Abstract
Six monoclonal antibodies (MAbs) from various laboratory sources (EB-CA1, EB-CA2, H5, AF1, C6, and 5B2), reacting with the polysaccharidic moieties of Candida albicans mannoproteins, were used for epitope mapping by an enzyme-linked immunosorbent assay (ELISA) with neoglycolipids and by Western blotting (immunoblotting) of a C. albicans germ tube extract. The ELISA involved neoglycolipids constructed from three families of oligomannosides released by sequential depolymerization of C. albicans phosphopeptidomannan by acid hydrolysis (NGLH), beta-elimination (NGLO), and acetolysis (NGLA). All of the MAbs exhibited low reactivities against NGLO. MAbs EB-CA1, EB-CA2, and H5 reacted mainly against NGLA, and MAbs C6 and AF1 recognized mainly NGLH, whereas MAb 5B2 reacted with both families of neoantigens. When this method was compared with Western blotting, strong reactivity to NGLA was associated with the presence of epitopes shared by high-molecular-weight mannoproteins, whereas strong reactivity to NGLH was associated with a reactivity to a family of 14- to 18-kDa antigens. The reactivity of MAb 5B2 was associated with both high-molecular-weight mannoproteins and the 14- to 18-kDa antigens. In relation to the present knowledge about the structure of the C. albicans phosphopeptidomannan oligomannosidic repertoire, these results provide preliminary data concerning the molecular basis of the recognition of mannopyranosyl sequences by MAbs and their distribution among C. albicans mannoproteins.
Full text
PDF

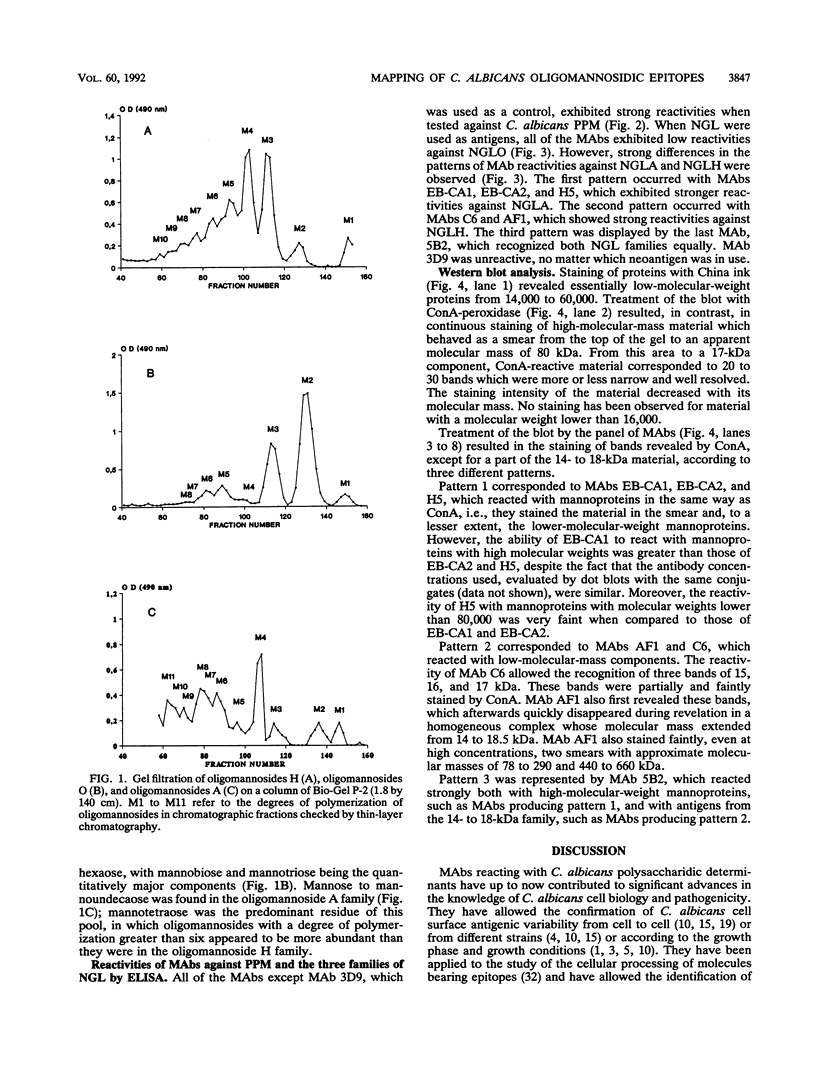
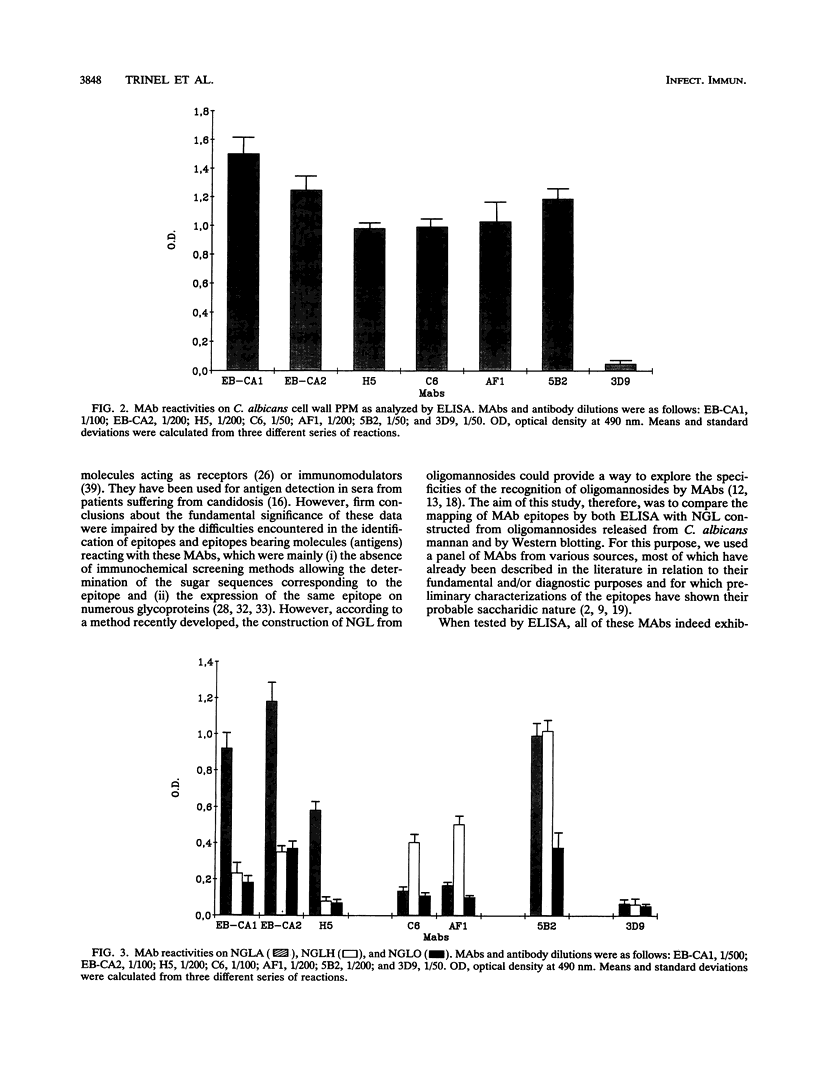
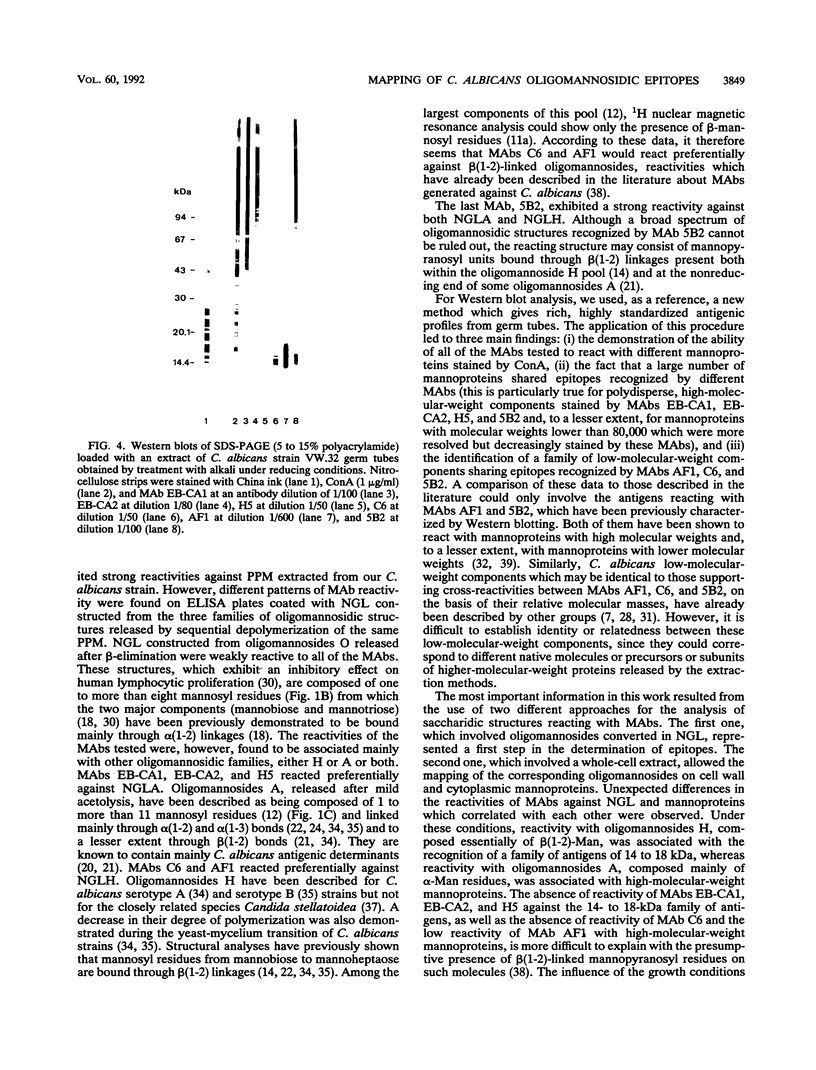


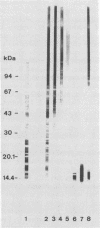
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brawner D. L., Cutler J. E., Beatty W. L. Caveats in the investigation of form-specific molecules of Candida albicans. Infect Immun. 1990 Feb;58(2):378–383. doi: 10.1128/iai.58.2.378-383.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Oral Candida albicans isolates from nonhospitalized normal carriers, immunocompetent hospitalized patients, and immunocompromised patients with or without acquired immunodeficiency syndrome. J Clin Microbiol. 1989 Jun;27(6):1335–1341. doi: 10.1128/jcm.27.6.1335-1341.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Ultrastructural and biochemical studies of two dynamically expressed cell surface determinants on Candida albicans. Infect Immun. 1986 Jan;51(1):327–336. doi: 10.1128/iai.51.1.327-336.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Variability in expression of a cell surface determinant on Candida albicans as evidenced by an agglutinating monoclonal antibody. Infect Immun. 1984 Mar;43(3):966–972. doi: 10.1128/iai.43.3.966-972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Variability in expression of cell surface antigens of Candida albicans during morphogenesis. Infect Immun. 1986 Jan;51(1):337–343. doi: 10.1128/iai.51.1.337-343.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailliez J. C., Poulain D. Analyse cytologique de l'expression d'un épitope porté par les glycoprotéines excrétées par Candida albicans. Ann Inst Pasteur Microbiol. 1988 Mar-Apr;139(2):171–188. doi: 10.1016/0769-2609(88)90003-8. [DOI] [PubMed] [Google Scholar]
- Casanova M., Chaffin W. L. Phosphate-containing proteins and glycoproteins of the cell wall of Candida albicans. Infect Immun. 1991 Mar;59(3):808–813. doi: 10.1128/iai.59.3.808-813.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M., Gil M. L., Cardeñoso L., Martinez J. P., Sentandreu R. Identification of wall-specific antigens synthesized during germ tube formation by Candida albicans. Infect Immun. 1989 Jan;57(1):262–271. doi: 10.1128/iai.57.1.262-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A., Torosantucci A., Boccanera M., Pellegrini G., Palma C., Malavasi F. Production and characterisation of a monoclonal antibody to a cell-surface, glucomannoprotein constituent of Candida albicans and other pathogenic Candida species. J Med Microbiol. 1988 Dec;27(4):233–238. doi: 10.1099/00222615-27-4-233. [DOI] [PubMed] [Google Scholar]
- Chaffin W. L., Skudlarek J., Morrow K. J. Variable expression of a surface determinant during proliferation of Candida albicans. Infect Immun. 1988 Feb;56(2):302–309. doi: 10.1128/iai.56.2.302-309.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faille C., Michalski J. C., Strecker G., Mackenzie D. W., Camus D., Poulain D. Immunoreactivity of neoglycolipids constructed from oligomannosidic residues of the Candida albicans cell wall. Infect Immun. 1990 Nov;58(11):3537–3544. doi: 10.1128/iai.58.11.3537-3544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faille C., Wieruszeski J. M., Lepage G., Michalski J. C., Poulain D., Strecker G. 1H-NMR spectroscopy of manno-oligosaccharides of the beta-1,2-linked series released from the phosphopeptidomannan of Candida albicans VW-32 (serotype A). Biochem Biophys Res Commun. 1991 Dec 31;181(3):1251–1258. doi: 10.1016/0006-291x(91)92073-s. [DOI] [PubMed] [Google Scholar]
- Fruit J., Cailliez J. C., Odds F. C., Poulain D. Expression of an epitope by surface glycoproteins of Candida albicans. Variability among species, strains and yeast cells of the genus Candida. J Med Vet Mycol. 1990;28(3):241–252. doi: 10.1080/02681219080000301. [DOI] [PubMed] [Google Scholar]
- Hayette M. P., Strecker G., Faille C., Dive D., Camus D., Mackenzie D. W., Poulain D. Presence of human antibodies reacting with Candida albicans O-linked oligomannosides revealed by using an enzyme-linked immunosorbent assay and neoglycolipids. J Clin Microbiol. 1992 Feb;30(2):411–417. doi: 10.1128/jcm.30.2.411-417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood V., Poulain D., Fortier B., Evans G., Vernes A. A monoclonal antibody to a cell wall component of Candida albicans. Infect Immun. 1986 Oct;54(1):222–227. doi: 10.1128/iai.54.1.222-227.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya K., Miyakawa Y., Fujihara H., Suzuki M., Soe G., Fukazawa Y. Immunologic significance of diverse specificity of monoclonal antibodies against mannans of Candida albicans. J Immunol. 1989 Nov 15;143(10):3353–3358. [PubMed] [Google Scholar]
- Kobayashi H., Shibata N., Mitobe H., Ohkubo Y., Suzuki S. Structural study of phosphomannan of yeast-form cells of Candida albicans J-1012 strain with special reference to application of mild acetolysis. Arch Biochem Biophys. 1989 Aug 1;272(2):364–375. doi: 10.1016/0003-9861(89)90230-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Shibata N., Nakada M., Chaki S., Mizugami K., Ohkubo Y., Suzuki S. Structural study of cell wall phosphomannan of Candida albicans NIH B-792 (serotype B) strain, with special reference to 1H and 13C NMR analyses of acid-labile oligomannosyl residues. Arch Biochem Biophys. 1990 Apr;278(1):195–204. doi: 10.1016/0003-9861(90)90248-w. [DOI] [PubMed] [Google Scholar]
- Kocourek J., Ballou C. E. Method for fingerprinting yeast cell wall mannans. J Bacteriol. 1969 Dec;100(3):1175–1181. doi: 10.1128/jb.100.3.1175-1181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan G., Pavliak V., Masler L. Structural studies of mannans from the cell walls of the pathogenic yeasts Candida albicans serotypes A and B and Candida parapsilosis. Carbohydr Res. 1988 Feb 1;172(2):243–253. doi: 10.1016/s0008-6215(00)90858-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linehan L., Wadsworth E., Calderone R. Candida albicans C3d receptor, isolated by using a monoclonal antibody. Infect Immun. 1988 Aug;56(8):1981–1986. doi: 10.1128/iai.56.8.1981-1986.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merson-Davies L. A., Hopwood V., Robert R., Marot-Leblond A., Senet J. M., Odds F. C. Reaction of Candida albicans cells of different morphology index with monoclonal antibodies specific for the hyphal form. J Med Microbiol. 1991 Dec;35(6):321–324. doi: 10.1099/00222615-35-6-321. [DOI] [PubMed] [Google Scholar]
- Ollert M. W., Calderone R. A. A monoclonal antibody that defines a surface antigen on Candida albicans hyphae cross-reacts with yeast cell protoplasts. Infect Immun. 1990 Mar;58(3):625–631. doi: 10.1128/iai.58.3.625-631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podzorski R. P., Gray G. R., Nelson R. D. Different effects of native Candida albicans mannan and mannan-derived oligosaccharides on antigen-stimulated lymphoproliferation in vitro. J Immunol. 1990 Jan 15;144(2):707–716. [PubMed] [Google Scholar]
- Ponton J., Jones J. M. Identification of two germ-tube-specific cell wall antigens of Candida albicans. Infect Immun. 1986 Dec;54(3):864–868. doi: 10.1128/iai.54.3.864-868.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D., Cailliez J. C., Dubremetz J. F. Secretion of glycoproteins through the cell wall of Candida albicans. Eur J Cell Biol. 1989 Oct;50(1):94–99. [PubMed] [Google Scholar]
- Shibata N., Fukasawa S., Kobayashi H., Tojo M., Yonezu T., Ambo A., Ohkubo Y., Suzuki S. Structural analysis of phospho-D-mannan-protein complexes isolated from yeast and mold form cells of Candida albicans NIH A-207 serotype A strain. Carbohydr Res. 1989 Apr 15;187(2):239–253. doi: 10.1016/0008-6215(89)80006-0. [DOI] [PubMed] [Google Scholar]
- Shibata N., Kobayashi H., Tojo M., Suzuki S. Characterization of phosphomannan-protein complexes isolated from viable cells of yeast and mycelial forms of Candida albicans NIH B-792 strain by the action of Zymolyase-100T. Arch Biochem Biophys. 1986 Dec;251(2):697–708. doi: 10.1016/0003-9861(86)90379-6. [DOI] [PubMed] [Google Scholar]
- Strockbine N. A., Largen M. T., Buckley H. R. Production and characterization of three monoclonal antibodies to Candida albicans proteins. Infect Immun. 1984 Mar;43(3):1012–1018. doi: 10.1128/iai.43.3.1012-1018.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo M., Shibata N., Ban Y., Suzuki S. Structure of the D-mannan of Candida stellatoidea IFO 1397 strain. Comparison with that of the phospho-D-mannan of Candida albicans NIH B-792 strain. Carbohydr Res. 1990 Jun 1;199(2):215–226. doi: 10.1016/0008-6215(90)84263-t. [DOI] [PubMed] [Google Scholar]
- Tojo M., Shibata N., Kobayashi M., Mikami T., Suzuki M., Suzuki S. Preparation of monoclonal antibodies reactive with beta-1,2-linked oligomannosyl residues in the phosphomannan-protein complex of Candida albicans NIH B-792 strain. Clin Chem. 1988 Mar;34(3):539–543. [PubMed] [Google Scholar]
- Torosantucci A., Boccanera M., Casalinuovo I., Pellegrini G., Cassone A. Differences in the antigenic expression of immunomodulatory mannoprotein constituents on yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1990 Jul;136(7):1421–1428. doi: 10.1099/00221287-136-7-1421. [DOI] [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]