Abstract
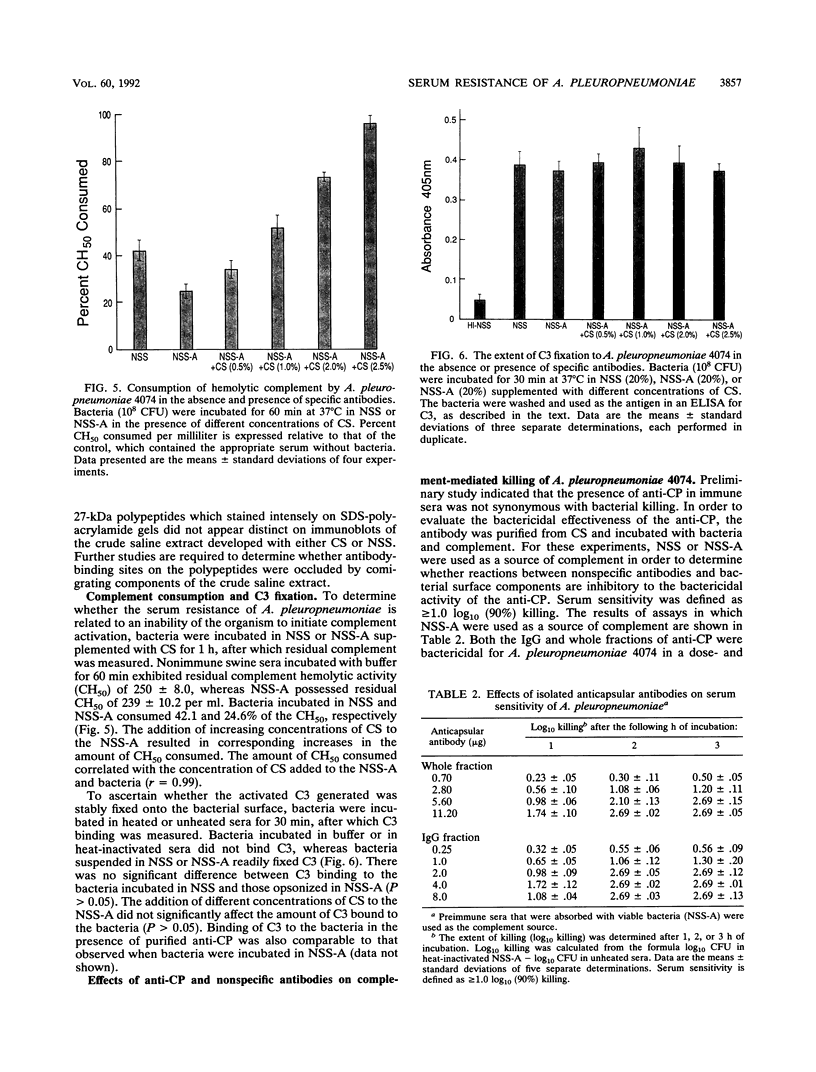
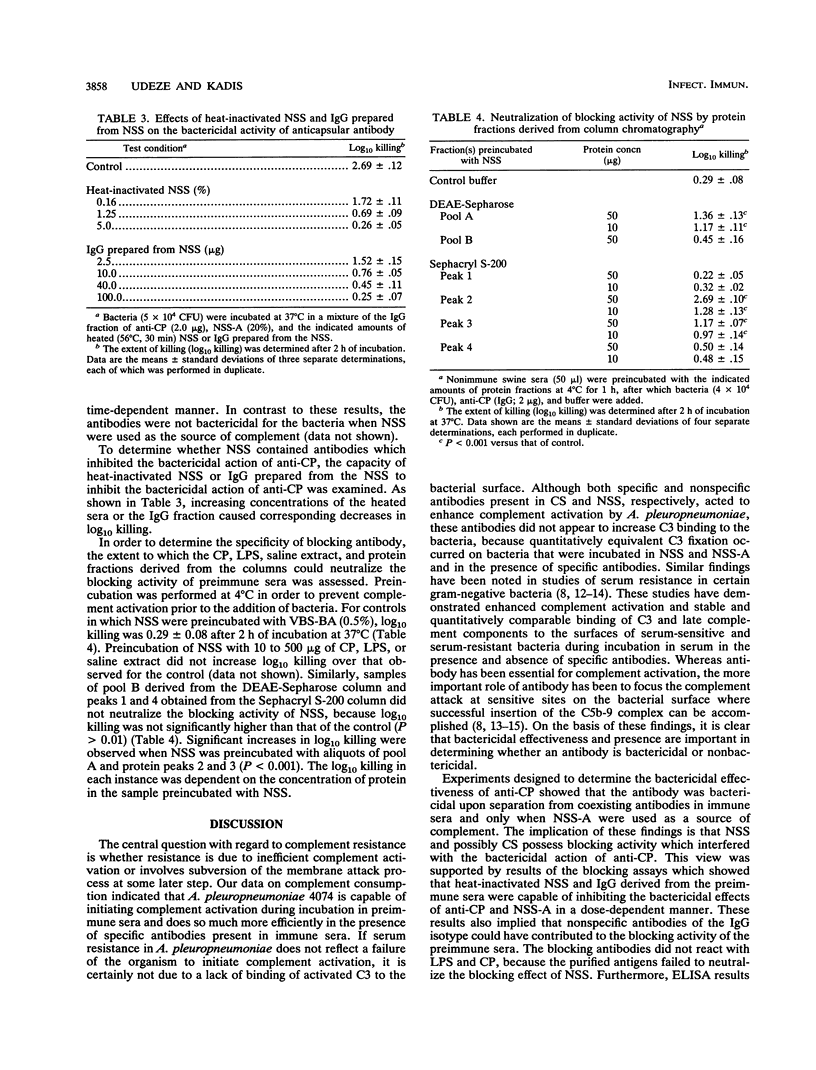
In an attempt to understand the mechanism of serum resistance in Actinobacillus pleuropneumoniae, in the present study we examined various interactions among the bacterial surface constituents, serum antibodies, and complement. Analysis of swine sera revealed the presence of anticapsular antibodies in convalescent-phase sera but not in preimmune sera. Both types of sera contained antibodies which reacted with each of 14 polypeptides present in saline extracts of the bacteria. Absorption of the preimmune sera with intact bacteria depleted antibodies to two of the polypeptides (27 and 32 kDa) and high-molecular-weight (greater than 97.4,000) components which did not stain with Coomassie blue. Data derived from complement consumption and C3-binding experiments indicated that the organism was capable of initiating complement activation and binding C3 during incubation in preimmune and immune sera. Experiments designed to evaluate the bactericidal effectiveness of anticapsular antibody revealed that the purified antibody was bactericidal only when preimmune sera absorbed with intact bacteria were used as a source of complement. The bactericidal effects of anticapsular antibody and absorbed preimmune sera were inhibited in a dose-dependent manner by heat-inactivated preimmune sera and immunoglobulin G derived from the sera. The inhibitory activity of the preimmune sera was neutralized by preincubating the sera with column fractions of the saline extract which contained either the 27- or the 32-kDa polypeptide. These results indicate that serum resistance in A. pleuropneumoniae 4074 could be related to inhibition of the bactericidal action of anticapsular antibody by nonspecific antibodies which recognize surface-exposed epitopes on the polypeptides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman E., Brisson J. R., Perry M. B. Structural studies of the capsular polysaccharide from Haemophilus pleuropneumoniae serotype 1. Biochem Cell Biol. 1986 Aug;64(8):707–716. doi: 10.1139/o86-097. [DOI] [PubMed] [Google Scholar]
- Bertram T. A. Quantitative morphology of peracute pulmonary lesions in swine induced by Haemophilus pleuropneumoniae. Vet Pathol. 1985 Nov;22(6):598–609. doi: 10.1177/030098588502200615. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cross A. S. The biologic significance of bacterial encapsulation. Curr Top Microbiol Immunol. 1990;150:87–95. doi: 10.1007/978-3-642-74694-9_5. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Protective role of complement in experimental Escherichia coli endocarditis. Infect Immun. 1977 Apr;16(1):213–217. doi: 10.1128/iai.16.1.213-217.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins R., Larivière S., Mittal K. R., Martineau G. P., Rousseau P., Cameron J. Evaluation of a Killed Vaccine Against Porcine Pleuropneumonia Due to Haemophilus pleuropneumoniae. Can Vet J. 1985 Feb;26(2):86–89. [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Ma J., Workman T., Gogolewski R. P., Anderson P. Virulence properties and protective efficacy of the capsular polymer of Haemophilus (Actinobacillus) pleuropneumoniae serotype 5. Infect Immun. 1988 Aug;56(8):1880–1889. doi: 10.1128/iai.56.8.1880-1889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques M., Foiry B., Higgins R., Mittal K. R. Electron microscopic examination of capsular material from various serotypes of Actinobacillus pleuropneumoniae. J Bacteriol. 1988 Jul;170(7):3314–3318. doi: 10.1128/jb.170.7.3314-3318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Goldman R. C., Hammer C. H., Leive L., Frank M. M. Studies of the mechanism of bacterial resistance to complement-mediated killing. V. IgG and F(ab')2 mediate killing of E. coli 0111B4 by the alternative complement pathway without increasing C5b-9 deposition. J Immunol. 1983 Nov;131(5):2563–2569. [PubMed] [Google Scholar]
- Joiner K. A., Scales R., Warren K. A., Frank M. M., Rice P. A. Mechanism of action of blocking immunoglobulin G for Neisseria gonorrhoeae. J Clin Invest. 1985 Nov;76(5):1765–1772. doi: 10.1172/JCI112167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Warren K. A., Tam M., Frank M. M. Monoclonal antibodies directed against gonococcal protein I vary in bactericidal activity. J Immunol. 1985 May;134(5):3411–3419. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacInnes J. I., Rosendal S. Analysis of major antigens of Haemophilus (Actinobacillus) pleuropneumoniae and related organisms. Infect Immun. 1987 Jul;55(7):1626–1634. doi: 10.1128/iai.55.7.1626-1634.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley J. R., Kadis S., Mayberry W. R. Isolation, purification, and partial characterization of a lipopolysaccharide from Haemophilus pleuropneumoniae. Infect Immun. 1986 Feb;51(2):501–506. doi: 10.1128/iai.51.2.501-506.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe W. R., Kaijser B., Olling S., Uwaydah M., Hanson L. A. Escherichia coli in bacteremia: K and O antigens and serum sensitivity of strains from adults and neonates. J Infect Dis. 1978 Jul;138(1):33–41. doi: 10.1093/infdis/138.1.33. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Kroll J. S. The role of bacterial polysaccharide capsules as virulence factors. Curr Top Microbiol Immunol. 1990;150:65–85. doi: 10.1007/978-3-642-74694-9_4. [DOI] [PubMed] [Google Scholar]
- Nielsen R. Pleuropneumonia of swine caused by Haemophilus parahaemolyticus. Studies on the protection obtained by vaccination. Nord Vet Med. 1976 Jul-Aug;28(7-8):337–348. [PubMed] [Google Scholar]
- Niven D. F., Donga J., Archibald F. S. Responses of Haemophilus pleuropneumoniae to iron restriction: changes in the outer membrane protein profile and the removal of iron from porcine transferrin. Mol Microbiol. 1989 Aug;3(8):1083–1089. doi: 10.1111/j.1365-2958.1989.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Olling S. Sensitivity of gram-negative bacilli to the serum bactericidal activity: a marker of the host-parasite relationship in acute and persisting infections. Scand J Infect Dis Suppl. 1977;(10):1–40. doi: 10.3109/inf.1977.9.suppl-10.01. [DOI] [PubMed] [Google Scholar]
- Rapp V. J., Ross R. F. Antibody response of swine to outer membrane components of Haemophilus pleuropneumoniae during infection. Infect Immun. 1986 Dec;54(3):751–760. doi: 10.1128/iai.54.3.751-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., McCormack W. M., Kasper D. L. Natural serum bactericidal activity against Neisseria gonorrhoeae isolates from disseminated, locally invasive, and uncomplicated disease. J Immunol. 1980 May;124(5):2105–2109. [PubMed] [Google Scholar]
- Rice P. A., Vayo H. E., Tam M. R., Blake M. S. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986 Nov 1;164(5):1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roantree R. J., Rantz L. A. A STUDY OF THE RELATIONSHIP OF THE NORMAL BACTERICIDAL ACTIVITY OF HUMAN SERUM TO BACTERIAL INFECTION. J Clin Invest. 1960 Jan;39(1):72–81. doi: 10.1172/JCI104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Carpenter D. S., Mitchell W. R., Wilson M. R. Vaccination against pleuropneumonia of pigs caused by Haemophilus pleuropneumoniae. Can Vet J. 1981 Feb;22(2):34–35. [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Miniats O. P., Sinclair P. Protective efficacy of capsule extracts of Haemophilus pleuropneumoniae in pigs and mice. Vet Microbiol. 1986 Sep;12(3):229–240. doi: 10.1016/0378-1135(86)90052-0. [DOI] [PubMed] [Google Scholar]
- Rycroft A. N., Cullen J. M. Complement resistance in Actinobacillus (Haemophilus) pleuropneumoniae infection of swine. Am J Vet Res. 1990 Sep;51(9):1449–1453. [PubMed] [Google Scholar]
- Rycroft A. N., Taylor D. J. Preparation and characterisation of envelope proteins from Haemophilus pleuropneumoniae. Vet Microbiol. 1987 Dec;15(4):303–314. doi: 10.1016/0378-1135(87)90018-6. [DOI] [PubMed] [Google Scholar]
- SHOPE R. E. PORCINE CONTAGIOUS PLEUROPNEUMONIA. I. EXPERIMENTAL TRANSMISSION, ETIOLOGY, AND PATHOLOGY. J Exp Med. 1964 Mar 1;119:357–368. doi: 10.1084/jem.119.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOPE R. E., WHITE D. C., LEIDY G. PORCINE CONTAGIOUS PLEUROPNEUMONIA. II. STUDIES OF THE PATHOGENICITY OF THE ETIOLOGICAL AGENT, HEMOPHILUS PLEUROPNEUMONIAE. J Exp Med. 1964 Mar 1;119:369–375. doi: 10.1084/jem.119.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Barrera O. Immunological characteristics of gonococcal outer membrane protein II assessed by immunoprecipitation, immunoblotting, and coagglutination. J Exp Med. 1983 May 1;157(5):1405–1420. doi: 10.1084/jem.157.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaits R. N., Kadis S. Immunogenicity of Actinobacillus pleuropneumoniae outer membrane proteins and enhancement of phagocytosis by antibodies to the proteins. Infect Immun. 1991 Feb;59(2):544–549. doi: 10.1128/iai.59.2.544-549.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosti K. L., Randall E. Sensitivity of serologically classified strains of escherichia coli of human origin to the serum bactericidal system. Am J Med Sci. 1970 Feb;259(2):114–119. doi: 10.1097/00000441-197002000-00005. [DOI] [PubMed] [Google Scholar]