over a 40 min period, and counted in a scintillation counter. 86Rb efflux was fitted by a single exponential and the reciprocal of the exponential time constant (rate constant) for each efflux experiment is then proportional to the K+ (Rb+) conductance of the islet membranes.
Insulin Release Experiments
Acutely, following isolation, or after overnight incubation in low-glucose (5.6 mM) CMRL-1066, islets (ten per well in 12-well plates) were preincubated for 30 min in glucose-free CMRL-1066 plus 3 mM D(+)-glucose, then incubated in CMRL-1066 plus different glucose concentration, as indicated. Islets were incubated for 60 min at 37 °C and medium removed and assayed for insulin content. Isolated islets were sonicated on ice prior to estimation of insulin content. Insulin was measured using rat insulin radioimmunoassay according to manufacturer's procedure (Linco, http://www.millipore.com). Experiments were repeated in triplicate.
Immunohistological Analysis
Pancreata from WT, Kir6.2 KO, and SUR1 KO mice treated for 56 d with placebo or glibenclamide pellets were fixed in 10% formalin and paraffin embedded for serial sectioning (5 μm thick). Hematoxylin-eosin (HE) staining was carried out as described previously [42]. For insulin and glucagon immunofluorescence, pancreatic sections were incubated overnight at 37 °C with a guinea pig anti-insulin primary antibody or a guinea pig anti-glucagon primary antibody (1:250 or 1:500, respectively; Linco). Primary antibodies were detected by incubating for 1.5 h at 25 °C with an anti-guinea pig secondary antibody conjugated with the Alexa 488 fluorescent dye (Molecular Probes, http://www.invitrogen.com) [42]. Apoptosis was assessed on paraffin sections using the TUNEL staining technique (ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit, Chemicon International, httpp://www.millipore.com). DNAase I recombinant (Roche) was used to damage DNA as a positive control for the experiment.
Statistics
Data are presented as mean ± standard error of the mean (SEM). Differences between the non-implanted control group and the pellet-implanted groups were assessed using analysis of variance (ANOVA) within each time point. When significant and within the framework of the ANOVA, the Duncan post hoc test was used to test specific hypotheses about the equivalence of the control group with each test group. When only two groups were compared, unpaired t-tests were used to assess significance. Differences were assumed to be significant in each case if p < 0.05. When the sample size was three or less, statistics were not performed and significance is not assigned. Given sample size limitations, we did not use a multilevel model to compare change over time between groups. Unless stated otherwise, asterisks in figures indicate significant difference between the test group and the control group within time point or condition, non-significant differences are not indicated.
Results
Glucose Tolerance Is Enhanced and Fed Glucose Is Reduced in WT Mice Acutely Injected with Glibenclamide
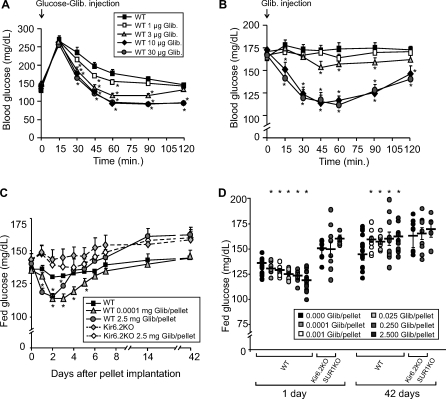
Six-week-old C57BL/6 WT mice simultaneously injected with 1.5 g/kg glucose and glibenclamide (0, 1, 3, 10, and 30 μg) showed a dose-dependent enhancement of glucose tolerance, with a more rapid glucose decline compared with control mice injected with only glucose (Figure 1A). Consistent with the well-known acute effect of SUs on blood sugars, and in correlation with the glibenclamide effect on the GTT, these acute injections caused a rapid, dose-dependent drop in blood glucose levels. Importantly, the two highest concentrations of glibenclamide (10 and 30 μg) caused a similar marked reduction of fed blood glucose after an extended period, consistent with a saturated effect of the drug in vivo (Figure 1B).
Figure 1. Acute and Chronic Effect of Glibenclamide on Glucose Tolerance and Blood Glucose Levels.
In each graph, asterisks indicate control group is significantly different (p < 0.05) from the test group at the specified time point.
(A) GTTs on 6-wk-old WT mice (n = 10) fasted for 12 h and intraperitoneally injected with 1.5 g/kg glucose and glibenclamide simultaneously.
(B) Blood glucose response of fed 6-wk-old mice (n = 10) to acute injection of glibenclamide.
(C) Fed blood glucose from WT and Kir6.2 knockout (Kir6.2KO) mice implanted with placebo or glibenclamide pellets (0.0001 or 2.5 mg/pellet) over time.
(D) Individual values of fed glucose (plus mean and SEM) from WT control and glibenclamide-pelleted mice, as well as Kir6.2-KO and SUR1-KO mice implanted with 2.5 mg pellets, at 1 and 42 d after implantation.
Each group in (C) and (D) contained ten to 15 mice.
Biphasic Blood Glucose Response of WT Mice Chronically Treated with Glibenclamide
As discussed in the Introduction, KATP KO mice show an unexpected phenotype that contradicts the simple prediction for complete inhibition of KATP channel activity [10–12]. We have previously shown that this phenotype depends on complete, or nearly complete loss of KATP, while intermediate loss induces insulin hypersecretion [14]. We suggest that this biphasic response profile is a direct consequence of loss of KATP and resultant hyperexcitability. In order to pharmacologically test this hypothesis, we took advantage of the availability of slow-release implantable drug pellets. Mice were chronically treated with glibenclamide, a specific KATP channel blocker, by pellet implantation at 6 wk of age. We could thus examine long-term (90 d) effects of the drug, avoiding the peak and valley effect produced by single injections and the stress of frequent injections.
During the first four days following implantation with high-dose slow-release glibenclamide pellets, fed blood glucose levels in WT mice were significantly lower than in placebo-implanted mice (Figure 1C), and the glucose-lowering effect was glibenclamide-dose–dependent. The circulating glibenclamide level is unknown in these experiments. However, therapeutic glibenclamide doses in humans are typically around 0.05 mg/kg/d. Pellets containing 0.1 mg released over 90 d should give a similar net dose in a 25 g mouse; thus, the implanted doses (0.0001–2.5 mg) should span therapeutic ranges. The initial decrease in fed blood sugars was lost within a few days, and fed blood glucose was significantly higher in implanted animals after 4–5 d, for all implanted doses except the very lowest 0.0001 mg dose (Figure 1C). Two weeks after pellet implantation, all glibenclamide-treated mice (with the exception of those with the 0.0001 mg pellet) showed similar elevated fed blood glucose, and this elevated level persisted without further impairment until 42 d after implantation (Figure 1C). The elevation was essentially the same as that seen in placebo-treated KATP KO mice, with no significant differences between glibenclamide- and placebo-treated KO mice (Figure 1C and 1D).
Impaired Glucose Tolerance in WT Mice Chronically Treated with High Doses of Glibenclamide
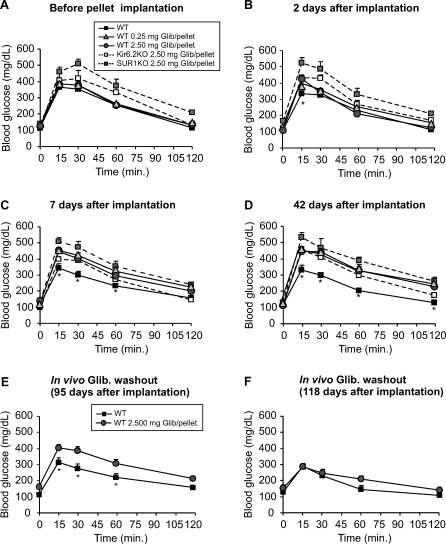
As previously reported, Kir6.2 KO and SUR1 KO mice are less glucose tolerant than WT mice [10–12], as illustrated by intraperitoneal GTT (Figure 2A). WT mice implanted with high-dose glibenclamide pellets (0.25 and 2.5 mg/pellet, equivalent to a release of 3 and 30 μg/d) showed very rapid progression to impaired glucose tolerance compared to WT placebo-treated mice (Figure 2B–2D). After only 7 d (Figure 2C), and sustained thereafter to at least 42 d postimplantation (Figure 2D), glibenclamide-treated mice were as glucose intolerant as KO mice. Moreover, KATP KO mice chronically treated with glibenclamide show no change in the preexisting glucose-intolerant response. Thus, pharmacological block of the KATP channel in WT mice essentially reiterates the loss of glucose tolerance seen in KATP channel KO mice.
Figure 2. Impaired Glucose Tolerance in Mice Implanted with High Dose Slow-Release Glibenclamide Pellets.
GTTs on WT mice before (A), 2 d (B), 7 d (C), 42 d (D), 95 d (E), and 118 d (F) after implantation of pellets containing high doses of glibenclamide. Each group contained ten mice, except five in (E) and (F). Mice were injected intraperitoneally with glucose (1.5 g/kg). Blood was taken at times indicated and assayed for glucose concentration. In each graph, asterisks indicate control group is significantly different (p < 0.05) from the test group at the specified time point.
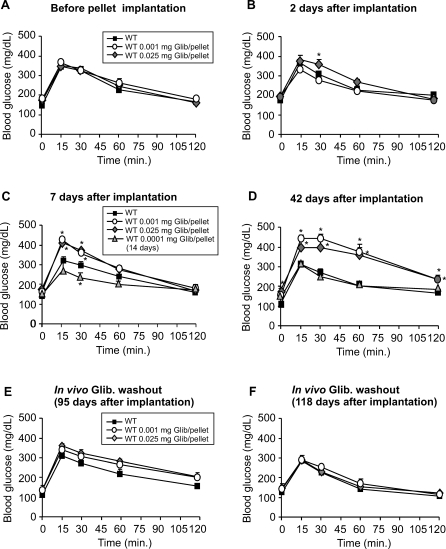
Enhanced Glucose Tolerance, but Crossover to a Progressive Impairment of Glucose Tolerance, in Mice Implanted with Low-Dose Glibenclamide Pellets
GTTs were also performed in mice implanted with pellets containing much lower doses of glibenclamide (Figure 3). An early (2 or 7 d after implantation) mild enhancement of glucose tolerance was detectable in mice implanted with a very low dose of glibenclamide (0.001 or 0.0001 mg/pellet, Figure 3B and 3C, respectively), but this was again followed by a progressive impairment in glucose tolerance over time (Figures 3C and 3D). Again, 42 d postimplantation, 0.001 mg/pellet–treated mice showed similar impairment in glucose tolerance to mice treated with high doses of glibenclamide for the same period of time (Figures 2D and 3D). However, this crossover was markedly delayed with the very lowest dose (0.0001 mg/pellet); at 7 d glucose tolerance was significantly enhanced, although at 42 d, blood glucose and glucose tolerance were similar to control. Thus, at very low doses, the “pelleted” mice resemble heterozygous KATP knockout mice [13,14,43], at least for a period of 2–7 d.
Figure 3. Enhancement and Crossover of Glucose Tolerance in Mice Implanted with Low Dose Slow-Release Glibenclamide Pellets.
In each panel, asterisks indicate control group is significantly different (p < 0.05) from the test group at the specified time point.
GTTs performed on WT mice before (A), 2 d (B), 7 d (C), 42 d (D), 95 d (E), and 118 d (F) after implantation of pellets containing high doses of glibenclamide. Groups contained ten mice each, except five in (E) and (F). Note: The lowest-dose pellets (0.0001 mg) were examined only at 14 and 42 d postimplant.
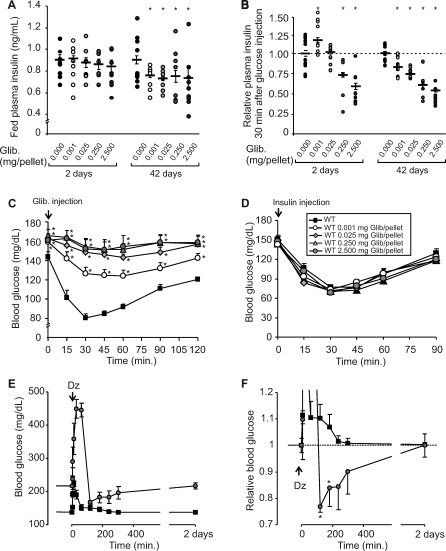
In Vivo Dose- and Time-Dependent Changes in Insulin Secretion in Response to Glucose Injection in Mice Chronically Treated with Glibenclamide Pellets
Two days after implantation, plasma insulin was very slightly elevated in mice treated with 0.001 mg pellets, but was dose-dependently reduced in all mice treated with higher doses for the same period. At 42 d after glibenclamide treatment, fed plasma insulin was significantly reduced in all implanted mice (Figure 4A), paralleling the marked increase in blood sugar (Figure 1D), independent of the glibenclamide dose. Glucose-stimulated insulin secretion (GSIS) in vivo was assessed from plasma insulin values 30 min after glucose challenge. Insulin secretion in response to glucose was increased in mice treated with the lowest dose of glibenclamide, consistent with the enhanced glucose tolerance observed in these mice 2 d after drug implantation. Also consistent with changes in glucose tolerance, all other glibenclamide-implanted mice showed a dose-dependent reduction in insulin secretion that worsened over time (Figure 4B).
Figure 4. Drug and Insulin Responsivity In Vivo.
In each graph, asterisks indicate control group is significantly different (p < 0.05) from the test group at the specified time point.
(A) Individual fed plasma insulin (plus mean and SEM) in glibenclamide-treated mice, 2 d and 42 d after glibenclamide implantation.
(B) Individual values (plus mean and SEM) of plasma insulin 30 min after glucose injection (normalized to control unpelleted) at either 2 d or 42 d.
(C and D) WT mice 5 wk implanted with different doses of glibenclamide were intraperitoneally injected with glibenclamide (C) or with insulin (D) and blood glucose was assessed over time.
(E and F) Control and high-dose glibenclamide-pelleted mice were intraperitoneally injected with 20 mg/kg diazoxide, and blood glucose was assessed over time. In (F), glucose is normalized to the precontrol value (indicated by broken line).
No Effect of Acute Glibenclamide Injection, and Normal Insulin Sensitivity in Mice Chronically Treated with Glibenclamide
To test whether the glucose-intolerant phenotype of chronically glibenclamide-treated mice is still correlated with loss of function of KATP channel activity, the response to acute injection of glibenclamide was measured at the times indicated. As expected for complete or near complete inhibition of the channel by the circulating drug levels, injection of glibenclamide produced no significant effect in mice chronically treated with high-dose pellets, and only a modest lowering of blood sugars in mice implanted with low-dose pellets (Figure 4C).
To assess insulin sensitivity in peripheral tissues, WT placebo- and glibenclamide-implanted mice were subjected to an ITT. Mice implanted with both low and high doses of glibenclamide showed similar insulin sensitivity to WT-placebo-implanted mice (Figure 4D).
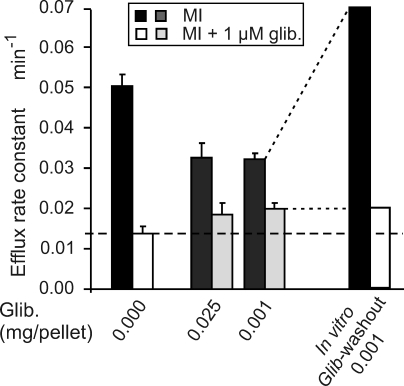
Glibenclamide-Treated Islets Exhibit a Marked Reduction of KATP Conductance
Macroscopic KATP channel density in freshly isolated (within 2 h) intact islets was assessed by 86Rb+ efflux under metabolic inhibition to lower cellular [ATP]:[ADP] and maximally activate available KATP channels (Figure 5). The efflux rate constant (proportional to available KATP conductance) was significantly reduced in glibenclamide-implanted mice compared with placebo-treated mice, indicating reduced K+ conductance. In WT placebo-treated islets, over 70% of the flux was glibenclamide-sensitive; however, WT glibenclamide-treated islets showed less than 40% glibenclamide-sensitive effluxes (Figure 5), indicating that KATP conductance was substantially reduced. Consistent with a reversible effect of the drug to inhibit the channel directly, maintenance of isolated islets from glibenclamide-implanted mice in the absence of the drug for 24 h (in vitro drug washout) show a dramatic recovery of glibenclamide-sensitive K+ conductance, without changes in glibenclamide-insensitive 86Rb+ effluxes (Figure 5).
Figure 5.
Glibenclamide-Treated Islets Exhibit a Reduction of Total KATP Channel Density Macroscopic KATP channel density in intact islets from WT placebo- and glibenclamide-treated mice was assessed by 86Rb+ effluxes under metabolic inhibition (MI) in the presence and absence of glibenclamide (glib.). Fluxes were fitted by single exponentials and reciprocal rate constant plotted in the figure (each case contained three animals; no significance assessed). Broken line indicates KATP-independent rate constant, determined by glibenclamide in control islets.
The assay of freshly isolated islets was made within 2 h of isolation, at which point there is clearly significant inhibition of KATP conductance in islets from implanted mice. However, realizing that full recovery occurs within 24 h, it seems likely that some recovery occurs even within this 2 h, so the measured KATP activity at that time is at best an upper limit estimate, and channel activity in vivo may have been completely suppressed by the circulating glibenclamide levels.
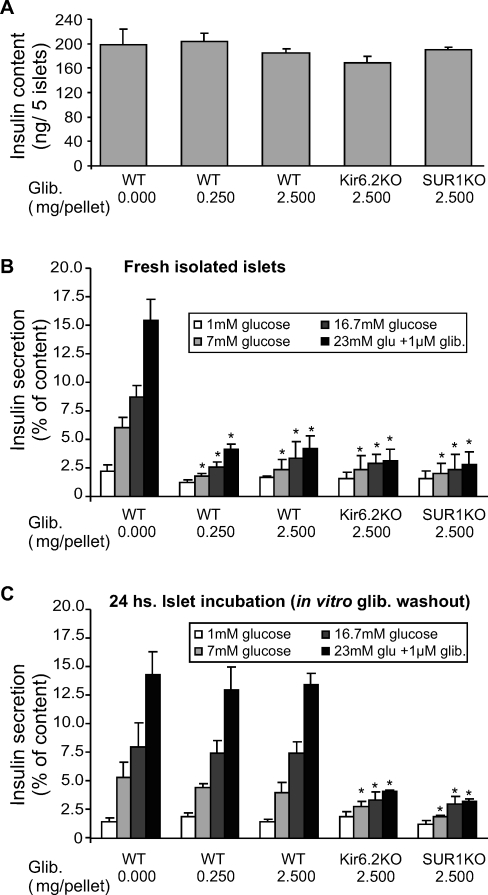
Chronically Glibenclamide-Treated Mice Show Reduced GSIS from Fresh Isolated Islets, but Restored Normal Insulin Secretion in Drug-Washout Islets
In order to assess the effect of long-term glibenclamide treatment on β-cell function directly, pancreatic islets were isolated from either WT or KATP KO mice after treatment with glibenclamide for 42 d. Insulin content of KO islets was slightly lower than WT islets, but insulin content was not significantly different between placebo- and pellet-implanted animals (Figure 6A). However, while freshly isolated islets from WT placebo-treated mice showed robust glucose- and glibenclamide-dependent insulin secretion, glibenclamide-treated WT and KATP KO islets both showed a similar, severely blunted response to both glucose and glibenclamide (Figure 6B). A 24 h incubation in CMRL medium (5.6 mM glucose, glibenclamide washout) restored normal glucose and glibenclamide responses in islets obtained from WT glibenclamide-treated mice (Figure 6C), paralleling the recovery of KATP conductance. However, as expected, insulin secretion was not improved in islets from glibenclamide-treated KATP KO mice.
Figure 6. Impaired Glucose-Dependence of Insulin Secretion in Fresh Isolated, but Not in 24-h Incubated Islets from Mice Chronically Treated with Glibenclamide.
(A) Insulin content from glibenclamide-pelleted mice.
(B and C) Glucose-sensitive insulin secretion from freshly isolated islets (B) and from islets incubated in 5.6 mM glucose for 24 h (C). Insulin release and content was measured by radioimmunoassay. Each group contained ten mice, and samples were assayed in triplicate. Significant differences between glibenclamide-implanted and control are indicated for each assay condition by asterisk.
There were no significant differences in (A).
Reversal of Phenotype Is also Observed In Vivo
The above results dramatically demonstrate rapid reversibility in vitro of the chronic glucose-desensitizing effects of glibenclamide. Removal of pellets to assess reversibility in vivo is impractical, so instead we examined glucose control in implanted mice, beyond the time at which release should cease (95 or 118 d). In this case, the animals should have been exposed to glibenclamide for the full 90-d release period, but then should have had either 5 d or 28 d without drug exposure. Partial restoration was detectable at 5 d after drug release (Figures 2E and 3E) was terminated, and even mice that had been implanted with 2.5 mg glibenclamide/pellet showed dramatic restoration of both insulin secretion and glucose tolerance 118 d after pellet implantation (i.e., 28 d after the drug release was terminated) (Figures 2F and 3F).
The restoration of secretion following the cessation of glibenclamide release raises the question of whether secretion might also be acutely restored by diazoxide treatment (to activate KATP and block the hyperexcitability) during the period of glibenclamide release. Acutely, diazoxide may cause glucose to rise, but in “resting” the β-cells may also transiently allow recovery of insulin “releasability,” leading to a transient restoration of normal glucose. We performed the experiment on control and high-dose (2.5 mg) pelleted animals. As predicted, there was a rise in glucose in both cases, but a marked subsequent prolonged undershoot only in the pelleted animals (Figure 4E and 4F).
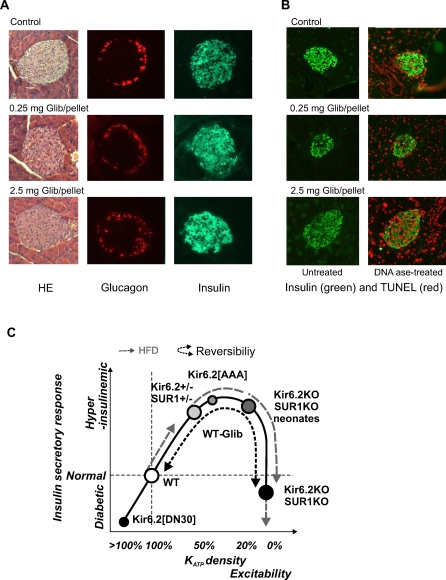
Morphology of Pancreas and α-/β-Cell Distribution within Islets from Glibenclamide-Implanted Mice Is Not Altered
The normal content of insulin and rapid recovery of secretion in isolated islets from glibenclamide-treated animals would seem to rule out apoptotic or necrotic effects of the drug on β-cells. However, to confirm this, immunohistochemical analyses were performed on sectioned pancreata from 9-wk glibenclamide-implanted mice. Hematoxylin-eosin staining showed similar pancreatic architecture in glibenclamide-implanted vs. placebo-treated mice (Figure 7A, left photomicrographs). Immunostaining for insulin and glucagon confirmed normal distribution of both insulin-containing β-cells and glucagon-containing α-cells, with no obvious changes in islet size (Figure 7A, middle and right photomicrographs). No marked redistribution of α-/β-cells or loss of β-cell mass (i.e., reduced insulin immunofluorescence) was observed in glibenclamide-treated mice, although there was a very slight tendency for α-cell infiltration in the core of glibenclamide-treated pancreatic islets. In order to specifically test for the possibility of enhanced β-cell death, in situ immunostaining for apoptosis was performed in paraffin-fixed sections using the TUNEL technique (Figure 7B). Pancreatic sections were costained with insulin (green) and TUNEL (red). The absence of red staining in the left images of Figure 7B indicates no obvious β-cell apoptosis was present in islets from control or high dose (0.25 and 2.5 mg/pellet) glibenclamide-pelleted mice. As positive control, consecutive pancreatic sections from each sample were treated with recombinant DNAase I, resulting in extensive TUNEL positivity in endocrine as well as exocrine cells (Figure 7B, right photomicrographs).
Figure 7. Morphological and Physiological Response to Chronic Hyperexcitability, and Proposed “Inverse U” Model Response for Enhanced β-Cell Excitability.
(A) Hematoxylin-eosin staining (left), glucagon (middle), and insulin (right) immunostaining of pancreatic paraffin sections from WT control and glibenclamide-implanted mice.
(B) Insulin (green) and TUNEL (red) staining of pancreatic sections from control and glibenclamide-pelleted mice. Left images show images from untreated sections; right images show islets from paraffin sections treated with recombinant DNAase I (TUNEL-positive controls).
(C) Proposed “Inverse U” model response for enhanced β-cell excitability: normal islets (white circle) secrete normally, but following a high-fat diet (HFD; grey dashed arrow) progress to insulin hypersecretion. Both Kir6.2[AAA] and heterozygous Kir6.2- and SUR1-KO mice (50%–70% decreased KATP activity with increased excitability) hypersecrete (grey circles, solid line) and are positioned on the “ascending limb” of the curve [13,14,43]. HFD causes further enhancement of excitability, beyond the threshold driving those islets “over the top” (dashed) to an undersecretory phenotype. Conversely, Kir6.2- and SUR1-KO islets (zero KATP channel activity), which have maximally enhanced excitability, hypersecrete as neonates (grey circle on solid line), but rapidly progress to an undersecretory phenotype (black circle on solid line), and are positioned on the “descending limb” [10–12]. Glibenclamide-treated WT mice rapidly progress from normal insulin secretion to undersecretion (white circle on “ascending limb” is converted to black circle on “descending limb”). This phenotype can be completely reversed when hyperexcitability is removed (small black dashed line). Finally, mice expressing mutant β-cell KATP channels with enhanced activity (Kir6.2[ΔN30]) [41] have a severely undersecreting phenotype, extending the ascending limb beyond the position of normal animals.
Discussion
In the present study, we examined the “inverse U” hypothesis to explain the response to enhanced excitability, in which excessive hyperexcitability drives β-cells to insulin secretory failure [14,42], using a novel approach of implanting mice with slow-release sulfonylurea (glibenclamide) pellets, to chronically inhibit β-cell KATP channels. Glibenclamide-implanted wild-type mice became progressively and consistently diabetic, with significantly reduced insulin secretion in response to glucose. After 1 wk of treatment, these mice were as glucose intolerant as adult KATP knockout mice, with similar loss of secretory capacity. However, secretory capacity was fully restored in these islets within hours of drug washout in vitro, or within 1 mo after glibenclamide treatment was terminated in vivo. Pancreatic immunostaining showed normal islet size and α-/β-cell distribution within the islet, and TUNEL staining showed no evidence of apoptosis.
Hyperexcitability and Hyperinsulinism in Animal Models and Humans: Key Features and Discrepancies
Glucose metabolism increases the β-cell [ATP]:[ADP] ratio, leading to closure of KATP channels, membrane depolarization and Ca2+ influx. The resultant rise in intracellular Ca2+ ([Ca2+]i) triggers insulin secretion. Reduced or absent KATP channel activity is expected to result in constitutive membrane depolarization, elevated [Ca2+]i, and hypersecretion of insulin. Consistent with this prediction, loss-of-function mutations of KATP channel subunits (SUR1, ABCC8 or Kir6.2, KCNJ11) underlie congenital HI in humans [9]. Conversely, gain-of-function mutations of Kir6.2 or SUR1 are expected to cause an undersecreting phenotype and hypoinsulinemia—and consistent with this prediction, activating mutations in KATP cause both permanent and transient neonatal diabetes [44,45].
Genetic manipulation of KATP channel subunits in mice has confirmed some of the basic expectations of the above paradigm and reiterated some features of human disease, although conflicting and contradictory findings illustrate additional complexities, particularly for models of reduced KATP channel activity. Mice that completely lack KATP channels do not reiterate the HI phenotype in any simple way. In these mice, hypersecretion reportedly occurs immediately after birth, but there is rapid progression to a relative undersecretion and glucose intolerance [10–12]. There is thus an apparent discrepancy between the outcome of genetic loss of KATP channels in mice and humans. One important potential caveat to consider is whether patients typically have a complete loss of KATP channels, and therefore whether complete KO mice are appropriate models for the disease. Both trafficking and functional mutations will probably tend to cause only a relative loss of KATP conductance. Mice expressing a dominant-negative Kir6.2[AAA] transgene in β-cells, as well as mice heterozygous for Kir6.2 or SUR1, may model such a condition, since they show substantial, but incomplete, reduction of KATP channel activity (∼60%–70%). All of these models demonstrate both an enhanced glucose stimulation of insulin secretion and hyperinsulinism that persists through adulthood [13,14,43].
Biphasic Response to KATP Inhibition: Pharmacological Verification of the “Inverse-U” Model for β-Cell Response to Hyperexcitability
We therefore propose that varying degrees of genetic suppression of KATP channels will all lead to enhanced excitability and insulin secretion, but with potentially very different long-term consequences depending on the severity of suppression: incomplete loss of KATP channels (e.g., in Kir6.2[AAA] or in heterozygous Kir6.2+/− or SUR1+/− mice) causes a persistent hyperinsulinism, whereas complete loss (e.g., in KATP KO mice) may transiently cause hypersecretion, but this is followed by a secretory deficit and reduced glucose tolerance (Figure 7C) [14,42].
We suggest that this model may be generalizable to hyperexcitability resulting from any other mechanism, such as loss of other K+ conductances, or gain of excitatory current. However, it is important to note that the model is developed solely from experiments with genetic manipulation of KATP channel subunits, and requires verification by direct manipulation of excitability in genetically normal animals. In the present study we have successfully performed just such an experiment by utilizing slow-release implanted drug pellets. This approach allowed us to pharmacologically block KATP channels over a prolonged period in a tractable in vivo model. The dramatic consequence is that although blood glucose levels are initially lowered and glucose tolerance is enhanced (Figures 1 and 3), within a few days the phenotype crosses over to hyperglycemia and glucose intolerance that is strikingly similar to the adult phenotype of KATP KO mice for all except the very lowest-dose pellets. Earlier studies of “long-term” treatment of animals with sulfonylureas have generally not gone beyond a few days [46], presumably due to the practical difficulties of injection regimens. Importantly, young mice injected daily with glibenclamide showed a degranulation effect in their β-cells, which might explain the reduced secretory capacity, although this effect was apparently not present in older mice [47]. Increased basal insulin secretion but reduced glucose- and glibenclamide-stimulated insulin secretion have been seen from isolated islets exposed in vitro to glibenclamide for 24 h [48,49], due to prolonged glibenclamide action and reduced insulin content [49]. Similarly, chronic exposure of rat pancreatic islets to sulfonylureas caused reversible impairment of glucose- and sulfonylurea-induced insulin release [50–52]. While short-term (24 h) exposure of insulinoma cell lines to SUs induced an increase in GLUT2 and GK mRNA, long-term (48–72 h) exposure induced a marked reduction of these glucose mobilizers [53], coupled with a reversible reduction in insulin content with no changes in Kir6.2 or SUR1 transcripts (72–144 h) [39]. Here we demonstrate that intact mice treated with chronic glibenclamide show no significant changes in islet insulin content, although a very mild reduction in insulin content was found in islets from mice implanted with the highest dose (Figure 6). Instead, our results indicate very specifically that chronic glibenclamide treatment in vivo induces β-cell desensitization (impairment of insulin secretion) rather than inhibition of insulin production [54]. 86Rb+-efflux experiments on fresh isolated islets from glibenclamide-implanted mice demonstrate that substantial inhibition of KATP activity is present (Figure 5), which predicts hyperexcitability and elevated [Ca2+], as has been seen in KATP KO animals. This would in turn stimulate insulin secretion, which suggests that the impairment of secretion is at a stage downstream of Ca2+, presumably at the level of insulin production or mobilization, or at the level of β-cell mass. There is a remarkable and rapid recovery of both KATP activity and normal insulin secretion within 24 h of islet isolation from these mice, arguing against β-cell apoptosis or other types of cell death induced by chronic glibenclamide treatment in vivo, and supported by the immunhistological analyses in Figure 7A and 7B.
Clearly, recovery of both KATP conductance and secretion is rapid (within hours of removal of islets from the animal), and although unassessable, the most reasonable explanation is that the recovery results simply from the washout of the drug and reversal of the resultant chronic depolarization. However, the formal possibility remains that the recovery is due to loss of other neuronal or hormonal inputs present in the intact animal. The level of KATP activity in Figure 5, assessed about 2 h after isolation, is an upper limit estimate, since some recovery may already have occurred and, in vivo, the degree of inhibition may be considerably greater. That this is likely is illustrated in Figure 5: except for the very lowest dose of implanted glibenclamide pellets, the glucose-lowering effect of injected glibenclamide, which reflects the degree of KATP inhibition possible, was negligible.
The “Inverse U” Model Is Reversible: Removal of the Hyperexcitable Stimulus Restores Normal Responsivity
In the genetic models of hyperexcitability, progression onto the “descending limb” of the “inverse U” response is permanent; glucose intolerance persists throughout the lifetime of KATP KO animals, and in reduced KATP animals when under dietary stress. However, it appears that this progression is rapidly reversible if excitability is normalized (as we show here), or if the dietary stress is removed [42]. As we discuss further below, various studies have suggested that “resting” pancreatic β-cells, by exogenous suppression of blood glucose, can restore function in diabetic states. This is the case for high-fat diet–induced glucose intolerance in Kir6.2+/−[AAA] mice [42]. Within 2 wk of restoration of normal diet, Kir6.2+/−[AAA] mice recover secretory capacity and glycemic control to near normal [42]. Genetic restoration of excitability has not been attempted in KO mice, but the present study allows us to begin to assess the lability of this effect. First, the pellets are designed to release at the appropriate dose for ∼90 d. The hyperglycemia and hypoinsulinism that develop within a few days of pellet implantation is maintained for at least 6 wk (42 d, Figures 1 and 4), and we did not routinely monitor animals after this time. However, we did assess a small number of animals at 95 and 118 d after implantation, i.e., 5 and 28 d after the pellets should have stopped releasing. By 1 mo, glucose tolerance is restored (Figures 2F and 3F) and even at only 5 d there is marked normalization of blood glucose and glucose tolerance (Figures 2E and 3E). These results strongly demonstrate that insulin secretory failure in response to loss-of-KATP activity is reversible, if hyperexcitability is removed.
More dramatically, isolated islets from implanted mice reveal rapid reversibility at the cellular level. Within a few hours of removal from the circulating glibenclamide, isolated islets regain not only essentially normal KATP activity but they regain essentially normal GSIS too. This means that whatever underlying mechanism causes the secretory failure, it is fully and rapidly reversible. Although 5.6 mM glucose is not particularly high, the finding that overnight incubation is sufficient to restore the secretory response to glucose in these islets may have parallels with the recent finding that overnight incubation of SUR1 KO islets in elevated (10 mM) glucose also restores insulin secretory capacity [55].
Relevance of the Present Findings for Type 2 Diabetes
The present findings have implications both for the potential etiology of type 2 diabetes and for pharmacological intervention. People with type 2 diabetes typically experience a gradual loss of secretory function. In many patients, SUs effectively control glycemia for an extended period [33], but over the long term (months to years) SU therapies often fail [33,56,57]. Several animal studies [28,58,59] and studies of isolated islets and cells [37,52] provide evidence that long-term SU treatment leads to impaired glucose tolerance and GSIS. The present study shows a markedly impaired secretory response to chronic glibenclamide that can be rapidly reversed following removal of the drug in vitro. In the implanted animals, glibenclamide release is continuous, and in patients, dosing is pulsatile. Conceivably, pulsatile presentation may provide islets with unstimulated periods that allow restoration of secretory capacity and responsivity. This rationale may suggest that lower-potency, shorter-acting SUs might be more efficacious therapeutically. In nonobese people with type 2 diabetes, including those in whom SUs secondarily fail, there are reports of significant restoration of β-cell secretory activity after a brief period of intensive insulin therapy [60,61]. This finding may argue for re-evaluation of short-acting analogs and give cause to further consider approaches [62] to reverse loss of secretory response in patients with type 2 diabetes following long-term SU treatment, and thereby prolong the use of pharmacotherapies and delay the need to switch to insulin.
Acknowledgments
SUR1 and Kir6.2 knockout mice were very graciously provided by Drs. Mark Magnuson (Vanderbilt University), and Susumu Seino and Takahashi Miki (Chiba University), respectively. We are grateful to Ms. Karen Steger-May (Division of Biostatistics, Washington University) for statistical advice.
Abbreviations
- GSIS
glucose-stimulated insulin secretion
- GTT
glucose tolerance test
- HI
hyperinsulinism
- ITT
insulin tolerance test
- KO
knock-out
- SEM
standard error of the mean
- SU
sulfonylurea
- TUNEL
terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling
- WT
wild type
Footnotes
Author contributions. CGN designed the experiments/the study and contributed to the writing of the paper. MSR designed the experiments/the study, collected data and did experiments for the study, analyzed the data, and wrote the first draft of the paper.
Funding: This work was supported by a NIH grant (DK69445) to CGN. Washington University Diabetes Research and Training Center (DK20579) provided reagent support. The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests: The authors have declared that no competing interests exist.
References
- Ashcroft FM, Gribble FM. ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia. 1999;42:903–919. doi: 10.1007/s001250051247. [DOI] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Thomas PM, Cote GJ, Hallman DM, Mathew PM. Homozygosity mapping, to chromosome 11p, of the gene for familial persistent hyperinsulinemic hypoglycemia of infancy. Am J Hum Genet. 1995;56:416–421. [PMC free article] [PubMed] [Google Scholar]
- Nestorowicz A, Wilson BA, Schoor KP, Inoue H, Glaser B, et al. Mutations in the sulonylurea receptor gene are associated with familial hyperinsulinism in Ashkenazi Jews. Hum Mol Genet. 1996;5:1813–1822. doi: 10.1093/hmg/5.11.1813. [DOI] [PubMed] [Google Scholar]
- Thomas P, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet. 1996;5:1809–1812. doi: 10.1093/hmg/5.11.1809. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP, et al. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- Nestorowicz A, Inagaki N, Gonoi T, Schoor KP, Wilson BA, et al. A nonsense mutation in the inward rectifier potassium channel gene, Kir6.2, is associated with familial hyperinsulinism. Diabetes. 1997;46:1743–1748. doi: 10.2337/diab.46.11.1743. [DOI] [PubMed] [Google Scholar]
- Aynsley-Green A, Polak JM, Bloom SR, Gough MH, Keeling J, et al. Nesidioblastosis of the pancreas: definition of the syndrome and the management of the severe neonatal hyperinsulinemic hypoglycemia. Arch Dis Child. 1981;56:496–508. doi: 10.1136/adc.56.7.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huopio H, Shyng SL, Otonkoski T, Nichols CG. K(ATP) channels and insulin secretion disorders. Am J Physiol Endocrinol Metab. 2002;283:E207–216. doi: 10.1152/ajpendo.00047.2002. [DOI] [PubMed] [Google Scholar]
- Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota C, Larsson O, Shelton KD, Shiota M, Efanov AM, et al. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem. 2002;277:37176–37183. doi: 10.1074/jbc.M206757200. [DOI] [PubMed] [Google Scholar]
- Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem. 2000;275:9270–9277. doi: 10.1074/jbc.275.13.9270. [DOI] [PubMed] [Google Scholar]
- Koster JC, Remedi MS, Flagg TP, Johnson JD, Markova KP, et al. Hyperinsulinism induced by targeted suppression of beta cell KATP channels. Proc Natl Acad Sci U S A. 2002;99:16992–16997. doi: 10.1073/pnas.012479199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedi MS, Rocheleau JV, Tong A, Patton BL, McDaniel ML, et al. Hyperinsulinism in mice with heterozygous loss of K(ATP) channels. Diabetologia. 2006;49:2368–2378. doi: 10.1007/s00125-006-0367-4. [DOI] [PubMed] [Google Scholar]
- Cartier EA, Conti LR, Vandenberg CA, Shyng SL. Defective trafficking and function of KATP channels caused by a sulfonylurea receptor 1 mutation associated with persistent hyperinsulinemic hypoglycemia of infancy. Proc Natl Acad Sci U S A. 2001;98:2882–2887. doi: 10.1073/pnas.051499698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng SL, Ferrigni T, Shepard JB, Nestorowicz A, Glaser B, et al. Functional analyses of novel mutations in the sulfonylurea receptor 1 associated with persistent hyperinsulinemic hypoglycemia of infancy. Diabetes. 1998;47:1145–1151. doi: 10.2337/diabetes.47.7.1145. [DOI] [PubMed] [Google Scholar]
- de Lonlay P, Fournet JC, Touati G, Groos MS, Martin D, et al. Heterogeneity of persistent hyperinsulinaemic hypoglycaemia. A series of 175 cases. Eur J Pediatr. 2002;161:37–48. doi: 10.1007/s004310100847. [DOI] [PubMed] [Google Scholar]
- Henwood MJ, Kelly A, MacMullen C, Bhatia P, Ganguly A, et al. Genotype-phenotype correlations in children with congenital hyperinsulinism due to recessive mutations of the adenosine triphosphate-sensitive potassium channel genes. J Clin Endocrinol Metab. 2005;90:789–794. doi: 10.1210/jc.2004-1604. [DOI] [PubMed] [Google Scholar]
- Huopio H, Vauhkonen I, Komulainen J, Niskanen L, Otonkoski T, et al. Carriers of an inactivating beta-cell ATP-sensitive K(+) channel mutation have normal glucose tolerance and insulin sensitivity and appropriate insulin secretion. Diabetes Care. 2002;25:101–106. doi: 10.2337/diacare.25.1.101. [DOI] [PubMed] [Google Scholar]
- Grimberg A, Ferry RJ, Jr., Kelly A, Koo-McCoy S, Polonsky K, et al. Dysregulation of insulin secretion in children with congenital hyperinsulinism due to sulfonylurea receptor mutations. Diabetes. 2001;50:322–328. doi: 10.2337/diabetes.50.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huopio H, Reimann F, Ashfield R, Komulainen J, Lenko HL, et al. Dominantly inherited hyperinsulinism caused by a mutation in the sulfonylurea receptor type 1. J Clin Invest. 2000;106:897–906. doi: 10.1172/JCI9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes. 2001;50(Suppl 1):S154–159. doi: 10.2337/diabetes.50.2007.s154. [DOI] [PubMed] [Google Scholar]
- Rifkin H. Current status of non-insulin-dependent diabetes mellitus (type II): management with gliclazide. Am J Med. 1991;90:3S–7S. doi: 10.1016/0002-9343(91)90411-p. [DOI] [PubMed] [Google Scholar]
- Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, et al. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet. 2003;362:1275–1281. doi: 10.1016/S0140-6736(03)14571-0. [DOI] [PubMed] [Google Scholar]
- Levetan C. Oral antidiabetic agents in type 2 diabetes. Curr Med Res Opin. 2007;23:945–952. doi: 10.1185/030079907x178766. [DOI] [PubMed] [Google Scholar]
- Karam JH, Sanz N, Salamon E, Nolte MS. Selective unresponsiveness of pancreatic beta-cells to acute sulfonylurea stimulation during sulfonylurea therapy in NIDDM. [erratum appears in Diabetes 1987 Mar; 36 (3): following 406] Diabetes. 1986;35:1314–1320. doi: 10.2337/diab.35.12.1314. [DOI] [PubMed] [Google Scholar]
- Filipponi P, Marcelli M, Nicoletti I, Pacifici R, Santeusanio F, et al. Suppressive effect of long term sulfonylurea treatment on A, B, and D cells of normal rat pancreas. Endocrinology. 1983;113:1972–1979. doi: 10.1210/endo-113-6-1972. [DOI] [PubMed] [Google Scholar]
- Dunbar JC, Foa PP. An inhibitory effect of tolbutamide and glibenclamide (glyburide) on the pancreatic islets of normal animals. Diabetologia. 1974;10:27–35. doi: 10.1007/BF00421411. [DOI] [PubMed] [Google Scholar]
- Groop LC, Pelkonen R, Koskimies S, Bottazzo GF, Doniach D. Secondary failure to treatment with oral antidiabetic agents in non-insulin-dependent diabetes. Diabetes Care. 1986;9:129–133. doi: 10.2337/diacare.9.2.129. [DOI] [PubMed] [Google Scholar]
- Genuth S. Insulin use in NIDDM. Diabetes Care. 1990;13:1240–1264. doi: 10.2337/diacare.13.12.1240. [DOI] [PubMed] [Google Scholar]
- Pontiroli AE, Calderara A, Pozza G. Secondary failure of oral hypoglycaemic agents: frequency, possible causes, and management. Diabetes Metab Rev. 1994;10:31–43. doi: 10.1002/dmr.5610100104. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group. Diabet Med. 1998;15:297–303. doi: 10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Efanova IB, Zaitsev SV, Zhivotovsky B, Kohler M, Efendic S, et al. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration. J Biol Chem. 1998;273:33501–33507. doi: 10.1074/jbc.273.50.33501. [DOI] [PubMed] [Google Scholar]
- Del Guerra S, Marselli L, Lupi R, Boggi U, Mosca F, et al. Effects of prolonged in vitro exposure to sulphonylureas on the function and survival of human islets. J Diabetes Complicat. 2005;19:60–64. doi: 10.1016/j.jdiacomp.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, et al. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab. 2005;90:501–506. doi: 10.1210/jc.2004-0699. [DOI] [PubMed] [Google Scholar]
- Kawaki J, Nagashima K, Tanaka J, Miki T, Miyazaki M, et al. Unresponsiveness to glibenclamide during chronic treatment induced by reduction of ATP-sensitive K+ channel activity. Diabetes. 1999;48:2001–2006. doi: 10.2337/diabetes.48.10.2001. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Nagashima K, Hamasaki A, Kuwamura N, Kawasaki Y, et al. Sulfonylurea and glinide reduce insulin content, functional expression of K(ATP) channels, and accelerate apoptotic beta-cell death in the chronic phase. Diabetes Res Clin Pract. 2007;77:343–350. doi: 10.1016/j.diabres.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Ball AJ, McCluskey JT, Flatt PR, McClenaghan NH. Chronic exposure to tolbutamide and glibenclamide impairs insulin secretion but not transcription of K(ATP) channel components. Pharmacol Res. 2004;50:41–46. doi: 10.1016/j.phrs.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Hambrock A, de Oliveira Franz CB, Hiller S, Osswald H. Glibenclamide-induced apoptosis is specifically enhanced by expression of the sulfonylurea receptor isoform SUR1 but not by expression of SUR2B or the mutant SUR1(M1289T) J Pharmacol Exp Ther. 2006;316:1031–1037. doi: 10.1124/jpet.105.097501. [DOI] [PubMed] [Google Scholar]
- Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell. 2000;100:645–654. doi: 10.1016/s0092-8674(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Remedi MS, Koster JC, Markova K, Seino S, Miki T, et al. Diet-induced glucose intolerance in mice with decreased (beta}-cell ATP-sensitive k+ channels. Diabetes. 2004;53:3159–3167. doi: 10.2337/diabetes.53.12.3159. [DOI] [PubMed] [Google Scholar]
- Rocheleau JV, Remedi MS, Granada B, Head WS, Koster JC, et al. Critical role of gap junction coupled KATP channel activity for regulated insulin secretion. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040026. doi: 10.1371/journal.pbio.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster JC, Remedi MS, Dao C, Nichols CG. ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes. 2005;54:2645–2654. doi: 10.2337/diabetes.54.9.2645. [DOI] [PubMed] [Google Scholar]
- Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–2513. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- Nguyen QA, Antoine MH, Ouedraogo R, Hermann M, Sergooris J, et al. In vitro and in vivo effects of new insulin releasing agents. Biochem Pharmacol. 2002;63:515–521. doi: 10.1016/s0006-2952(01)00880-2. [DOI] [PubMed] [Google Scholar]
- Guiot Y, Henquin JC, Rahier J. Effects of glibenclamide on pancreatic beta-cell proliferation in vivo. Eur J Clin Pharmacol. 1994;261:157–161. doi: 10.1016/0014-2999(94)90314-x. [DOI] [PubMed] [Google Scholar]
- Chen J, Jeppesen PB, Nordentoft I, Hermansen K. Stevioside counteracts the glyburide-induced desensitization of the pancreatic beta-cell function in mice: studies in vitro. Metab Clin Exp. 2006;55:1674–1680. doi: 10.1016/j.metabol.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Anello M, Gilon P, Henquin JC. Alterations of insulin secretion from mouse islets treated with sulphonylureas: perturbations of Ca2+ regulation prevail over changes in insulin content. Br J Pharmacol. 1999;127:1883–1891. doi: 10.1038/sj.bjp.0702731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawalich WS. Phosphoinositide hydrolysis and insulin secretion in response to glucose stimulation are impaired in isolated rat islets by prolonged exposure to the sulfonylurea tolbutamide. Endocrinology. 1989;125:281–286. doi: 10.1210/endo-125-1-281. [DOI] [PubMed] [Google Scholar]
- Bolaffi JL, Heldt A, Lewis LD, Grodsky GM. The third phase of in vitro insulin secretion. Evidence for glucose insensitivity. Diabetes. 1986;35:370–373. doi: 10.2337/diab.35.3.370. [DOI] [PubMed] [Google Scholar]
- Gullo D, Rabuazzo AM, Vetri M, Gatta C, Vinci C, et al. Chronic exposure to glibenclamide impairs insulin secretion in isolated rat pancreatic islets. J Endocrinol Invest. 1991;14:287–291. doi: 10.1007/BF03346813. [DOI] [PubMed] [Google Scholar]
- Porzio O, Marlier LN, Federici M, Hribal ML, Magnaterra R, et al. GLUT2 and glucokinase expression is coordinately regulated by sulfonylurea. Mol Cell Endocrinol. 1999;153:155–161. doi: 10.1016/s0303-7207(99)00073-8. [DOI] [PubMed] [Google Scholar]
- Rustenbeck I. Desensitization of insulin secretion. Biochem Pharmacol. 2002;63:1921–1935. doi: 10.1016/s0006-2952(02)00996-6. [DOI] [PubMed] [Google Scholar]
- Szollosi A, Nenquin M, Henquin JC. Overnight culture unmasks glucose-induced insulin secretion in mouse islets lacking ATP-sensitive K+ channels by improving the triggering Ca2+ signal. J Biol Chem. 2007;282:14768–14776. doi: 10.1074/jbc.M701382200. [DOI] [PubMed] [Google Scholar]
- Bretzel RG, Voigt K, Schatz H. The United Kingdom Prospective Diabetes Study (UKPDS) implications for the pharmacotherapy of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 1998;106:369–372. [PubMed] [Google Scholar]
- Birkeland KI, Furuseth K, Melander A, Mowinckel P, Vaaler S. Long-term randomized placebo-controlled double-blind therapeutic comparison of glipizide and glyburide. Glycemic control and insulin secretion during 15 months. Diabetes Care. 1994;17:45–49. doi: 10.2337/diacare.17.1.45. [DOI] [PubMed] [Google Scholar]
- Filipponi P, Gregorio F, Marcelli M, Cristallini S, Santeusanio F, et al. Effects of long-term glibenclamide administration on gastrointestinal and pancreatic hormones in normal fasting rats. Diabetes Res Clin Pract. 1989;6:83–87. doi: 10.1016/0168-8227(89)90110-1. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Fujiyama K, Hoshino T, Takeuchi T, Mashiba H, et al. Effect of long-term administration of insulin and glibenclamide on pancreatic A and B cell function. Exp Clin Endocrinol. 1990;95:237–241. doi: 10.1055/s-0029-1210958. [DOI] [PubMed] [Google Scholar]
- Guldstrand M, Grill V, Bjorklund A, Lins PE, Adamson U. Improved beta cell function after short-term treatment with diazoxide in obese subjects with type 2 diabetes. Diabetes Metab. 2002;28:448–456. [PubMed] [Google Scholar]
- Torella R, Salvatore T, Cozzolino D, Giunta R, Quatraro A, et al. Restoration of sensitivity to sulfonylurea after strict glycaemic control with insulin in non-obese type 2 diabetic subjects. Diabete Metabol. 1991;17:443–447. [PubMed] [Google Scholar]
- Melander A, Lebovitz HE, Faber OK. Sulfonylureas. Why, which, and how. Diabetes Care. 1990;13(Suppl 3):18–25. doi: 10.2337/diacare.13.3.18. [DOI] [PubMed] [Google Scholar]