Abstract
Laminins are glycoproteins expressed in the basement membrane of multiple epithelial tissues. Previously described purification procedures for the human laminin variants laminin-5 (LN-332) and laminin-10 (LN-511) use tissue as starting material and have multiple steps. We demonstrate a two-step laminin immunoaffinity purification method to produce consistent quantities of intact and biologically active LN-332 and LN-511 from human keratinocyte (HaCaT) and human lung carcinoma (A549) cell lines, respectively. The purification of LN-332 and LN-551 was demonstrated by PAGE analysis, silver staining and Western blot analysis. The purification procedure includes instruction on removing a cell adhesion contaminant known as galectin-3 binding protein from purified LN-511. The biological activity of purified laminin was tested in a standard cell adhesion assay and compared to commercially available LN-111. This rapid and reproducible purification method will contribute to understanding the role of LN-332 and LN-511 in cell behavior, signaling and gene expression.
Keywords: laminin-322, laminin-511, purification
INTRODUCTION
Laminins are a family of basement membrane glycoproteins implicated in diverse biological activities including promotion of cell adhesion, migration, proliferation, differentiation and survival. Laminins are disulfide-linked heterotrimeric glycoproteins comprised of three distinct chains termed α-, β- and γ-which form the well known cruciform structure. There are five α-, three β-, and three γ-chains which formulate at least 15 laminin isoforms. Laminin-332 (also referred to as laminin-5 and laminin-α3β3γ2) is a major adhesive component of epidermal basement membranes and other epithelial tissues [1; 2] and is comprised of the α3, β3 and γ2 subunits. The α5 subunit containing laminin, laminin-511 (also known as laminin-10 or laminin α5β1γ1), is comprised of the α5,β1 and γ1 subunits and is widely expressed in adult tissues and is also a major component of basement membranes [3; 4].
The most widely studied laminin, laminin-111 (also known as laminin-1), had been exclusively investigated due to the ease in which it is obtained from mouse Engelbreth-Holm-Swarm (EHS) tumors. However, studies involving most other laminin family members have been hampered due to inefficient methods for extracting intact laminin from tissue or cell culture systems. For example, Wondimu et al. recently published a report outlining concerns with the lack of consistency of commercially available laminin preparations from human placental tissue which was directly related to variation in purification protocols [5]. Additional investigators have claimed that yield of purified endogenous laminin from cultured cell lines was extremely low and therefore pioneered methodology for overexpressing LN-332 or LN-511 in cell culture model systems to produce high quantities of recombinant LN-332 and LN-511 [6; 7]. However, the use of these techniques for reproducible and consistent purification of laminin may be difficult due to differences with expression vectors and instability of genetically altered cell lines. Therefore, the development of efficient methods for purifying LN-332 and LN-511 from human cell lines that naturally secrete these proteins was developed.
We and others have previously reported the influence of LN-332 [8; 9; 10; 11] and LN-511 [11; 12; 13] on cancer cell migration and gene expression using purified components. Our purpose is to provide the details of our standard and reproducible protocol for purifying LN-332 and-511 from cultured cell lines. We devised a two-step scheme to isolate biologically active LN-332 and LN-511 from conditioned medium of human immortalized keratinocytes and human lung adenocarcinoma cells, respectively, based in part, on previously described laminin purification methods.
MATERIALS AND METHODS
Cell Lines and Culture Conditions
HaCaT immortalized keratinocytes were obtained from Dr. Norbert E. Fusenig, (German Cancer Research Center, Heidelberg) as described in [14], A549 human lung adenocarcinoma (obtained from ATCC) and DU145H cells (selected for overexpression of the integrin laminin receptor, A6 integrin [15] ) were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2 with Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum, and 100 U/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA).
Antibodies and reagents
The LN-332 anti-α3 monoclonal antibody, BM165 and the LN-332 anti-β3 monoclonal (used at 1:5000 for Western Blotting) were kind gifts from Dr. Robert Burgeson (Massachusetts General Hospital, Boston, MA). The LN-332 anti-α3 monoclonal 12C4 antibody (used at 1:100 for Western blotting) was a kind gift from Dr. Jonathan Jones (Northwestern University, Chicago, IL) and the monoclonal LN-332 anti-γ2 antibody (used at 1:5000 for Western blotting) was from Santa Cruz Biotechnologies (Santa Cruz, CA). The monoclonal antibody 4C7 against the α5 subunit of LN-511 was a kind gift from Dr. Eva Engvall (The Burnham Institute, La Jolla, CA). The LN-511 anti-α5 monoclonal 15H5 antibody (used at 1:5000 for Western Blotting) was a kind gift from Dr. Kiyotoshi Sekiguchi (Osaka University, Oskaka, Japan) and the 4E10 and 2E8 monoclonal antibodies against LN-511 β1 and γ1 chains, respectively (used at 1:2000 and 1:5000 for Western blotting) were from Millipore (Billerica, MA). The goat anti-mouse-HRP antibody was from Transduction Laboratories (Lexington, KY). The alpha-lactose and alpha-lactose agarose were from Sigma-Aldrich (St. Louis, MO). Laminin-1 used in the cell adhesion assay was supplied by BD Biosciences (Franklin Lakes, NJ).
Purification of Laminin-5
The human immortalized keratinocyte HaCaT cell line was grown in 175-cm2 culture flasks. After the cells reached confluence, the media was removed and fresh pre-warmed media without serum was added at a minimum volume for 18 hours. The resulting conditioned medium was harvested, intact cells removed by centrifugation and the resulting supernatant collected. Protease activity was inhibited by the addition of 5 mM EDTA, 50 μM phenylmethysulfonyl floride, and 50 μM N-ethylmaleimide. The supernatant was cooled to 4 degrees, clarified and applied to a prepared antibody affinity column. All subsequent procedures were done at 4 degrees. The BM165-Sepharose CL-4B affinity column was prepared by coupling purified anti-LN-332 α3 chain monoclonal antibody BM165 to cyanogen-activated Sepharose CL-4B from GE Life Sciences (Piscataway, NJ) according to manufacturer’s suggestions. The diameter of the column was 10 mm, and the column volume was 4 ml with a flow rate of 1 ml per minute. After loading the starting material onto the column, the column was washed with two column volumes of column buffer (50 mM Tris-HCl, 150 mM NaCl pH 7.5). The LN-332 was eluted from the affinity column with 0.1 M glycine (pH 2.7) in 0.5 ml fractions and the fractions were neutralized by addition of 1M Tris-HCl (pH 8.0). The protein concentration was determined with Advanced Protein Assay Reagent (Cytoskeleton, Inc., Denver, CO). The fractions containing protein were pooled. The approximate yield was 60 μg of purified protein per liter of conditioned media. The resulting protein was stored in aliquots at −80 degrees for up to 6 months. The use of human LN-332 purified according to this method was previously described by us [9; 11; 16].
Purification of Laminin-10 with Galectin-3 Binding Protein Removal
The human lung carcinoma A549 cell line was grown in 175-cm2 culture flasks in DMEM supplemented with 1% fetal bovine serum and 30 mM α-lactose for 3 days. The inclusion of lactose in the media suppressed the secretion of galectin and galectin-3 binding protein. After the cells reached confluence, the conditioned medium was harvested, intact cells removed by centrifugation and the resulting supernatant collected. Protease activity was inhibited by the addition of 5 mM EDTA, 50 μM phenylmethysulfonyl floride, and 50 μM N-ethylmaleimide. The A549 conditioned medium was applied to an α-lactose-agarose HR 10/10 column (GE Life Sciences) to remove contaminating galectin-3 binding protein. The column diameter was 10 mm, and the column volume was 4 ml with a flow rate of 1 ml per minute. The eluent was then applied to a 4C7-Sepharose CL-4B affinity column prepared by coupling the anti-LN-511 α5 chain monoclonal antibody 4C7 to cyanogen-activated Sepharose CL-4B (GE Life Sciences). LN-511 was eluted from the affinity column with 0.1 M glycine (pH 2.7) and neutralized by addition of 1 M Tris-HCl (pH 8.0). The protein concentration was determined with Advanced Protein Assay Reagent (Cytoskeleton, Inc., Denver, CO). The approximate yield was 50 μg of purified protein per liter of conditioned media. The use of human LN-511 purified in this manner was previously described by us [11; 12; 13].
Gel Electrophoresis and Protein Analysis
The purified LN-332 or LN-511 proteins were analyzed by SDS-PAGE on 4 or 6% polyacrylamide gels under reducing conditions, and the separated proteins were stained with silver stain. For Western blot analysis, proteins resolved by SDS-PAGE were transferred to a Millipore Immobilon-P polyvinylidene fluoride (PVDF) membrane (Millipore) and blots were developed using ECL chemiluminescence (ECL Western Blotting Detection System, Amersham Bioscience).
Cell Adhesion Assay
The cell adhesion assay was performed by coating 96-well microtiter plates supplied by VWR International (West Chester, PA) with LN-111, LN-332 and LN-511 and allowed to dry at room temperature overnight. Following coating, the plates were washed and non-specific sites blocked with phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) for 2 hours at 37°C. DU145H cells were harvested with 2 mM EDTA and 0.05% BSA and suspended in serum free DMEM at a density of 4 × 105 cells/ml; and 0.1 ml of the cell suspension was added to each well followed by incubation at 37°C for 2 hours. The wells were washed two times with PBS and 0.1 ml of 2.5% formalin was added to each well for 10 minutes at room temperature. The wells were washed with distilled water and adherent cells were stained with 0.5% crystal violet in 20% methanol (w/v) for 5 minutes at room temperature. After washing with distilled water, the stain was extracted with 0.1 M citric acid. The absorbance of each well was read at 570 nm with a model ELX800® microplate reader (Bio-Tek Instruments, Inc., Winooski, VM).
RESULTS AND DISCUSSION
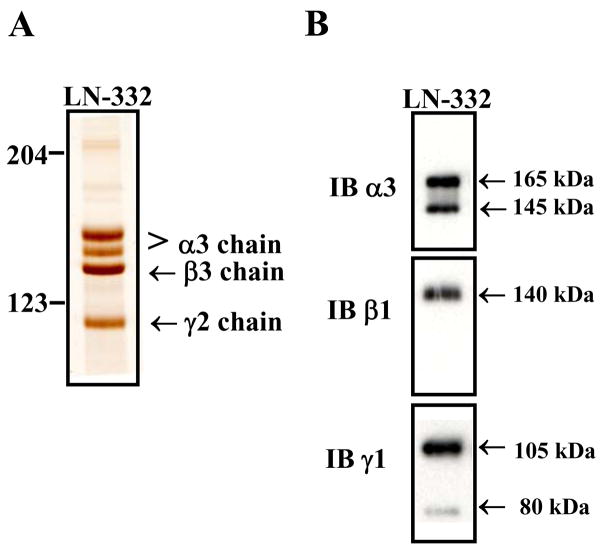
In this method, we purified intact human LN-332 and LN-511 from the conditioned medium of cultured human cell lines. The HaCaT and A549 cell lines were chosen for purification of LN-332 and LN-511, respectively, based on evidence of the elevated expression levels of each laminin in these cell lines compared to other cultured cell lines (data not shown). The protein isolated from the HaCaT conditioned medium via the BM165-Sepharose column gave four bands with the molecular masses of 165, 145, 140 and 105 kDa under reducing conditions (Figure 1A). The ability of the isolated chains to assemble into a trimer was observed as a single band at approximately 400 kDa in non-reducing conditions (data not shown). For additional verification of LN-322 chains, western blot analysis using monoclonal antibodies to the α-, β- and γ-subunits of LN-332 was performed under reducing conditions as demonstrated in Figure 1B. The top panel indicates the presence of the α3 subunit, the middle panel the β3 subunit and the bottom panel the γ2 subunit of LN-332. The results in Figure 1 demonstrate the isolation of purified LN-332 with the absence of detectable contaminating proteins from a cell line that naturally produces and secretes the protein.
Figure 1. Purification of LN-332 from HaCaT conditioned medium.
LN-332 was purified from serum free conditioned medium from confluent immortalized keratinocyte HaCaT cells using an affinity column containing the mAb BM165 α3 chain specific antibody. (A) Purified LN-322 was analyzed by a 6% SDS-PAGE gel under reducing conditions and silver stained. LN-332 chains of the trimer were observed with absence of any substantial contaminants. (B) Gel electrophoresis and Western blot analysis of purified LN-332 using monoclonal antibodies against the α3 (12C4), β3 (BM145) and γ2 (SC-7652) subunits.
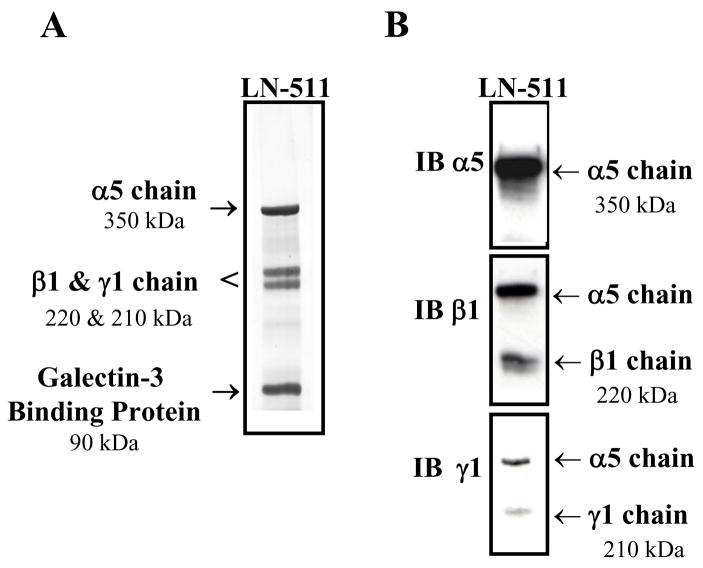
The protein isolated from A549 conditioned medium using only the 4C7- Sepharose column yielded four discrete protein bands with the molecular masses of 350, 220, 210 and 90 kDa under reducing conditions (Figure 2A) and a single band of approximately 800 kDa in non-reducing conditions (data not shown). The identical banding pattern of the larger molecular weight bands (including the 350, 220 and 210 kDa bands) for purified LN-511 from A549 conditioned medium has previously been reported in the literature [11; 12; 13]. However, the 90 kDa band within the eluted LN-511 fraction had not been indentified in these studies although it was consistently found in all purified LN-511 from the A549 cell line. Subsequently, we identified this protein as galectin-3 binding protein by mass spectrometry analysis [17]. The method presented here provides an expansion on previously established LN-511 purification procedures, as it includes steps to remove the lower molecular weight (90 kDa) galectin-3 binding protein contaminant. The affinity of galectin-3 binding protein for lactose has been described previously [18]. To further verify the presence of all three LN-511 subunits, western blot analysis under reducing conditions using monoclonal antibodies for the three LN-511 chains was performed as shown in Figure 2B. The top panel indicates the presence of the α5 subunit; the middle and bottom panels exhibit the β1 and γ1 subunits, respectively. Further verification of galectin-3 binding protein was also assessed by western blot analysis (data not shown).
Figure 2. Purification of LN-511 from A549 conditioned medium.
Serum free conditioned medium from confluent lung adenocarcinoma A549 cells was affinity purified using the 4C7 α5 chain specific antibody. (A) Purified LN-511 was analyzed using a 4% SDS-PAGE gel under reducing conditions and silver stained. The gel indicates the presence of the complete LN-511 trimer with an additional 90 kDa band designated as Galectin-3 binding protein. (B) Gel electrophoresis and Western blot analysis of purified LN-511 using monoclonal antibodies against the α5 (15H5), β1 (4E10) and γ1 (2E8) subunits of LN-511.
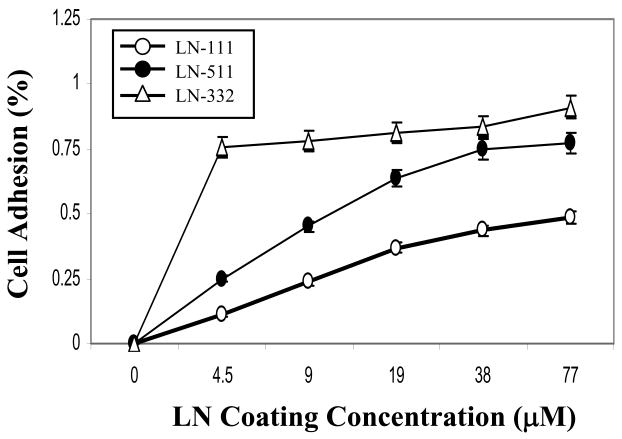
The cell adhesive activity of the purified LN-332 and 511 in comparison to recombinant LN-111 was determined using DU145 H cells. Quantitative analysis indicated that the cells increasingly preferred adhesion to the ligands in the following order: LN-332 more than LN-511 more than LN-111 (Figure 3). These results are consistent with the selection of DU145 H cells, expressing high cell surface levels of the A6 and A3 integrin [15]. These results indicate the biological activity of each purified laminin in an adhesion assay, demonstrating the functionality of using the purified proteins as laboratory reagents for understanding laminin function and biology.
Figure 3. Adhesion of DU-145H cells to purified LN-511 and LN-332.
DU-145H cells were placed into 96-well microtiter plate wells coated with increasing concentrations of recombinant LN-111 and purified LN-511 and LN-332 at 37°C for 2 hours. Following incubation and washing, adherent cells were quantified by crystal violet staining as described in “Materials and Methods”. Each point represents the average value of triplicate determinations, with an error of approximately 5% as shown.
In this report we have demonstrated a time and cost effective procedure for purifying LN-332 and LN-511 from cultured human cell lines, which secrete large amounts of these proteins constitutively. This natural system allows for a highly reproducible and consistent purification procedure and could be easily implemented by investigators requiring purified laminin as a reagent for their 3D culture studies [19].
Acknowledgments
The work was supported in part by CA56666, CA23074. The expert technical assistance of the mass spectrometry service staff was much appreciated. The contribution of the monoclonal antibodies and their careful characterization by several other investigators was essential for the successful completion of the work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aberdam D, Galliano MF, Vailly J, Pulkkinen L, Bonifas J, Christiano AM, Tryggvason K, Uitto J, Epstein EH, Jr, Ortonne JP, et al. Herlitz’s junctional epidermolysis bullosa is linked to mutations in the gene (LAMC2) for the gamma 2 subunit of nicein/kalinin (LAMININ-5) Nat Genet. 1994;6:299–304. doi: 10.1038/ng0394-299. [DOI] [PubMed] [Google Scholar]
- 2.Nagle RB, Hao J, Knox JD, Dalkin BL, Clark V, Cress AE. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol. 1995;146:1498–507. [PMC free article] [PubMed] [Google Scholar]
- 3.Miner JH, Lewis RM, Sanes JR. Molecular cloning of a novel laminin chain, alpha 5, and widespread expression in adult mouse tissues. J Biol Chem. 1995;270:28523–6. doi: 10.1074/jbc.270.48.28523. [DOI] [PubMed] [Google Scholar]
- 4.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–84. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 5.Wondimu Z, Gorfu G, Kawataki T, Smirnov S, Yurchenco P, Tryggvason K, Patarroyo M. Characterization of commercial laminin preparations from human placenta in comparison to recombinant laminins 2 (alpha2beta1gamma1), 8 (alpha4beta1gamma1), 10 (alpha5beta1gamma1) Matrix Biol. 2006;25:89–93. doi: 10.1016/j.matbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Doi M, Thyboll J, Kortesmaa J, Jansson K, Iivanainen A, Parvardeh M, Timpl R, Hedin U, Swedenborg J, Tryggvason K. Recombinant human laminin-10 (alpha5beta1gamma1). Production, purification, and migration-promoting activity on vascular endothelial cells. J Biol Chem. 2002;277:12741–8. doi: 10.1074/jbc.M111228200. [DOI] [PubMed] [Google Scholar]
- 7.Kariya Y, Ishida K, Tsubota Y, Nakashima Y, Hirosaki T, Ogawa T, Miyazaki K. Efficient expression system of human recombinant laminin-5. J Biochem. 2002;132:607–12. doi: 10.1093/oxfordjournals.jbchem.a003263. [DOI] [PubMed] [Google Scholar]
- 8.Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol. 2000;148:615–24. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–76. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazaki K, Kikkawa Y, Nakamura A, Yasumitsu H, Umeda M. A large cell-adhesive scatter factor secreted by human gastric carcinoma cells. Proc Natl Acad Sci U S A. 1993;90:11767–71. doi: 10.1073/pnas.90.24.11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calaluce R, Beck SK, Bair EL, Pandey R, Greer KA, Hoying AM, Hoying JB, Mount DW, Nagle RB. Human laminin-5 and laminin-10 mediated gene expression of prostate carcinoma cells. Prostate. 2006;66:1381–90. doi: 10.1002/pros.20393. [DOI] [PubMed] [Google Scholar]
- 12.Bair EL, Chen ML, McDaniel K, Sekiguchi K, Cress AE, Nagle RB, Bowden GT. Membrane type 1 matrix metalloprotease cleaves laminin-10 and promotes prostate cancer cell migration. Neoplasia. 2005;7:380–9. doi: 10.1593/neo.04619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kikkawa Y, Sanzen N, Sekiguchi K. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. laminin-10/11 mediates cell adhesion through integrin alpha3 beta1. J Biol Chem. 1998;273:15854–9. doi: 10.1074/jbc.273.25.15854. [DOI] [PubMed] [Google Scholar]
- 14.Breitkreutz D, Schoop VM, Mirancea N, Baur M, Stark HJ, Fusenig NE. Epidermal differentiation and basement membrane formation by HaCaT cells in surface transplants. Eur J Cell Biol. 1998;75:273–86. doi: 10.1016/S0171-9335(98)80123-4. [DOI] [PubMed] [Google Scholar]
- 15.Rabinovitz I, Nagle RB, Cress AE. Integrin alpha 6 expression in human prostate carcinoma cells is associated with a migratory and invasive phenotype in vitro and in vivo. Clin Exp Metastasis. 1995;13:481–91. doi: 10.1007/BF00118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Udayakumar TS, Chen ML, Bair EL, Von Bredow DC, Cress AE, Nagle RB, Bowden GT. Membrane type-1-matrix metalloproteinase expressed by prostate carcinoma cells cleaves human laminin-5 beta3 chain and induces cell migration. Cancer Res. 2003;63:2292–9. [PubMed] [Google Scholar]
- 17.Bair EL, Nagle RB, Ulmer TA, Laferte S, Bowden GT. 90K/Mac-2 binding protein is expressed in prostate cancer and induces promatrilysin expression. Prostate. 2006;66:283–93. doi: 10.1002/pros.20343. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg I, Cherayil BJ, Isselbacher KJ, Pillai S. Mac-2-binding glycoproteins. Putative ligands for a cytosolic beta-galactoside lectin. J Biol Chem. 1991;266:18731–6. [PubMed] [Google Scholar]
- 19.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–65. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]