Abstract
Objective
To test the co-occurrence and interrelation of ictal activity and cortical spreading depressions (CSDs) - including the related periinfarct depolarisations in acute brain injury caused by trauma, and spontaneous subarachnoid and/or intracerebral haemorrhage.
Methods
63 patients underwent craniotomy and electrocorticographic (ECoG) recordings were taken near foci of damaged cortical tissue for up to 10 days.
Results
32 of 63 patients exhibited CSDs (5 to 75 episodes), and 11 had ECoGraphic seizure activity (1-81 episodes). Occurrence of seizures was significantly associated with CSD, as 10 of 11 patients with seizures also had CSD (p=0.007, 2-tailed Fishers exact test).
Clinically overt seizures were only observed in one patient. Each patient with CSD and seizures displayed one of four different patterns of interaction between CSD and seizures. In four patients CSD was immediately preceded by prolonged seizure activity. In three patients the two phenomena were separated in time: multiple CSDs were replaced by ictal activity. In one patient seizures appeared to trigger repeated CSDs at the adjacent electrode. In two patients ongoing repeated seizures were interrupted each time CSD occurred.
Conclusions
Seizure activity occurs in association with CSD in the injured human brain.
Significance
ECoG recordings in brain injury patients provide insight into pathophysiological mechanisms that is not accessible by scalp EEG recordings.
Keywords: Brain injury, Secondary damage, Seizures, Peri-infarct depolarisations, cortical spreading depression
Introduction
It is well-known from animal experiments that hyperexcitation or damage to the cortex may lead to either cortical spreading depression (CSD) (Leao, 1944) or seizure activity, depending on the conditions under which the phenomenon is elicited. CSD is a dramatic loss of brain ion homeostasis (Martins-Ferreira et al., 2000) resulting in depolarization of cortical neurons and astrocytes (Sugaya et al., 1975) and large scale changes of local blood flow and metabolism (Bures et al., 1974; Lauritzen, 1994). CSD is transient and spontaneously reversible, in contrast to anoxic and hypoglycaemic depolarization (Kraig and Nicholson, 1978). The mechanisms and consequences of CSD depend on the circumstances under which the reaction is elicited (Hansen and Lauritzen, 1984). CSD has been identified as the most likely explanation for migraine with aura (Lauritzen, 1994; Hadjikhani et al., 2001), and in the otherwise normal rodent brain, the phenomenon has previously been considered harmless, leaving no signs of cell damage (Nedergaard and Hansen, 1988). In comparison, CSD or CSD-like events such as periinfarct depolarisation following brain trauma, subarachnoid haemorrhage or stroke dynamically perturb brain metabolism in animals (Hopwood et al., 2005) as well as humans (Parkin et al., 2005), which leads to progressive ischemic damage in animals (Busch et al., 1996) and possibly in humans (Dreier et al., 2006). Prevention of CSD or periinfarct depolarisation with blockers of the glutamate NMDA-receptor reduces the extent of permanent brain damage in animal models of stroke (Mies et al., 1993; Park et al., 1994).
We have carried out a series of electrocorticographic (ECoG) studies of the cerebral cortex in acutely brain-injured patients to examine the neurophysiological basis of damage following the initial insult. In ~50 % of the patients we identified transient depressions of ECoG activity which spread across the cortical mantle with a speed of 0.4-10 mm/min (Strong et al., 2002; Fabricius et al., 2006). The ECoG depressions were accompanied by large slow potential changes (Fabricius et al., 2006), providing unambiguous evidence that the slowly propagating waves of ECoG silence were identical to Leao’s CSD (Leao, 1944). In several cases, the slow potential changes spread in otherwise electrically silenced tissue as a manifestation of periinfarct/ periinjury depolarisation (Fabricius et al., 2006).
Acute seizures are well-recognized secondary insults following primary brain injury and have the potential to exacerbate tissue damage (Vespa et al., 2003), and continuous EEG monitoring in the neurointensive unit has shown that the majority of acute seizures occur without overt motor manifestations (Claassen et al., 2004). Therefore, it is important to obtain reliable records of seizure activity in these patients and treat them. Here we examined the temporal and spatial association of CSD and seizures using ECoG in patients with acutely injured cerebral cortex. We report for the first time the co-occurrence and patterns of CSD and seizures in humans. We suggest that ECoG monitoring is useful in these patient categories to study pathophysiological mechanisms and may eventually provide a rational basis for treatment in order to prevent disease progression and improve outcome.
Methods
This paper describes in detail ECoG features from ten patients out of 63 consecutive patients that entered the CoOperative Study on Brain Injury Depolarisations (COSBID) at King’s College London, UK (27), Medical College of Virginia, USA (19), Klinikum Charité, Berlin,Germany (9), and Glostrup Hospital, Copenhagen, Denmark (8). COSBID is an international scientific cooperation that studies the importance of CSD and periinfarct depolarisation for secondary brain injury, metabolic and vascular changes and final clinical outcome in brain injury patients. The inclusion criteria and methods of COSBID have been described in detail previously (Strong et al., 2002; Fabricius et al., 2006). Clinical and research consents were obtained complying with the standards in force in each country. The research monitoring protocol was approved by the local research ethics committees. ECoG data from 27 of the patients have been published in part in previous studies as described in the Supplementary Appendix, but the analysis of the relation between CSD episodes and seizure activity is novel.
Electrocorticography
The 6-electrode (linear array) subdural strip (Wyler; platinum, 5-mm diameter, 10-mm interval between electrode centres; Ad-Tech Medical, Racine, Wisconsin) was placed along a gyrus, partly on mildly contused or haemorrhagic cortex and partly on the cortex of healthy appearance, adjacent to the lesion or – in case of SAH – in the region affected by the ruptured aneurysm. Part of the strip was usually outside the area of cortex exposed by the craniotomy. Electrode 1 (most remote from lesion) was used as ground. Electrodes 2-6 yielded four bipolar ECoG channels, named A, B, C, and D (D being closest to the lesion / haemorrhage). The ECoG was recorded continuously for periods of up to 10 days. ECoG from the initial 14 patients including patient 8 were recorded by means of four CED 1902 preamplifiers (frequency limits 0.5-70 Hz). The recordings from the most recent 49 patients, including patients 1-7 and 9–11, were performed using two two-channel Bioamps or one eight-channel GT205 amplifier (both from ADInstruments, New South Wales, Australia). The sampling rate was 200/s and the upper frequency limit 100 Hz. The lower frequency limit of these AC amplifiers was 0.02 Hz, but frequencies as low as 0.002 Hz were detectable (20 dB attenuation). Accordingly, slow potential changes in the near-DC frequency range lasting for 1-2 minutes could be recorded during CSD. Episodes of CSD were defined as sequential onset at adjacent recording sites of a rapidly developing reduction of ECoG amplitude of ≥ 50%, followed by gradual recovery (Strong et al., 2002). Recordings from the most recent 49 patients were also evaluated for slow potential changes as described previously (Fabricius et al., 2006). A slow potential change of large (>1 mV) amplitude and duration of 1-2 min spreading synchronously with the ECoG depression is regarded as the gold standard for identifying CSD (Leao, 1951; Kraig and Nicholson, 1978). Periinfarct depolarisation was defined as a slow potential change spreading through areas where locally generated ECoG activity in the Berger bands was absent (Fabricius et al., 2006).
Seizure activity was defined as rhythmic discharges with spikes or sharp waves of at least 10 second duration. This activity was quantified as the total number of seizure periods in any channel. In one patient (pt. 6) dynamic periodic epileptiform discharges with marked spikes was classified as seizure activity. In another patient (pt. 1) sharp, pointed rhythmic delta activity that was confined to two channels for two minutes before the arrival of CSD was classified as seizure. Rythmic delta activity as such was not considered ictal, as arousal phenomena can have a similar appearance.
Results
In 30 of the 63 patients EcoG recordings revealed neither signs of seizures, CSD nor periinfarct depolarisation. 22 patients had CSD and/or periinfarct depolarisation, but no seizure activity. Only one patient had seizure activity (non-convulsive status), but no CSD or periinfarct depolarisation. Ten patients (designated patient 1-10) displayed repeated episodes of CSD as well as seizure activity. The detailed analysis of the interaction between CSD and seizures in these ten patients constitutes the focus of this paper. The co-occurrence of CSD and seizure points to a significant interdependence of the two phenomena (p=0.007, 2-tailed Fishers exact test). The positive predictive value of ECoG seizure activity for co-occurring CSDs was 91%. Median age of the 10 patients was 43 (range: 20-68); 6 patients were male and 4 female. All required acute craniotomy. Four patients had aneurysmal SAH, one had intracerebral haematoma due to an arterio-venous malformation, and 5 had traumatic head injury. Clinical and ECoG data from the ten patients that had both CSD and seizure activity are summarised in Table 1 and are described in detail in a Supplementary Appendix. Five of the patient stories are summarised in the figure legends (Fig 2-6).
Table 1.
Summary of patient data, monitoring, and outcome
| Pt. no | age (yrs) sex | Injury | Preop. GCS |
6 month eGOS |
Start of monitoring hrs | Duration of monitoring hrs. | No. CSDs | Speed of spread mm/min | Duration of depression min | No. seizures | Type | Phenytoin | Epilepsy after discharge |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22,m | Parietal contusion- assault |
3 | 3 upper severe | 43 | 35 | 9 | 3.3 | 6 –7 increasing to 86 | 6 | ictal 1.5 Hz | Yes, from onset | Generalized tonic-clonic |
| 2 | 65,f | SAH, MCA aneurysm |
10 | 1 Dead | 37 | 111 | 16 | 1.3-3.0 | 2-10 | 3 | 1-2 Hz and spikes | No | N.a. |
| 3 | 66,f | SAH, PCA aneurysm |
14 | 7 upper good | 75 | 119 | 14 | 2.2-3.4 | 3.7-7.5 | 1 | 1.5 Hz spike-waves |
No | No |
| 4 | 46,m | SAH, MCA aneurysm |
3 | 7 upper good | 40 | 150 | 11 | 4.7-6.3 | 7 | 7 | 2 Hz polyspike-waves |
No | Generalized tonic-clonic |
| 5 | 57, f | SAH, MCA aneurysm |
15 | 5 upper moderate | 12 days | 55 | 34 | 1.1-8 | 2.2-8 increasing to 24 | 50 | 1-1.5 Hz spike-waves |
No | No |
| 6 | 40,m | SAH, ICH, aSDH, RTA |
7 | 1 Dead | 17 | 102 | 75 | 2.2-6.2 | 1.9 - 19 | 6 + status | Dynamic PEDs with spikes |
Yes, from onset | N.a. |
| 7 | 20,m | SAH, ICH, aSDH, fall |
8 | 7 upper good | 13 | 79 | 34 | 4-4.5 | 2.9 - 22.4 | 1 | 0.5-1.5 Hz polyspike-waves |
Yes, from onset | No |
| 8 | 68, f | aSDH, fall | 7 | 7 upper good | 26 | 43 | 5 | 0.9-2.4 | 4-15 | 61 | 5 Hz and spikes | Yes, from onset | No |
| 9 | 55,m | biFrontal perforating gunshot |
3 | 1 Dead | 7.1 | 86 | 7 | 1-2.4 | 5.7-10 | 80 | 1-2 Hz spikes | Yes, from onset + Levetiracetam 42 hours later | N.a. |
| 10 | 21,m | ICH, A-V malformation |
15 | 5 upper mode-rate | 72 | 43 | 34 | 2-5 | 1-9 | 81 | 2 Hz and spikes | Single prophyl. + after seizures | Focal motor |
aSDH: acute subdural haematoma; CSD: cortical spreading depression; eGOS: extended Glasgow outcome scale; GCS: Glasgow coma scale; ICH: intracerebral haematoma; MCA: middle cerebral artery; N.a.: non available; PCA: posterior communicating artery; RTA: road traffic accident SAH: subarachnoid haemmorrhage
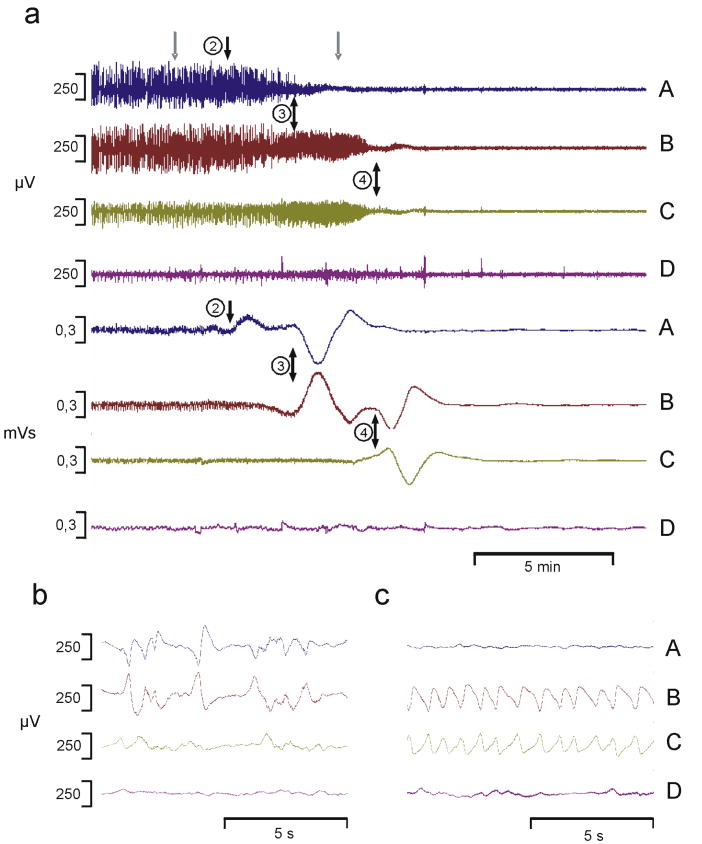
Figure 2. Ictal delta activity in front of approaching CSD.
Pt.1, a 22 year old male sustained a depressed skull fracture with left frontal and parietal contusions, and subsequent diffuse brain swelling. Phenytoin was administered for anticonvulsant prophylaxis. Two days later the contusion was evacuated. The subdural ECoG electrode strip was placed along left middle frontal gyrus. Over the initial 64 hours, 9 episodes of CSD occurred at 1.2 to 18.6 hour intervals. Seizure activity of ~ 2 min duration and characterized by trains of sharp 1-1.5 Hz rhythmic slow waves occurred only in relation to CSD episodes. At 8 month follow up, he was quite severely disabled and post traumatic generalized tonic-clonic seizures were well-controlled on carbamazepine.
(a) Upper traces: Time compressed traces of the raw ECoG signal (analogue frequency limits 0.02-100 Hz) showing the spread of a CSD from channel A to channels B and C. Lower traces: The amplitude integral of the same ECoG signal, enhancing very low frequencies. Each depression in the upper traces was accompanied by a stereotyped baseline change reflecting a slow potential change. Electrode numbers are indicated (black arrows) to mark the onset of depolarisation at that particular electrode as evidenced by phase reversal. In electrode 4 the seizure activity (c) started ~2 min before onset of the slow potential change and thereby onset of CSD. Grey arrows above channel A indicate time points for the detailed traces (b and c, same filtering as upper traces).
(b) Baseline activity before the CSD episode. ECoG activity showed irregular delta activity of 2-400 μV amplitude and short periods of flattening in channels A, B, and C.
(c) 1-1.5 Hz seizure activity in channels B and C at the time point indicated by the right grey arrow in (a). Seizure activity started at the same time in channel B and C, i.e. at electrode number 4. 70 sec later the pattern changed into complete depression of the ECoG (not shown in detail).
Figure 6. Seizure activity at one electrode preceding CSD at neighbouring electrodes.
Pt.10, a 21 year old male, had sudden onset of severe headache whilst weight-lifting, and developed dense paresis of the left arm. He was operated for a large right posterior frontal intracerebral haematoma and a small right frontal arteriovenous malformation. The electrode strip was placed anteriorly over the frontal convexity. A loading dose phenytoin was administered. During the first 34 hours, 34 episodes of CSD and 81 seizure episodes were recorded. Seizure frequency gradually decreased from 12 per hour to 1-2 per hour. CSD frequency remained stable at approximately 1.6 CSD episodes per hour during the first 15 hours of recording at which time clinically overt seizure activity was noticed and the patient was restarted on phenytoin. This abolished seizure activity altogether, and the incidence of CSD declined to 0.6 episodes per hour. At 6 month the paresis persisted and there were repeated focal seizures affecting the right arm.
(a) Time compressed recording (analogue frequency limits 0.02-100 Hz, high pass digital filter 0.5 Hz) showing 3 episodes of CSD spreading from channel C to A with a velocity of 3.3 - 5 mm per min. An amplitude reduction of 50-75% evolved over 20-60s, and lasted for 3-6 min. Duration of suppression was longest in channel C, which was closest to the electrode in which seizure activity was observed. 4 episodes of seizure activity recorded in electrode D are marked by arrows. The seizures lasted for 64–128 s. Note the constant time interval between seizure activity in channel D and the ECoG suppression in electrodes A-C. The grey arrow in channel D depicts the seizure shown in (b): an ictal pattern of rhythmic 1-2 Hz activity with spikes. Channel A-C show irregular 1-3 Hz activity.
ECoG activity: seizures and CSD
The ECoG recordings showed four patterns of abnormal activity as summarized in a schematic form in Figure 1. The patterns were consistent within recordings from the same patient, but differed between patients.
Figure 1.
Schematic drawing of four different patterns of the time relation between cortical spreading depression (CSD) and seizure activity as encountered in patients 1-10 for selected times. The number of hours indicated for each pattern gives the duration of the recording time, which is illustrated.
Pattern I: Close interaction of ictal activity and CSD:
At one or several electrodes, the slow potential change and depression of cortical activity was preceded by ictal activity for 1-2 minutes. In patients 1 and 2, this ictal activity started at the time when the approaching CSD reached the neighbouring electrode (upper line in the drawing) (Fig 2). In patient 3 and 4, seizure activity of a few minutes duration spread at a slow rate to the next electrodes immediately followed by CSD (lower two lines in the drawing). The seizure activity subsequently stopped as CSD reached each electrode.
Pattern II: Segregation of CSD and seizures in time:
a) Frequent CSD episodes recurred for ~24 hours without any seizure activity. After spontaneous arrest of CSDs, frequent seizures (pt.5) or periodic epileptiform discharges (PEDs) (pt.6) were noted for the next 24-31 hours.
b) Frequent CSDs stopped when patient was cooled. After 7 hours a single seizure occurred and 30 minutes later CSD activity resumed (pt.7).
Pattern III: Temporary blocking of seizures by CSD:
Frequent seizures were interrupted by CSDs at the same electrode, each time causing a temporary block of seizure activity.
Pattern IV: Segregation of CSD and seizures in space + triggering of CSD by seizures
Frequent brief seizures and CSDs coexisted, but at separate electrodes (see also Fig 6). Most CSDs were preceded by timelocked seizure activity suggesting triggering. When seizures were blocked by phenytoin (arrowhead), the incidence of CSD episodes decreased as well.
Pattern 1 (Patients 1-4) showed that local ictal activity was recorded for 30-123 s before arrival of a wave of CSD. In patients 1 and 2, CSD without previous ictal activity was recorded at one electrode at the same time as ictal activity started at the neighbouring electrode. At the latter electrode ictal activity stopped a few minutes later as the wave of CSD arrived (Fig 1 and 2). In patients 3 and 4, seizure activity spread from electrode to electrode at the same slow speed as CSD, but preceded it by several minutes. This is noteworthy, since the march of seizure activity under other conditions spreads much faster than a CSD. Apparently CSD in one electrode triggers a seizure in another electrode, and as the CSD spreads to this electrode, it triggers a seizure in a third electrode. In patient 4, seizures continued to spread at a similar slow rate to even more distant electrodes, while the CSD was confined to the initial electrodes. We have grouped these four patients together, since they show similar phenomena, while the variations may depend on the actual position of the five electrodes.
Pattern II (Patients 5-7) showed multiple CSDs followed by ictal activity, but segregated in time. In patients 5 and 6 we observed that CSD episodes dominated the ECoG for the first 24 hours and then stopped. Subsequently, multiple seizures (patient 5) or dynamic periodic epileptiform discharges (PEDs) with prominent spikes (patient 6, Fig 3) were recorded for the following 31 hours. Thus, in these patients the two reactions were manifest in the same tissue area, but at different times after the insult. Possibly, altered balance between excitation and inhibition may promote CSDs under certain circumstances and seizures or PEDs under other conditions. In patient 7, 11 CSD episodes occurring at 12-152 minutes interval were recorded 14-23 hours after a traumatic brain injury. After cooling of the patient, no further episodes occurred for the next seven hours. Then a single 0.5-1.5 Hz polyspike-wave seizure lasting 60 s was recorded (Fig 4). This heralded a new series of CSDs for the next 2.5 hours.
Figure 3. Multiple CSDs replaced by PEDs.
Pt. 6, a 40 year old male struck by a car, developed severe and diffuse bilateral contusions, scattered intracerebral hemmorhages, acute subdural hematoma, and diffuse sub-arachnoid hemorrhage. Bilateral craniectomy was performed 13 hr after injury. ECoG was recorded from the partietal lobe for 5 days. 200 mg phenytoin was administered twice daily. Initially ECoG showed flattening and pseudoperiodic 1-2 Hz activity at 2-5 s intervals. During the first 34.5 hours of recording, 73 episodes of CSD occurred at 10-40 minutes interval. After the last CSD, a continuous pattern of dynamically fluctuating PEDs with prominent spikes occurred at 1-4 seconds intervals to resolve gradually only after 25.5 hours. Patient died after 19 days.
(a): Highly compressed recording, analogue frequency limits 0.02-100 Hz. Upper four traces show an 0.5 Hz high pass digitally filtered trace while the lower four traces show the same recording after 0.05 Hz low pass digital filtering. 13 episodes of CSD are evidenced by loss of local ECoG activity accompanied by a quite stereotyped slow potential change spreading between electrodes at a rate of 2-6 mm/min as indicated by boxes. Arrows indicate time points for 0.5 Hz high pass filtered traces (b): two small double arrows and (c): big double arrow.
(b): A CSD has just started in channel A, but not reached channel B and C, while channel D persistently has little or no locally generated ECoG activity.
(c): Continuous PED activity in channel A, B, and C.
Figure 4. Comparison of seizure- and cortical spreading depression (CSD)-induced changes as recorded in a single bipolar electrode.
Pt. 7, a 20 year old male who suffered a closed head injury and was operated for a frontal sub-dural hemorrhage, extensive sub-arachnoid blood, and right frontal intraparenchymal hemorrhage. The electrode strip extended from the inferior frontal gyrus to inferior parietal cortex. 200 mg phenytoin was administered twice daily. 6 month recovery was good.
(a) Upper traces: low pass digital filter (< 0.05 Hz) lower traces: same channel, high pass digital filter (> 0.5 Hz), all traces analogue frequency limits 0.02-100 Hz. At the left, a 220 s lasting spike/polyspike-and-wave seizure augments to a 1.8 mV amplitude then stops abruptly followed by a 1 minute lasting post seizure depression. No significant changes are detected in the low pass filtered curve (note: the recording is bipolar, electrodes 1 cm apart).
At the right, 38 minutes later, a CSD evidenced by a slow potential change of 2.5 mV amplitude and lasting 2 minutes is accompanied by a gradual loss of amplitude lasting 4.5 minutes. The slow potential change spread to the neighbouring channels at a speed of 3.4 mm/min (not shown).
Both episodes were followed by a gradual recovery of an irregular, but continuous 1.5-3 Hz ECoG activity.
(b): 2 × 13 s recording (same electrode) from the onset and from the end of the seizure shown in (a, lower trace) as indicated by arrows.
Pattern III (Patients 8-9) showed multiple ECoG seizures at usually 2-5 min intervals at the same electrodes which showed evidence of CSD. The frequent seizure activity was interrupted by repeated episodes of CSD. Each time ECoG recovered after 4-10 minutes. Seizure activity, however, did not resume until at least 17 minutes later. This may suggest that seizure activity was temporarily blocked by the CSD (Fig 5). In patient 8, CSD episodes propagated away from the lesion before seizures occurred, while the CSD episodes propagated towards the lesion during seizures. It is not clear if this indicated that seizures triggered CSD, or that CSD and seizure activity occurred independently.
Figure 5. Prolonged blockade of ECoG seizure activity by CSD.
Pt 8, a 68 year-old woman, suffered a head injury after falling down a staircase. After evacuation of an acute right subdural haematoma, the electrode strip was placed over the pre-central gyrus. At 10 - 30 hours after start of recording we observed 5 episodes of CSD. A total of 61 seizure episodes were recorded at 24 to 32 hours. Most of the time, the seizures recurred approximately every 5 minutes. Corresponding to the 3 CSDs occurring in this period, seizure activity was blocked for 19, 17, and 72 min. respectively, while ECoG was fully depressed for 8, 4, and 10 min. 6 month recovery was good.
(a) In the middle of the traces (analogue frequency limits 0.5-70 Hz), a CSD spreads from channel A–D with a velocity of 1-1.8 mm/min (duration of depression 5.5 – 11 min). Rates and ECoG recovery times were comparable to CSD {Leao, 1944 6242 /id}. Arrows depict 16 episodes of seizure activity in channel A and B occurring before and after, but not during the CSD. The grey arrow above channel A marks the seizure shown in detail in (b): Ictal pattern of rhythmic 5 Hz activity with spikes.
Pattern IV (Patient 10) demonstrated (clinically overt) focal seizures close to the site of the lesion. The ECoG seizure activity occurred adjacent to, but did not invade the area under the electrodes where the CSD episodes were recorded. CSD propagated away from the seizure focus in all instances and was time-locked to seizure activity in 63% of the events (Fig 1, 6, and 7). This may suggest that seizure activity triggered CSD. Treatment with phenytoin stopped seizures altogether, while CSD episodes were still observed albeit with a strong reduction in frequency.
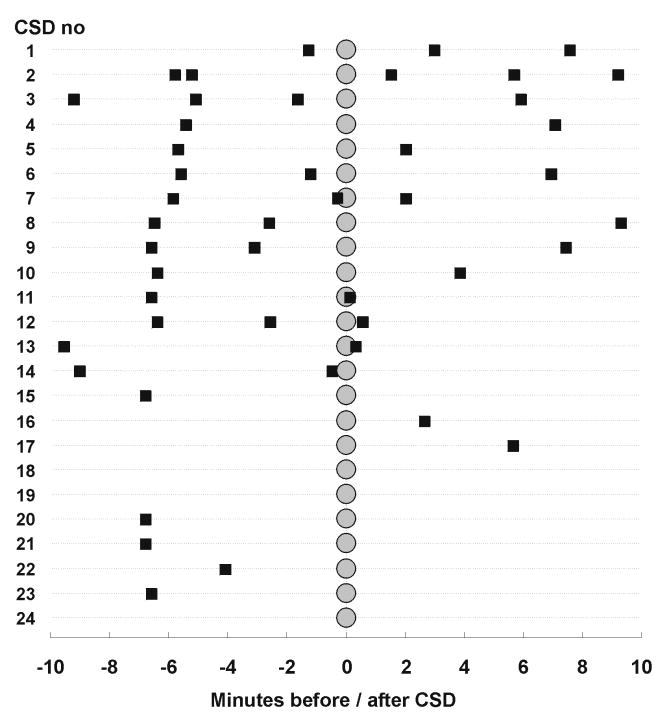
Figure 7. Time lock between ECoG seizure episodes and CSD in pt. 10 may suggest causal link between cortical seizure activity and CSD.
The figure shows for the initial 24 CSD episodes the incidence and timing of seizure episodes (■) within10 min before and after onset of the CSD (○) arranged in a top-down sequence. Seizures were of 1-2 minutes duration. 15 CSD episodes were preceded at 5-7 minutes by a seizure, suggesting that in these instances the seizure triggered the CSD.
Each patient displayed only one type of pattern. Within the group of 10 patients there was no statistical correlation when the number of seizures for each patient was plotted against the number of CSDs, nor was there when the total duration of seizure activity in each patient was plotted against the total duration of depressed ECoG activity of CSD. Comparison of the four patterns (Table 1) suggested that independent occurrence of seizures and CSD (pattern II or IV) predicted a higher incidence of both seizures and CSD episodes, while fewer episodes were observed when seizures and CSD occurred interdependently (pattern I). The number of CSDs/periinfarct depolarisations did not differ between the group of patients with both seizures and CSD (median: 15, range 5-75, n=10) and patients with CSD/ periinfarct depolarisation alone (median: 9, range 2-96, n=22), (P= 0.6, t-test).
The extent to which the subdural strip was placed on partially healthy / partially damaged tissue was documented by CT-scan and 3D reconstruction. The baseline ECoG patterns confirmed in each case that the tissue under at least some of the electrodes was able to generate neuronal activity, while electrodes 5 and 6 corresponding to channel D often showed no or very sparse ECoG activity, probably corresponding to penumbral conditions (Figs 2 and 3). Under these electrodes, spreading depolarisations had the character of periinfarct depolarisations as previously defined (Fabricius et al., 2006).
Other ECoG activity
In most patients, initial ECoG baseline activity was continuous or discontinuous 0.5-3 Hz delta activity with amplitude of 300-1100 μV. The background activity often became more continuous and/or mixed with theta activity as anaesthesia waned. One patient had predominantly theta activity with many interictal short trains of spikes or spike-and-waves, but otherwise interictal spiking was not a common finding. CSDs occurred multiple times in the ten patients (Table 1) and often at 20-30 minutes interval. The depressions usually lasted less than 10 minutes, but two patients (patient 1 and 5) experienced gradual prolongation of recovery of the ECoG activity up to 86 and 24 minutes respectively. After recovery from repeated CSDs the predominant activity was either a burst suppression pattern or periodic discharges with repetition intervals of 1-5 seconds. This demonstrates that in these patients repetitive CSDs leave a disrupted background activity in their wake. Various seizure patterns occurred, but were stereotyped within the individual patient usually consisting of trains of rhythmic activity with or without spikes/polyspikes (Table 1). The duration of seizure activity ranged between 15 and 175 sec, but in two patients they lasted for up to 14 min. Seizures often recurred at regular intervals of 2-15 minutes. In patient 6, a multitude of CSDs (n=75) was replaced by a continuous pattern of dynamic periodic epileptiform discharges with spikes and polyspikes, amplitude up to 900 μV.
Comparison of post seizure depression and CSD
Post seizure depression occurred after seizures in patient 3, 4 and 7. The seizure activity stopped abruptly and was followed by 1-2 minutes of severely depressed local ECoG activity (Fig 4), then a gradual recovery. There was no manifest slow potential change during the seizure or depression. In contrast, CSD showed a more gradual decay of activity over 1-2 minutes corresponding to spread of the wave of depression over the area recorded by the electrode. The depression of activity was accompanied by a massive change of the direct current potential as evidenced by a 1-2- minute lasting deflection of 2-4 mV amplitude.
Anticonvulsive treatment
The study protocol did not include standards for prophylactic anticonvulsant treatment. Treatment was given according to the department guidelines and tradition. Of the eleven patients with ECoG seizure activity (Table1), five patients received no anti-convulsant treatments while six patients received phenytoin as prophylaxis. Eight of the eleven patients survived to 6 months. Three patients (38 %) developed post-traumatic epilepsy: two of these patients had been treated with phenytoin.
Discussion
We here provide evidence for the co-occurrence of CSD and ECoG seizure activity in acutely injured human cerebral cortex in vivo. CSD was more often encountered than seizures, since there were twice as many patients with CSD/ periinfarct depolarisation alone than with CSD/ periinfarct depolarisation plus seizures. 10 of 11 patients with seizure activity also had CSD. Clinical overt seizures were only observed in 1 of the 11 patients, while seizures were not suspected on clinical grounds in the other 10 patients. Taken together, this confirms previous observations indicating that continuous monitoring of EEG or ECoG activity in recently operated patients with acute brain injury provides information that is not otherwise available for the planning of treatment (Claassen et al., 2004).
Our ECoG recordings allowed unambiguous distinction between CSD and seizures (Fig. 4). The cortical DC potential was stable during seizures (smaller changes may have been cancelled out in the bipolar recordings), and when post seizure depression occurred, ECoG activity was abruptly depressed. CSD, in contrast, showed a more gradual depression of background EcoG activity and a massive, but transient, change in the DC potential spreading at a slow apparent rate of 0.9-6 mm/min. This slow potential change is the hallmark of CSD. In patient 1-4 where ictal activity preceded and spread in front of the CSD, the two patterns were closely linked in time, but components corresponding to ictal activity and CSD could be distinguished (Fig. 2). In routine ECoG recordings, CSD may be overlooked if low frequencies are removed by filtering or curves are inspected in 10 s frames, which may explain why CSD has not been observed in clinical practice for so many years. In surface EEG, post seizure depression is a common phenomenon. Some of these patterns may in fact be due to CSD which may be one mechanism of the post-ictal depression of cortical electrical activity (Van Harreveld and Stamm, 1955). At present this can only be resolved by invasive measurements.
In 1944, Leao suggested that CSD and propagating focal seizures were related phenomena, generated by the same cellular elements (Leao, 1944). Since then, the co-occurrence of CSD and seizure activity has been observed in animal studies (Van Harreveld and Stamm, 1953; Koroleva and Bures, 1983), and in human neocortical tissue slices upon induction of CSD (Avoli et al., 1991; Gorji and Speckmann, 2004). The occurrence of CSD and seizures we observed in the present study relates well with previous observations reported in studies of animal and isolated human cortex:
Pattern I (Fig.1 and 2) showed a 1-2 minute train of ictal activity immediately before CSD. This might represent a prolongation of the paroxysmal ‘epileptoid’ activity during the initial 1-5 seconds of a CSD (Buresova et al., 1963; Morlock et al., 1964; Rosenblueth and Garcia Ramos, 1966) or a manifestation of the conversion of CSD to ‘spreading convulsions’ (Van Harreveld and Stamm, 1953). Following the initiation of CSD, we observed that seizure activity ceased at successive electrodes following the invasion of CSD into cortical tissue, as did van Harreveld in the rabbit (Van Harreveld and Stamm, 1955).
In pattern II (Fig 1 and 3) multiple CSDs were recorded before ictal activity erupted. It is possible that repeated CSD activity may lower the seizure threshold or build up increased excitability to promote a change into epileptic status. In rodents, CSD can both elicit and block epileptic discharges (Van Harreveld and Stamm, 1953). Traumatic brain injury in the rat provokes CSD in virtually all animals and subsequent seizures in a lesser fraction (Williams et al., 2006). In isolated human brain tissue, seizure activity may be enhanced by repeated CSD episodes (Gorji and Speckmann, 2004), possibly by selective suppression of GABAergic function (Kruger et al., 1996).
In pattern III (Fig 1 and 5), seizures occurring at 2-5 min. intervals paused as CSD passed (Van Harreveld and Stamm, 1953). Obviously, a fully depolarised cortex cannot support seizure activity, but even after recovery of local ECoG activity seizures did not resume for another 10-15 minutes, suggesting a refractory phase for seizures after CSD. In patient 4 however, seizures propagated beyond CSD without being blocked. This apparent paradox may be explained by the fact that a CSD may penetrate to different degrees into adjacent cortical layers (Munoz-Martinez, 1970; Richter and Lehmenkuhler, 1993). If elicited at the cortical surface a CSD may remain in the superficial layers, and a CSD triggered in the deeper cortical layers may remain there and propagate independent of a CSD elicited at the cortical surface. This may allow seizure activity to continue during CSD.
In pattern IV (patient 10, Fig 1, 6, and 7)), seizures appeared to trigger CSD, but in segregated areas of the cortex (Fig 6 and 7). The latter corresponds well with experimental observations by Koroleva and Bures where CSDs did not invade epileptic foci from outside (Koroleva and Bures, 1979). Generalized tonic-clonic seizures may trigger CSD in rabbits (Van Harreveld and Stamm, 1955), and single cortical spikes may trigger CSD in rats (Koroleva and Bures, 1983). Audiogenic seizures in the rat provoked CSDs which in turn marked the transition into a kindled state, and subsequent spontaneous seizures were followed by CSDs (Vinogradova et al., 2006).
We found a significant co-occurrence of CSD and seizures among the 63 patients, but there was no obvious correlation of the intensity of the two phenomena with respect to frequency or duration. Multiple episodes of both phenomena were seen only in three of the patients (patient 5, 6, and 10) where they occurred separately in time or space. This may suggest that the two phenomena may both trigger and limit each other. The assessment of a cause-effect relationship between CSD and seizures in our patients are associated with difficulties that require careful consideration. First of all, the speed of spread of a CSD, and hence the time correlation between the wave of depolarization and seizures, is influenced by a number of factors including the functional anatomy of the underlying grey matter (Bures et al., 1974). The speed of spread of CSD is both faster and more reproducible on a gyrus than in a sulcus, which may be considered as a barrier for CSD (Bowyer et al., 1999). Therefore, if part of the electrode strip was located over a sulcus this would lead to long and variable intervals between CSD episodes and seizure activity and in turn difficulties in the correlation analysis. This is hard to exclude because part of the electrode strip was placed outside of the neurosurgeons operative field, radiating away from the lesioned area to ensure that the electrodes were in contact with presumably viable cortex (Strong et al., 2002). The speed of spread of a CSD and the amplitude of the propagated signal also varies with the functional state of the underlying tissue, i.e. the extracellular microenvironment (Martins-Ferreira et al., 2000), while the duration of the depolarization changes are dependent on how close the CSD is to the brain lesion (Nedergaard and Hansen, 1993). These problems limit our ability to demonstrate if the reactions triggered one another in each of the patients.
Conditions that facilitate activation of glutamate NMDA receptors, i.e. exposure to low extracellular magnesium, favour the occurrence of both seizure activity and CSD in rat and human neocortical slices (Mody et al., 1987; Avoli et al., 1991). Likewise, both CSD and seizure activity may be evoked in response to attenuation of the inhibitory GABAergic tone (Amemori et al., 1987; Kohling et al., 2003). Recently, modelling of the regulation by glial cells of extracellular potassium levels have shown increased susceptibility towards elicitation of both seizures and CSD when glial function was impaired (Somjen et al., 2008). Therefore, the cerebral grey matter does support both seizures and CSD at the same time, and the two reactions may interact to change the excitability of the tissue and the susceptibility of the cerebral grey matter to support either of the two reactions.
In patient 10, CSD episodes were still observed after seizures had been blocked with phenytoin, albeit with a strong reduction in frequency. This could suggest that seizure activity does increase the susceptibility of the tissue for CSD, but that seizures are not necessary for the generation of CSD. An alternative interpretation is that phenytoin decreases the excitability of the tissue by a mechanism that influences both reactions. The effect of phenytoin in preventing seizures in patients with traumatic brain injury is well-known (The Brain Trauma Foundation., 2000), while the ability of phenytoin to block CSD in animal experiments is less clear. Phenytoin reduced the speed of propagation of CSD in the chick retina (Chebabo and Do Carmo, 1991), and increased the threshold for CSD elicitation in rodents (Reid et al., 1988), although huge doses of phenytoin were unable to block CSD in acute experiments in rodents (Marrannes et al., 1988). This problem will have to be addressed in further studies designed to assess the susceptibility of CSD and seizure activity to various treatments.
In conclusion, CSD and seizure activity co-occur in acutely injured human cerebral cortex. We hypothesize that the two reactions have a common basis - enhanced excitation, decreased inhibition, or both. The data show that the same small piece of cortical tissue may support both phenomena in vivo. In one patient, acute treatment with phenytoin blocked seizures while the incidence of CSD episodes was reduced afterwards. Our findings are consistent with experimental data obtained in studies of human brain slices which suggest that increases in cortical excitability favour the production of both seizures and CSD independently (Avoli et al., 1991; Kohling et al., 2003; Gorji and Speckmann, 2004). Six of the patients with seizures received seizure prophylaxis, but obviously without sufficient effect. However, the seizure activity was first noticed a day or more after they occurred, and no acute change of treatment was performed based on the ECoG. Efficient means of online detection of seizures are therefore needed. We emphasise that had we not monitored the ECoG we would not have known about most of the ECoG seizures. This gives an additional benefit to ECoG monitoring beyond the detection of CSD/ periinfarct depolarisation events.
Supplementary Material
Acknowledgments
Supported by grants of Novo Nordisk Fonden and Fonden for Neurologisk Forskning to Dr. Fabricius and the Deutsche Forschungsgemeinschaft to Dr. Dreier (SFBTr3D10, DFG DR 323/3-1)
Footnotes
No conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Amemori T, Gorelova NA, Bures J. Spreading depression in the olfactory bulb of rats: reliable initiation and boundaries of propagation. Neuroscience. 1987;22:29–36. doi: 10.1016/0306-4522(87)90195-3. [DOI] [PubMed] [Google Scholar]
- Avoli M, Drapeau C, Louvel J, Pumain R, Olivier A, Villemure JG. Epileptiform activity induced by low extracellular magnesium in the human cortex maintained in vitro. Ann Neurol. 1991;30:589–596. doi: 10.1002/ana.410300412. [DOI] [PubMed] [Google Scholar]
- Bowyer SM, Tepley N, Papuashvili N, Kato S, Barkley GL, Welch KM, Okada YC. Analysis of MEG signals of spreading cortical depression with propagation constrained to a rectangular cortical strip. II. Gyrencephalic swine model. Brain Res. 1999;843:79–86. doi: 10.1016/s0006-8993(99)01893-4. [DOI] [PubMed] [Google Scholar]
- Bures J, Buresova O, Krivanek J. The mechanism and applications of Leao’s spreading depression of electroencephalographic activity. Academia; Prague: 1974. [Google Scholar]
- Buresova O, SHIMA I, Bures J, Fifkova E. Unit activity in regins affected by the spreading depression. Physiol Bohemoslov. 1963;12:488–494. [PubMed] [Google Scholar]
- Busch E, Gyngell ML, Eis M, Hoehn-Berlage M, Hossmann KA. Potassium-induced cortical spreading depressions during focal cerebral ischemia in rats: contribution to lesion growth assessed by diffusion-weighted NMR and biochemical imaging. J Cereb Blood Flow Metab. 1996;16:1090–1099. doi: 10.1097/00004647-199611000-00002. [DOI] [PubMed] [Google Scholar]
- Chebabo SR, Do Carmo RJ. Phenytoin and retinal spreading depression. Brain Res. 1991;551:16–19. doi: 10.1016/0006-8993(91)90907-d. [DOI] [PubMed] [Google Scholar]
- Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, Lehmann TN, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Fuhr S, Bhatia R, Boutelle M, Hashemi P, Strong AJ, Lauritzen M. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129:778–790. doi: 10.1093/brain/awh716. [DOI] [PubMed] [Google Scholar]
- Gorji A, Speckmann EJ. Spreading depression enhances the spontaneous epileptiform activity in human neocortical tissues. Eur J Neurosci. 2004;19:3371–3374. doi: 10.1111/j.0953-816X.2004.03436.x. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Sanchez dR, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Tootell RB, Sorensen AG, Moskowitz MA. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA. 2001;98:4687–4692. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AJ, Lauritzen M. The role of spreading depression in acute brain disorders. An Acad Bras Cienc. 1984;56:457–479. [PubMed] [Google Scholar]
- Hartings JA, Gugliotta M, Gilman C, Strong AJ, Tortella FC, Bullock R. Repetitive cortical spreading depolarizations in a case of severe brain trauma. Neurol Res. 2008 doi: 10.1179/174313208X309739. In press. [DOI] [PubMed] [Google Scholar]
- Hopwood SE, Parkin MC, Bezzina EL, Boutelle MG, Strong AJ. Transient changes in cortical glucose and lactate levels associated with peri-infarct depolarisations, studied with raperiinfarct depolarisation-sampling microdialysis. J Cereb Blood Flow Metab. 2005;25:391–401. doi: 10.1038/sj.jcbfm.9600050. [DOI] [PubMed] [Google Scholar]
- Kohling R, Koch UR, Hagemann G, Redecker C, Straub H, Speckmann EJ. Differential sensitivity to induction of spreading depression by partial disinhibition in chronically epileptic human and rat as compared to native rat neocortical tissue. Brain Res. 2003;975:129–134. doi: 10.1016/s0006-8993(03)02600-3. [DOI] [PubMed] [Google Scholar]
- Koroleva VI, Bures J. Circulation of cortical spreading depression around electrically stimulated areas and epileptic foci in the neocortex of rats. Brain Res. 1979;173:209–215. doi: 10.1016/0006-8993(79)90622-x. [DOI] [PubMed] [Google Scholar]
- Koroleva VI, Bures J. Cortical penicillin focus as a generator of repetitive spike- triggered waves of spreading depression in rats. Exp Brain Res. 1983;51:291–297. doi: 10.1007/BF00237205. [DOI] [PubMed] [Google Scholar]
- Kraig RP, Nicholson C. Extracellular ionic variations during spreading depression. Neuroscience. 1978;3:1045–1059. doi: 10.1016/0306-4522(78)90122-7. [DOI] [PubMed] [Google Scholar]
- Kruger H, Luhmann HJ, Heinemann U. Repetitive spreading depression causes selective suppression of GABAergic function. Neuroreport. 1996;7:2733–2736. doi: 10.1097/00001756-199611040-00065. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain. 1994;117:199–210. doi: 10.1093/brain/117.1.199. see comments. [DOI] [PubMed] [Google Scholar]
- Leao AA. The slow voltage variation of cortical spreading depression of activity. Electroenceph Clin Neurophysiol. 1951;3:315–321. doi: 10.1016/0013-4694(51)90079-x. [DOI] [PubMed] [Google Scholar]
- Leao AAP. Spreading depression of activity in cerebral cortex. J Neurophysiol. 1944;7:359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Marrannes R, Willems R, De Prins E, Wauquier A. Evidence for a role of the N-methyl-D-aspartate (NMDA) receptor in cortical spreading depression in the rat. Brain Res. 1988;457:226–240. doi: 10.1016/0006-8993(88)90690-7. [DOI] [PubMed] [Google Scholar]
- Martins-Ferreira H, Nedergaard M, Nicholson C. Perspectives on spreading depression. Brain Res Rev. 2000;32:215–234. doi: 10.1016/s0165-0173(99)00083-1. [DOI] [PubMed] [Google Scholar]
- Mies G, Iijima T, Hossmann KA. Correlation Between Peri-Infarct DC Shifts and Ischaemic Neuronal Damage in Rat. Neuroreport. 1993;4:709–711. doi: 10.1097/00001756-199306000-00027. [DOI] [PubMed] [Google Scholar]
- Mody I, Lambert JD, Heinemann U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol. 1987;57:869–888. doi: 10.1152/jn.1987.57.3.869. [DOI] [PubMed] [Google Scholar]
- Morlock NL, Mori K, WARD AA., Jr A study of single cortical neurons during spreading depression. J Neurophysiol. 1964;27:1192–1198. doi: 10.1152/jn.1964.27.6.1192. [DOI] [PubMed] [Google Scholar]
- Munoz-Martinez EJ. Facilitation of cortical cell activity during spreading depression. J Neurobiol. 1970;2:47–60. doi: 10.1002/neu.480020105. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Hansen AJ. Spreading depression is not associated with neuronal injury in the normal brain. Brain Res. 1988;449:395–398. doi: 10.1016/0006-8993(88)91062-1. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Hansen AJ. Characterization of cortical depolarizations evoked in focal cerebral ischemia. J Cereb Blood Flow Metab. 1993;13:568–574. doi: 10.1038/jcbfm.1993.74. [DOI] [PubMed] [Google Scholar]
- Park CK, McCulloch J, Kang JK, Choi CR. Pretreatment with a competitive NMDA antagonist D-CPPene attenuates focal cerebral infarction and brain swelling in awake rats. Acta Neurochir(Wien) 1994;127:220–226. doi: 10.1007/BF01808770. [DOI] [PubMed] [Google Scholar]
- Parkin MC, Hopwood SE, Jones DA, Hashemi P, Landolt H, Fabricius M, Lauritzen M, Boutelle MG, Strong AJ. Dynamic changes in brain glucose and lactate in pericontusional areas of the human cerebral cortex, monitored with raperiinfarct depolarisation sampling on-line microdialysis: relationship with depolarisation-like events. J Cereb Blood Flow Metab. 2005;25:402–413. doi: 10.1038/sj.jcbfm.9600051. [DOI] [PubMed] [Google Scholar]
- Reid KH, Marrannes R, Wauquier A. Effects of phenytoin and flunarizine on the rise in extracellular potassium induced by repetitive stimulation of rat cerebral cortex. Physiol Bohemoslov. 1988;37:193–202. [PubMed] [Google Scholar]
- Richter F, Lehmenkuhler A. Spreading depression can be restricted to distinct depths of the rat cerebral cortex. Neurosci Lett. 1993;152:65–68. doi: 10.1016/0304-3940(93)90484-3. [DOI] [PubMed] [Google Scholar]
- Rosenblueth A, Garcia Ramos J. Some phenomena usually associated with spreading depression. Acta Physiol Lat Am. 1966;16:141–179. [PubMed] [Google Scholar]
- Somjen GG, Kager H, Wadman WJ. Computer simulations of neuron-glia interactions mediated by ion flux. J Comput Neurosci. 2008 doi: 10.1007/s10827-008-0083-9. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Fabricius M, Boutelle MG, Hibbins SJ, Hopwood SE, Jones R, Parkin MC, Lauritzen M. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002;33:2738–2743. doi: 10.1161/01.str.0000043073.69602.09. [DOI] [PubMed] [Google Scholar]
- Sugaya E, Takato M, Noda Y. Neuronal and glial activity during spreading depression in cerebral cortex of cat. J Neurophysiol. 1975;38:822–841. doi: 10.1152/jn.1975.38.4.822. [DOI] [PubMed] [Google Scholar]
- The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Role of antiseizure prophylaxis following head injury. J Neurotrauma. 2000;17:549–553. doi: 10.1089/neu.2000.17.549. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A, Stamm JS. Spreading cortical convulsions and depressions. J Neurophysiol. 1953;16:352–366. doi: 10.1152/jn.1953.16.4.352. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A, Stamm JS. Cortical responses to metrazol and sensory stimulation in the rabbit. Electroenceph Clin Neurophysiol Suppl. 1955;7:363–370. doi: 10.1016/0013-4694(55)90005-5. [DOI] [PubMed] [Google Scholar]
- Vespa PM, O’Phelan K, Shah M, Mirabelli J, Starkman S, Kidwell C, Saver J, Nuwer MR, Frazee JG, McArthur DA, Martin NA. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441–1446. doi: 10.1212/01.wnl.0000063316.47591.b4. [DOI] [PubMed] [Google Scholar]
- Vinogradova LV, Vinogradov VY, Kuznetsova GD. Unilateral cortical spreading depression is an early marker of audiogenic kindling in awake rats. Epilepsy Res. 2006;71:64–75. doi: 10.1016/j.eplepsyres.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Hartings JA, Lu XC, Rolli ML, Tortella FC. Penetrating ballistic-like brain injury in the rat: differential time courses of hemorrhage, cell death, inflammation, and remote degeneration. J Neurotrauma. 2006;23:1828–1846. doi: 10.1089/neu.2006.23.1828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.