Abstract
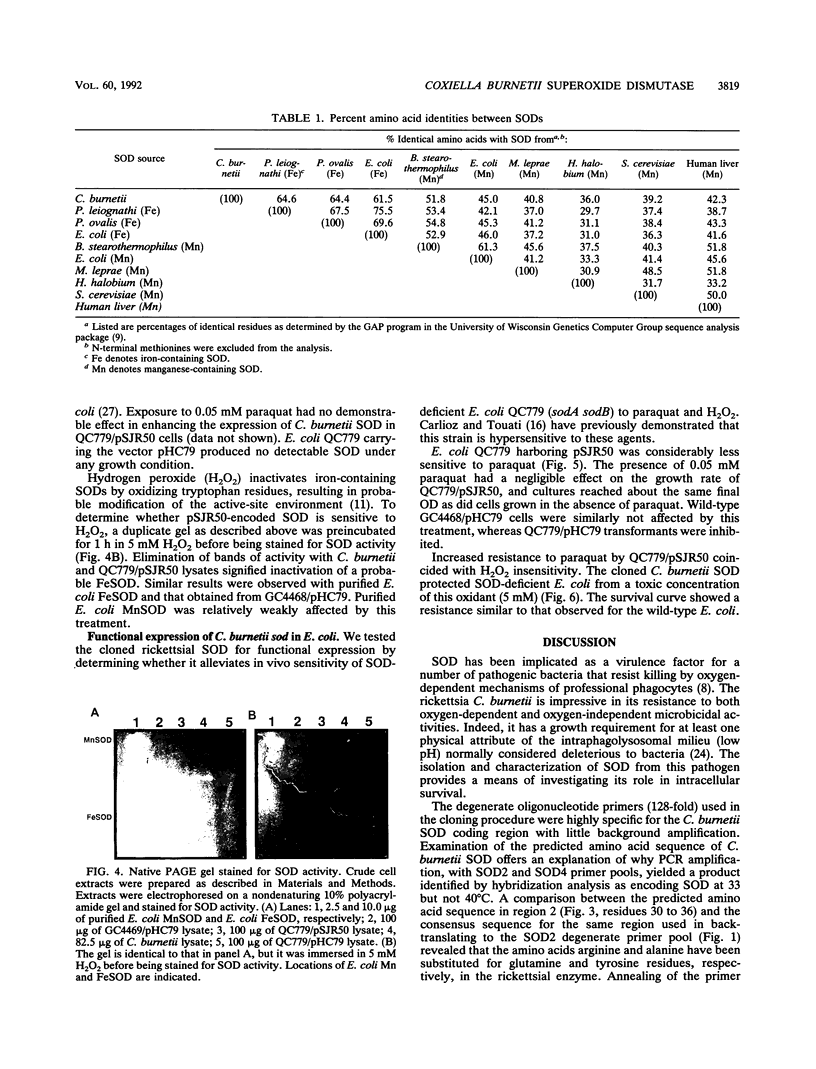
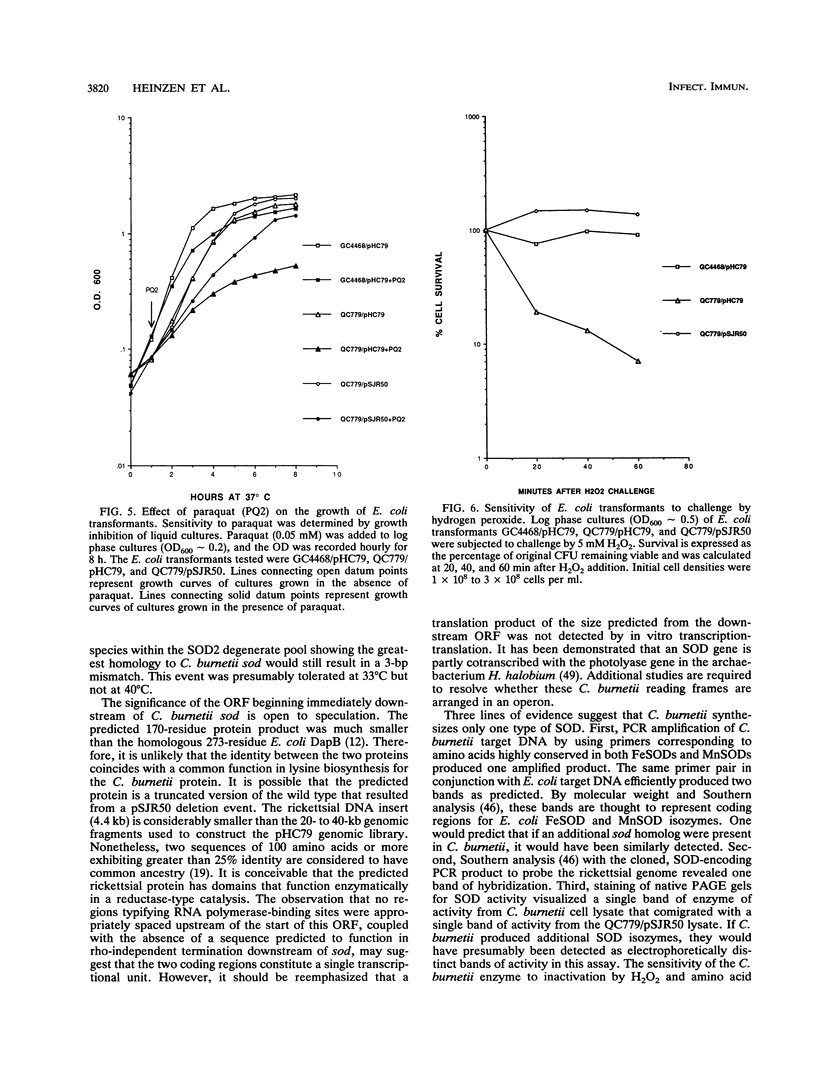
A superoxide dismutase (SOD) gene from the obligate intracellular bacterium Coxiella burnetii has been cloned, and its DNA sequence has been determined and expressed in Escherichia coli. The gene was identified on pSJR50, a pHC79-derived genomic clone, by using the polymerase chain reaction with degenerate oligonucleotide primers corresponding to conserved regions of known SODs. Sequences resembling conventional E. coli ribosomal and RNA polymerase-binding sites preceded the C. burnetii 579-bp SOD open reading frame. An E. coli SOD-deficient double mutant (sodA sodB) that carried pSJR50 had growth and survival responses similar to those of the wild type when the transformant was challenged with 0.05 mM paraquat and 5 mM hydrogen peroxide, respectively. These observations indicated that the C. burnetii gene was functionally expressed in E. coli. Staining of native polyacrylamide gels for SOD activity demonstrated that pSJR50 insert DNA codes for an SOD that comigrates with an SOD found in C. burnetii cell lysates. The enzyme was inactivated by 5 mM hydrogen peroxide, which is indicative of an iron-containing SOD. Additionally, the predicted amino acid sequence was significantly more homologous to known iron-containing SODs than to manganese-containing SODs. Isolation of the C. burnetii SOD gene may provide an opportunity to examine its role in the intracellular survival of this rickettsia.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akporiaye E. T., Baca O. G. Superoxide anion production and superoxide dismutase and catalase activities in Coxiella burnetii. J Bacteriol. 1983 Apr;154(1):520–523. doi: 10.1128/jb.154.1.520-523.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akporiaye E. T., Stefanovich D., Tsosie V., Baca G. Coxiella burnetii fails to stimulate human neutrophil superoxide anion production. Acta Virol. 1990 Feb;34(1):64–70. [PubMed] [Google Scholar]
- Archibald F. S., Duong M. N. Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect Immun. 1986 Feb;51(2):631–641. doi: 10.1128/iai.51.2.631-641.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra D., Schinina M. E., Simmaco M., Bannister J. V., Bannister W. H., Rotilio G., Bossa F. The primary structure of human liver manganese superoxide dismutase. J Biol Chem. 1984 Oct 25;259(20):12595–12601. [PubMed] [Google Scholar]
- Barra D., Schininà M. E., Bannister W. H., Bannister J. V., Bossa F. The primary structure of iron-superoxide dismutase from Photobacterium leiognathi. J Biol Chem. 1987 Jan 25;262(3):1001–1009. [PubMed] [Google Scholar]
- Beaman B. L., Black C. M., Doughty F., Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. Monoclonal antibodies demonstrate that superoxide dismutase contributes to protection of Nocardia asteroides within the intact host. Infect Immun. 1990 Sep;58(9):3122–3128. doi: 10.1128/iai.58.9.3122-3128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. The role of oxygen and its derivatives in microbial pathogenesis and host defense. Annu Rev Microbiol. 1984;38:27–48. doi: 10.1146/annurev.mi.38.100184.000331. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beyer W. F., Jr, Fridovich I. Effect of hydrogen peroxide on the iron-containing superoxide dismutase of Escherichia coli. Biochemistry. 1987 Mar 10;26(5):1251–1257. doi: 10.1021/bi00379a008. [DOI] [PubMed] [Google Scholar]
- Bouvier J., Richaud C., Richaud F., Patte J. C., Stragier P. Nucleotide sequence and expression of the Escherichia coli dapB gene. J Biol Chem. 1984 Dec 10;259(23):14829–14834. [PubMed] [Google Scholar]
- Brock C. J., Walker J. E. Superoxide dismutase from Bacillus stearothermophilus. Complete amino acid sequence of a manganese enzyme. Biochemistry. 1980 Jun 24;19(13):2873–2882. doi: 10.1021/bi00554a009. [DOI] [PubMed] [Google Scholar]
- Burton P. R., Stueckemann J., Welsh R. M., Paretsky D. Some ultrastructural effects of persistent infections by the rickettsia Coxiella burnetii in mouse L cells and green monkey kidney (Vero) cells. Infect Immun. 1978 Aug;21(2):556–566. doi: 10.1128/iai.21.2.556-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A., Ludwig M. L., Stallings W. C., Fee J. A., Steinman H. M., Touati D. Iron superoxide dismutase. Nucleotide sequence of the gene from Escherichia coli K12 and correlations with crystal structures. J Biol Chem. 1988 Jan 25;263(3):1555–1562. [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Searching through sequence databases. Methods Enzymol. 1990;183:99–110. doi: 10.1016/0076-6879(90)83008-w. [DOI] [PubMed] [Google Scholar]
- Ferencík M., Schramek S., Kazár J., Stefanovic J. Effect of Coxiella burnetii on the stimulation of hexose monophosphate shunt and on superoxide anion production in human polymorphonuclear leukocytes. Acta Virol. 1984 May;28(3):246–250. [PubMed] [Google Scholar]
- Franzon V. L., Arondel J., Sansonetti P. J. Contribution of superoxide dismutase and catalase activities to Shigella flexneri pathogenesis. Infect Immun. 1990 Feb;58(2):529–535. doi: 10.1128/iai.58.2.529-535.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci U S A. 1981 May;78(5):3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J Biol Chem. 1977 Nov 10;252(21):7667–7672. [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzen R. A., Frazier M. E., Mallavia L. P. Nucleotide sequence of Coxiella burnetii superoxide dismutase. Nucleic Acids Res. 1990 Nov 11;18(21):6437–6437. doi: 10.1093/nar/18.21.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Isobe T., Fang Y. I., Muno D., Okuyama T., Ohmori D., Yamakura F. Amino acid sequence of iron-superoxide dismutase from Pseudomonas ovalis. FEBS Lett. 1987 Oct 19;223(1):92–96. doi: 10.1016/0014-5793(87)80516-1. [DOI] [PubMed] [Google Scholar]
- Keele B. B., Jr, McCord J. M., Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970 Nov 25;245(22):6176–6181. [PubMed] [Google Scholar]
- Kusunose E., Ichihara K., Noda Y., Kusunose M. Superoxide dismutase from Mycobacterium tuberculosis. J Biochem. 1976 Dec;80(6):1343–1352. doi: 10.1093/oxfordjournals.jbchem.a131407. [DOI] [PubMed] [Google Scholar]
- Lynch R. E., Cole B. C. Mycoplasma pneumoniae: a prokaryote which consumes oxygen and generates superoxide but which lacks superoxide dismutase. Biochem Biophys Res Commun. 1980 Sep 16;96(1):98–105. doi: 10.1016/0006-291x(80)91186-9. [DOI] [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978 Jul 10;253(13):4697–4699. [PubMed] [Google Scholar]
- May B. P., Dennis P. P. Unusual evolution of a superoxide dismutase-like gene from the extremely halophilic archaebacterium Halobacterium cutirubrum. J Bacteriol. 1990 Jul;172(7):3725–3729. doi: 10.1128/jb.172.7.3725-3729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Niederhoffer E. C., Naranjo C. M., Bradley K. L., Fee J. A. Control of Escherichia coli superoxide dismutase (sodA and sodB) genes by the ferric uptake regulation (fur) locus. J Bacteriol. 1990 Apr;172(4):1930–1938. doi: 10.1128/jb.172.4.1930-1938.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrod P., Morse S. A. Absence of superoxide dismutase in some strains of Neisseria gonorrhoeae. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1287–1294. doi: 10.1016/0006-291x(79)91176-8. [DOI] [PubMed] [Google Scholar]
- Parker M. W., Blake C. C. Iron- and manganese-containing superoxide dismutases can be distinguished by analysis of their primary structures. FEBS Lett. 1988 Mar 14;229(2):377–382. doi: 10.1016/0014-5793(88)81160-8. [DOI] [PubMed] [Google Scholar]
- Reschke D. K., Frazier M. E., Mallavia L. P. Transformation of Rochalimaea quintana, a member of the family Rickettsiaceae. J Bacteriol. 1990 Sep;172(9):5130–5134. doi: 10.1128/jb.172.9.5130-5134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Touati D. Cloning of the iron superoxide dismutase gene (sodB) in Escherichia coli K-12. J Bacteriol. 1984 Jul;159(1):418–420. doi: 10.1128/jb.159.1.418-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., Duke M. V., Oesterhelt D., Ma D. P. Cloning and determination of the nucleotide sequence of the Mn-containing superoxide dismutase gene from Halobacterium halobium. Gene. 1988 Oct 15;70(1):153–159. doi: 10.1016/0378-1119(88)90113-8. [DOI] [PubMed] [Google Scholar]
- Samuel J. E., Frazier M. E., Mallavia L. P. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect Immun. 1985 Sep;49(3):775–779. doi: 10.1128/iai.49.3.775-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinman H. M. The amino acid sequence of mangano superoxide dismutase from Escherichia coli B. J Biol Chem. 1978 Dec 25;253(24):8708–8720. [PubMed] [Google Scholar]
- Takahashi M. A., Asada K. Superoxide anion permeability of phospholipid membranes and chloroplast thylakoids. Arch Biochem Biophys. 1983 Oct 15;226(2):558–566. doi: 10.1016/0003-9861(83)90325-9. [DOI] [PubMed] [Google Scholar]
- Takao M., Kobayashi T., Oikawa A., Yasui A. Tandem arrangement of photolyase and superoxide dismutase genes in Halobacterium halobium. J Bacteriol. 1989 Nov;171(11):6323–6329. doi: 10.1128/jb.171.11.6323-6329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Avila H. Structure and gene expression of the E. coli Mn-superoxide dismutase gene. Nucleic Acids Res. 1986 Jun 11;14(11):4577–4589. doi: 10.1093/nar/14.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaraj H. S., Lamb F. I., Davis E. O., Jenner P. J., Jeyakumar L. H., Colston M. J. Identification, sequencing, and expression of Mycobacterium leprae superoxide dismutase, a major antigen. Infect Immun. 1990 Jun;58(6):1937–1942. doi: 10.1128/iai.58.6.1937-1942.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati D. Cloning and mapping of the manganese superoxide dismutase gene (sodA) of Escherichia coli K-12. J Bacteriol. 1983 Sep;155(3):1078–1087. doi: 10.1128/jb.155.3.1078-1087.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]
- Zhang Y., Lathigra R., Garbe T., Catty D., Young D. Genetic analysis of superoxide dismutase, the 23 kilodalton antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991 Feb;5(2):381–391. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]




