Abstract
Recently, it was shown that that the orexigenic effect of melanin concentrating hormone (MCH) is attenuated by estradiol treatment in ovariectomized (OVX) rats. This suggests that female rats may be less responsive than male rats to the behavioral effects of MCH. To investigate this hypothesis, the effects of lateral ventricular infusions of MCH on food intake, water intake, meal patterns, and running wheel activity were examined in male and female rats. To further characterize the impact of estradiol on MCH-induced food intake, female rats were OVX and tested with and without 17-β-estradiol benzoate (EB) replacement. In support of our hypothesis, food and water intakes following MCH treatment were greater in male rats, relative to female rats. Specifically, the orexigenic effect of MCH was maximal in male rats and minimal in EB-treated OVX rats. In both sexes, the orexigenic effect of MCH was mediated by a selective increase in meal size, which was attenuated in EB-treated OVX rats. MCH induced a short-term (2 h) decrease in wheel running that, unlike its effects on ingestive behavior, was similar in males and females. Thus, estradiol decreases some, but not all, of the behavioral effects of MCH. To examine the influence of endogenous estradiol, food intake was monitored following MCH treatment in ovarian-intact, cycling rats. As predicted by our findings in OVX rats, the orexigenic effect of MCH was attenuated in estrous rats, relative to diestrous rats. We conclude that the female rat’s reduced sensitivity to the orexigenic effect of MCH may contribute to sex- and estrous cycle-related differences in food intake.
Keywords: food intake, water intake, meal size, estradiol, locomotor activity
1. Introduction
Melanin concentrating hormone (MCH) is a 19-amino acid peptide that is synthesized in the mammalian lateral hypothalamus (LH) and zona incerta (ZI) [1]. In rodents, MCH exerts its varied actions through the MCH-1 receptor, a G protein-coupled receptor that is widely expressed throughout the central nervous system [2]. First identified for its role in regulating the aggregation of melanin pigment in teleost fish [3], more recent studies involving rodents implicate MCH in the control of food intake and the regulation of fluid and energy balance. For example, acute hypothalamic or ventricular (i.c.v.) administration of MCH increases food intake [4–7], and chronic treatment with an MCH-1 receptor agonist or transgenic over-expression of MCH promotes hyperphagia, weight gain, and lipogenesis [8, 9]. In addition to its potent orexigenic effect, MCH has been reported to stimulate water intake, independent of food intake, in male rats [4, 10]. Finally, i.c.v. infusion of MCH decreases core body temperature [11, 12], and targeted deletion of the genes encoding either pre-pro MCH or the MCH-1 receptor stimulates locomotor activity, metabolic energy expenditure, and thermogenesis [13–15].
At present, only two studies have investigated whether the orexigenic effect of MCH is mediated by an increase in meal size, meal number, or both. Because food intake is the product of meal size and meal number [16], this represents an important initial step in characterizing the mechanism by which MCH influences ingestive behavior in the rat. In one study involving diet-induced obese rats, the hypophagia induced by T-226296, a selective MCH-1 receptor antagonist, was mediated by a decrease in meal size, not meal number [17]. This suggests that endogenous MCH influences feeding by selectively affecting the controls of meal size. However, in a more recent study, acute administration of MCH increased food intake primarily via an increase in the number of meals consumed by lean, male rats [10]. Thus, additional studies are necessary to reconcile the discrepant findings regarding meal number.
Also requiring additional research is the notion that the behavioral response to MCH may differ in male and female rats. While the majority of rodent studies investigating the behavioral effects of MCH have been conducted in male rats, a recent study involving female rats provided the first evidence that the orexigenic and dipsogenic effects of MCH may be sexually dimorphic. In this study, the hyperphagia induced by acute i.c.v. administration of MCH was decreased by estradiol treatment in ovariectomized (OVX) rats [18]. Unlike that observed in male rats [4, 10], MCH failed to stimulate water intake, independent of food intake, in either oil- or estradiol-treated OVX rats [18].
The primary aim of this study was to investigate whether the behavioral response to MCH is reduced in female rats, relative to male rats. To investigate this hypothesis, the effects of MCH on food and water intakes, meal patterns, and locomotor activity were examined in male and female rats. To further characterize the impact of estradiol on the behavioral effects of MCH, female rats were OVX and treated with a physiological regimen of estradiol or oil vehicle replacement. In a second experiment, ovarian-intact rats were studied at different stages of the estrous cycle in order to examine the influence of endogenous estradiol on the orexigenic effect of MCH.
2. Methods
2.1. Animals and housing
Twenty-three female and seven male Long-Evans rats (Charles River Breeding Laboratory, Raleigh, NC), weighing 225–250 g at study onset, were housed individually in custom-designed cages. In Experiment 1, the cages were connected to running wheels (Wahmann; 35 cm in diameter) and equipped with feeding niches that provided access to spill-resistant food cups mounted on weight-sensitive load beams. Infrared beams, located on either side of the feeding niches and centered above the feeding cups, were also used to signal the occurrence of feeding behavior. Any food spillage was collected on a platform surrounding the food cup. Water bottles containing drip-resistant sipper tubes were located ~ 20 cm from the feeding niches. Dipole magnets, attached to the barrels of the running wheels, were used to monitor the occurrence of wheel revolutions. In Experiment 2, rats were housed individually in Plexiglas cages equipped with feeding niches that provided access to spill-resistant food cups. In both experiments, rats were given free access to powdered rat chow (Purina 5001) and tap water, except as otherwise noted. The testing room was maintained at 20 ± 2°C with a 12:12 h light-dark cycle (dark onset = 1300 h). Animal usage and all procedures were approved by the Florida State University Institutional Animal Care and Use Committee.
2.2. Surgery
Male and female rats were anesthetized with intraperitoneal (i.p.) injections of a mixture of ketamine (50 mg/ml; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (4.5 mg/ml; Rompun, Mobay, Shawnee, KS) and then implanted with stainless-steel guide cannulas (26 gauge, Plastics One, Roanoke, VA) aimed at the right lateral ventricle. Stereotaxic coordinates (AP: -0.6 mm relative to bregma; ML: -1.7 mm from the midline; DV: -3.5 mm from the surface of the skull) were based on the atlas of Paxinos and Watson [19]. Immediately after cannula implantation, female rats, used in Experiment 1, were bilaterally OVX using an intra-abdominal approach. Following surgery, each rat received an i.p. injection of butorphanol (0.5 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) and a subcutaneous (s.c.) injection of gentamicin (10 mg/ml; Pro Labs Ltd, St. Joseph, MO) to minimize post-surgical pain and the risk of infection, respectively.
Following 7 days of postoperative recovery, a behavioral assay was used to verify each rat’s cannula placement. Water intake was monitored in individual rats following an i.c.v. infusion of 50 ng of angiotensin II (Sigma-Aldrich, St. Louis, MO) delivered in 5 µl of saline vehicle over a period of 1 min. Only those rats that consumed at least 5 ml of water in 20 min were included in the study. Out of 33 animals, 3 (1 male and 2 female) did not meet this requirement and were excluded from the study.
2.3. Behavioral measures
In Experiment 1, outputs from the magnets, load beams, and photo beams were fed via an interface into a computer located in an adjacent room. Custom-designed software (ESP 500; R. Henderson; Florida State University) recorded the occurrence of wheel revolutions (± 0.5 rev) as well as the weights of load beams (± 0.001 g) and the activity of photo beams at 30 s intervals. Additional software (Meal Weight Analysis; R. Henderson, Tallahassee FL) was used to assess food intake and wheel running at particular intervals and to convert individual bouts of ingestive behavior into discrete meals. A meal was defined as any feeding bout of at least 0.35 g that was separated from other feeding bouts by at least 15 min. In previous studies, these criteria accounted for 97–99 % of daily food intake (e.g., [20]). Water intake was monitored by weighing water bottles (± 0.1 g) at specific intervals. In Experiment 2, food intake was monitored by weighing the food cups (± 0.1 g) at specific intervals.
2.4. Experiment 1: behavioral response to MCH in male and female rats
Following i.c.v. surgery, male and OVX female rats were allowed at least 1 week to adapt to the custom-designed cages. Data collection did not commence until stable levels of ingestive behavior and locomotor activity were observed. Each day, the computerized system monitoring food intake and running wheel activity was stopped at 0930 h. At this time, the rats’ body weights were recorded, food cups and water bottles were refilled, and behavioral data were downloaded from the computer. At 1030 h, the computer system was restarted. At study onset, rats received either cyclic estradiol or vehicle treatment for 3 consecutive weeks. Each week, OVX rats (n = 8) received s.c. injections of 4 µg 17-β-estradiol-3-benzoate (EB, Sigma, St. Louis, MO), delivered in 0.1 ml sesame oil vehicle, on Monday and Tuesday at 1000 h. A second group of OVX rats (n = 8) and a group of male rats (n = 7) received the same schedule of s.c. injections of sesame oil vehicle. This procedure was used because it models the changes in estradiol secretion observed across the 4-day estrous cycle in ovarian-intact rats (plasma estradiol levels rise from ~40 pmol/L to ~ 275 pmol/L) and it decreases food intake and increases locomotor activity on Thursday, the day that models behavioral estrus [21]. Each week on Thursday, food and water were removed from the rats’ cages 1 h prior to dark onset. Using a within-subjects, randomized design, rats received weekly i.c.v. infusions, via a handheld 25 µl syringe (Hamilton, Reno, NV), of either 0, 1 or 5 µg MCH (Bachem Biosceince Inc., King of Prussia, PA) dissolved in 2.5 µl saline over a period of 1 min. These infusions were administered 30 min prior to dark onset. At dark onset (1300 h), food and water were returned to the rats’ cages and food intake, water intake, and wheel running were assessed at the first two 2-h intervals after dark onset (i.e., from 1300 – 1500 h and 1500 – 1700 h) and during the subsequent 16.5 h period (from 1700 – 0930 h).
2.5. Experiment 2: effect of the estrous cycle on MCH-induced food intake
Following i.c.v. surgery, seven female rats were allowed at least 1 week to adapt to the custom-designed cages. Each day at 0930 h, the rats’ body weights were recorded, food cups and water bottles were refilled and vaginal cytology samples were collected. Stage of the estrous cycle (diestrus 1, diestrus 2, proestrus, or estrus) was determined by examining the appearance and abundance of cells within vaginal cytology samples. Diestrus 1 was characterized by leukocytes and clusters of cornified cells, diestrus 2 was characterized by leukocytes and nucleated epithelial cells, proestrus was characterized primarily by nucleated epithelial cells, and estrus was characterized by an abundance of cornified cells. Cycle stage labels were assigned to the 24 h period ending at the time of sampling. Using this strategy, proestrus included the light-phase peak in estradiol and luteinizing hormone secretion, and estrus included the subsequent dark phase when female rats ovulate and display proceptive behaviors [22]. Data collection did not commence until stable levels of food intake were observed and all rats had displayed a minimum of 2 consecutive 4-day estrous cycles. Rats were tested during diestrus 2, a time following low estradiol secretion, and during estrus, a time following high estradiol secretion [22]. For two estrous cycles, food and water were removed from the rats’ cages 1 h prior to dark onset on test days (either diestrus 2 or estrus). Using a within-subjects, crossover design, rats received i.c.v. infusions of either 0 or 5 µg MCH dissolved in 2.5 µl saline over a period of 1 min. These infusions were administered 30 min prior to dark onset. At dark onset (1300 h), food and water were returned to the rats’ cages and food intake was monitored hourly for the first 2 h of the dark phase.
2.6. Data analyses
Data, presented as means ± SE throughout, were analyzed with the BMDP (SOLO V. 6.0; SPSS; Chicago, IL) statistical package. To determine the time course over which MCH influenced ingestive behavior in male and female rats in Experiment 1, the effects of MCH on food and water intake during the first 4 h of the dark phase were analyzed using two-factor, mixed-design ANOVAs, with group (OVX-oil, OVX-EB, or Male-oil) as the between-subjects variable and drug dose (0, 1, and 5 µg MCH) as the within-subjects variable. This interval was based on a review of the current literature suggesting that an acute infusion of MCH can increase food intake for up to 4 h in male rats [2]. These initial analyses revealed that the duration of MCH’s orexigenic effect was longer in male rats, relative to female rats (4 h vs 2 h, respectively). Thus, the maximal increase in food/water intake following MCH treatment, relative to that consumed following saline vehicle treatment was determined for 4 h in male rats and for 2 h in female rats. This and subsequent analyses were limited to the highest dose of MCH because the lower dose of MCH failed to influence food intake in EB-treated OVX rats. The resulting data were analyzed for group differences (OVX-oil, OVX-EB, or Male-oil) using one-way ANOVAs. To determine whether MCH stimulated water intake, independent of food intake, the ratio of water intake to food intake was calculated in vehicle and MCH-treated rats during the intervals (either 2 h or 4 h) corresponding to the maximal orexigenic/dipsogenic effect of MCH. Meal patterns were assessed by examining the size of the first meal following drug treatment, as well as the number and average meal size throughout the duration of MCH’s orexigenic effect. Finally, running wheel activity during the first 4 h following drug treatment was examined. The effects of MCH on the ratio of water intake to food intake, meal patterns, and running wheel activity were analyzed using mixed-design ANOVAs with group (OVX-oil, OVX-EB, or Male- oil) as the between-subjects variable and drug (0 and 5 µg MCH) as the within-subjects variable. In Experiment 2, MCH increased food intake for 2 h. An examination of non-cumulative, hourly intake revealed that the hyperphagia occurred primarily during the first h following MCH treatment. Thus, the effect of MCH on 1 h food intake in cycling rats was analyzed using a 2-factor, repeated-measures ANOVA with cycle stage (D2 and E) and drug (0 and 5 µg MCH) as the within-subjects variables. Newman Keuls post-hoc tests were used to investigate differences between groups following significant ANOVA effects (p < 0.05).
3. Results
3.1. Experiment 1
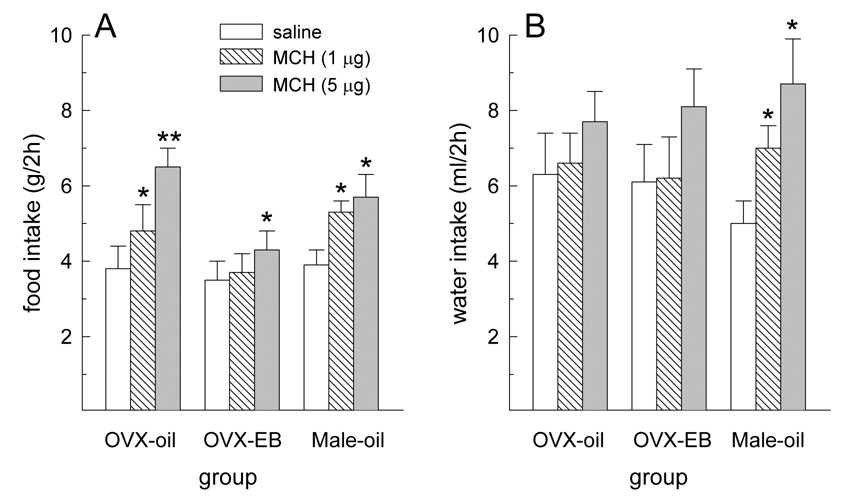
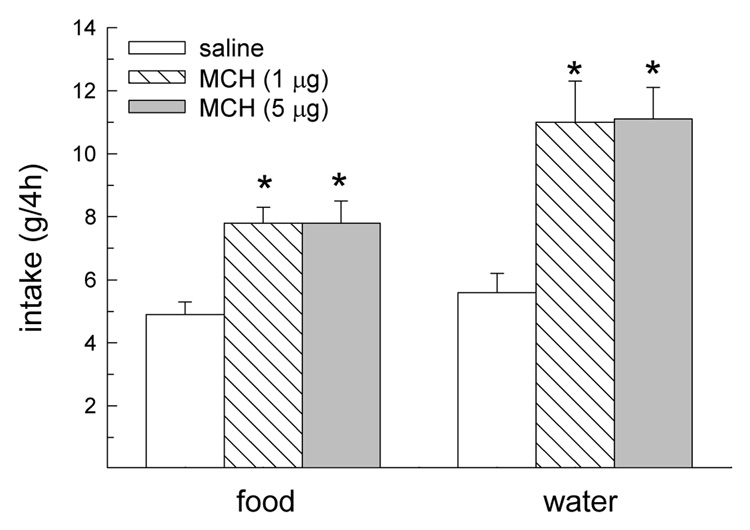
During the first 2 h following drug treatment, MCH influenced dark-phase food and water intakes, F(2,40) = 21.37 and 9.62, respectively, Ps < 0.05 (Fig. 1A,B). At this time, both doses of MCH increased food intake in oil-treated OVX and male rats, Ps < 0.05 (Fig. 1A). In contrast, only the largest dose of MCH increased food intake in EB-treated OVX rats, P < 0.05. During this same 2-h interval, both doses of MCH increased water intake in male rats, Ps < 0.05 (Fig. 1B). Although there was a tendency for the higher dose of MCH to increase water intake in female rats, this failed to reach statistical significance. During the second 2 h interval following drug treatment, MCH continued to influence dark-phase food and water intake in male rats. Because a similar effect was not observed in female rats, analysis of 4-h food intake was limited to male rats. During the 4 h following drug treatment, MCH influenced dark-phase food and water intakes, F(2,6) = 7.45 and 10.69, respectively, Ps < 0.01 (Fig. 2). Both doses of MCH increased 4-h food and water intake in male rats, Ps < 0.01.
Fig. 1.
Effect of MCH on food and water intake during the first 2 h following drug treatment. (A) Both doses of MCH increased food intake in oil-treated OVX and male rats. Only the larger dose of MCH increased food intake in EB-treated OVX rats. (B) Both doses of MCH increased water intake in male rats. A similar effect was not observed in female rats. *Greater than saline treatment, P < 0.05. **Greater than 1 µg MCH treatment, P < 0.05.
Fig. 2.
Effect of MCH on food and water intake during the first 4 h following drug treatment in male rats. Both doses of MCH increased food and water intake for 4 h in male rats. *Greater than saline treatment, P < 0.01.
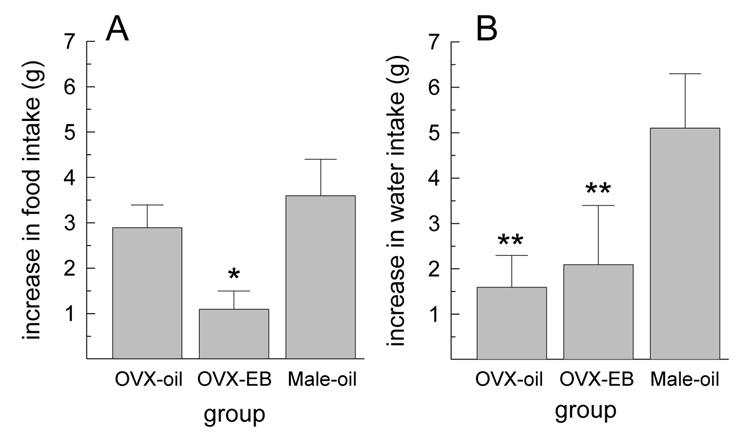
The increase in food and water intakes following treatment with 5 µg MCH, relative to that consumed following saline vehicle, was influenced by group, F(2,20) = 4.72 and 3.96, respectively, Ps < 0.05 (Fig. 3). The orexigenic effect of MCH was attenuated in EB-treated OVX rats, relative to oil-treated OVX and male rats, Ps < 0.05 (Fig. 3A). The dipsogenic effect of MCH was attenuated in oil- and EB-treated OVX rats, relative to male rats, Ps < 0.05 (Fig. 3B).
Fig. 3.
Increase in food and water intake following the larger (5 µg) dose of MCH, relative to that consumed following saline treatment. (A) The orexigenic effect of MCH was attenuated in EB-treated OVX rats, relative to oil-treated OVX and male rats. (B) The dipsogenic effect of MCH was attenuated in female rats, relative to male rats. *Less than OVX-oil and Male-oil groups, P < 0.05. **Less than Male-oil group, P < 0.05.
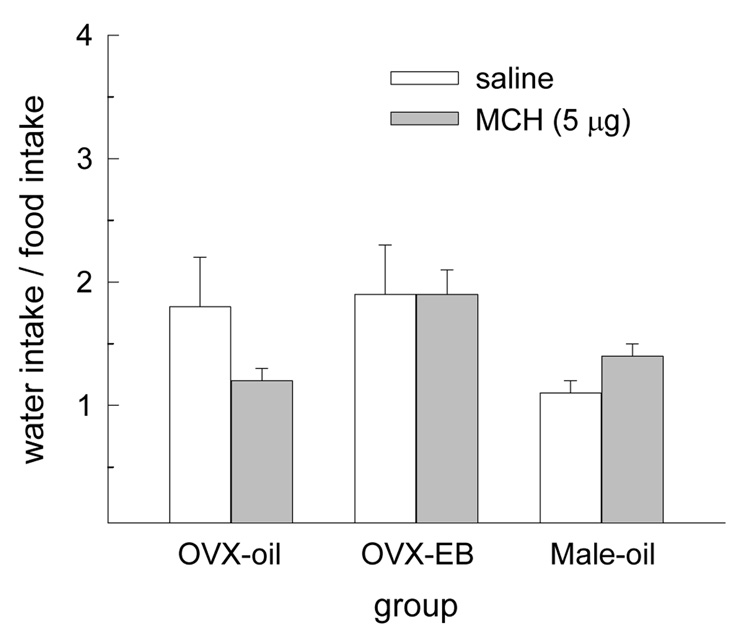
To determine whether MCH stimulated water intake, independent of food intake, the ratio of dark-phase water intake to food intake was calculated following infusion of saline vehicle and 5 µg MCH in male and female rats. The ratio of water intake to food intake was not influenced by either a main effect of drug treatment or group or by an interactive effect of drug treatment and group (Fig. 4).
Fig. 4.
MCH did not alter the ratio of water intake to food intake in any group.
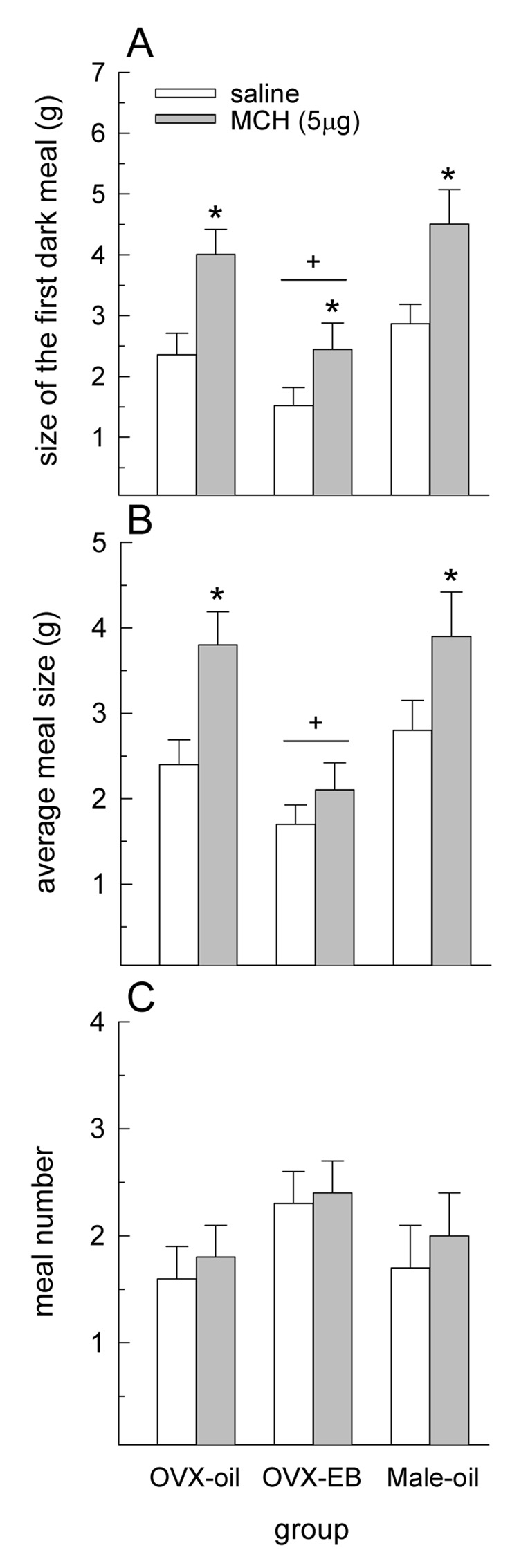
Meal pattern analyses revealed that the size of the first dark meal was influenced by main effects of drug treatment and group, F(2,20) = 26.54 and 7.19, respectively Ps < 0.001 (Fig. 5A). MCH increased the size of the first meal in each group, P < 0.05. Regardless of drug treatment, the size of the first meal was smaller in EB-treated OVX rats, relative to oil-treated OVX and male rats, Ps < 0.05. Average meal size throughout the duration of MCH’s orexigenic effect was also influenced by drug treatment, F(2,20) = 15.23, P < 0.0005 (Fig. 5B). MCH increased average meal size in oil-treated OVX and male rats, Ps < 0.05. A similar response was observed in EB-treated OVX rats, but this failed to reach statistical significance. The average meal size throughout the duration of MCH’s orexigenic effect was influenced by a main effect of group, F(2,20) = 7.22, P < 0.005 (Fig. 5B). Regardless of drug treatment, average meal size was lower in EB-treated OVX rats, relative to oil-treated OVX and male rats (Ps < 0.05). Meal number was not influenced by either a main effect of drug treatment or group or by an interactive effect of drug treatment and group (Fig. 5C).
Fig. 5.
The effect of MCH on meal patterns. (A) MCH increased the size of the first meal consumed following drug treatment in all groups. Regardless of drug treatment, meal size was lower in EB-treated OVX rats, relative to oil-treated OVX and male rats. (B) Throughout the duration of MCH’s orexigenic effect, an increase in average meal size was observed in oil-treated OVX and male rats. A similar effect was not observed in EB-treated OVX rats. Regardless of drug treatment, average meal size was lower in EB-treated OVX rats, relative to oil-treated OVX and male rats. (C) MCH did not influence meal number in any group. *Greater than saline treatment, P < 0.01. +OVX-EB group less than OVX-oil and Male-oil groups, P < 0.05.
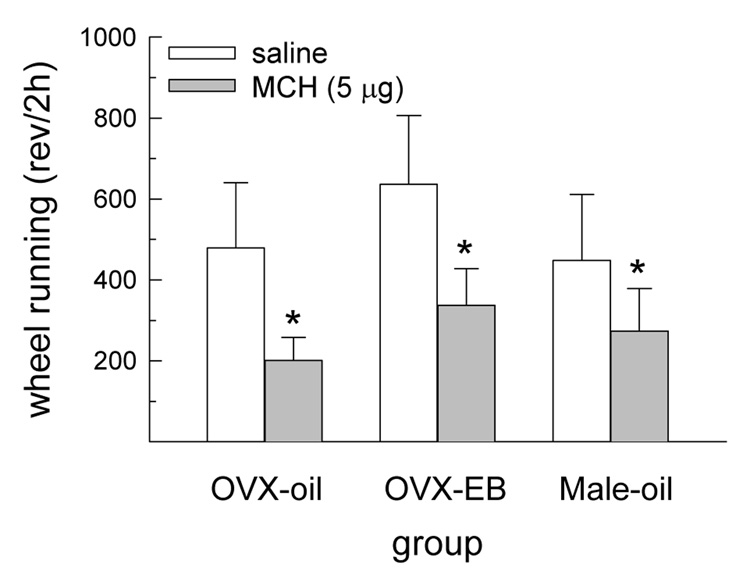
During the first 2 h following drug treatment, running wheel activity was influenced by a main effect of drug treatment, F(1,20) = 6.56, P < 0.05 (Fig. 6). At this time, the largest dose of MCH decreased wheel running in all groups, P < 0.05. Wheel running was not influenced by either a main effect of group or an interactive effect of drug treatment and group during this first 2-h interval. During the subsequent 2-h interval, wheel running was not influenced by either a main effect of drug treatment or group or by an interactive effect of drug treatment and group.
Fig. 6.
MCH decreased wheel running during the first 2 h following drug treatment. This effect was similar in all groups. *Less than saline treatment, P < 0.05.
3.2. Experiment 2
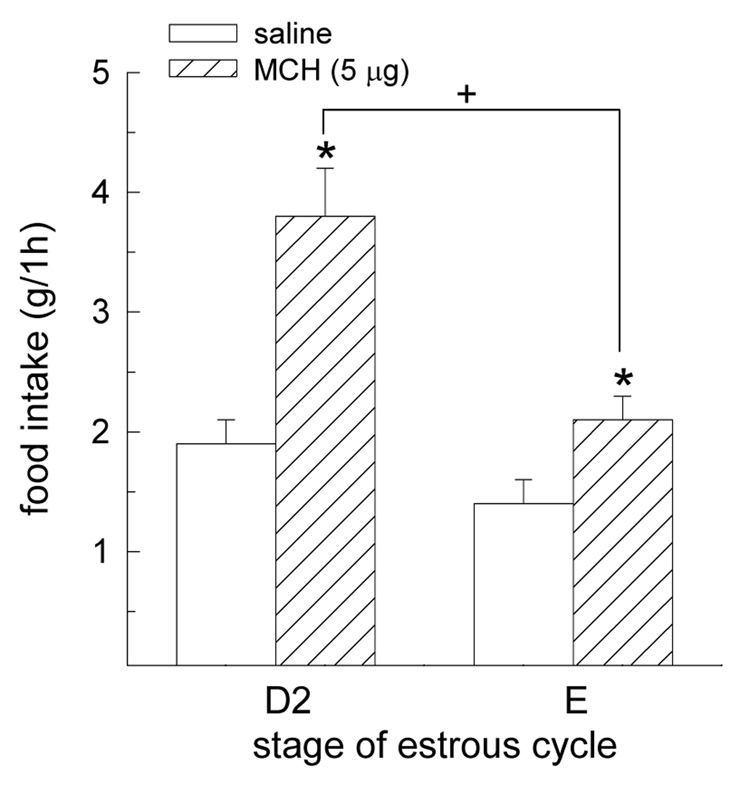
In intact, female rats, the orexigenic effect of MCH was influenced by an interactive effect of cycle stage and drug treatment, F(1,6) = 6.87, P < 0.05 (Fig 7). While MCH increased food intake during both stages of the estrous cycle, P < 0.05, the magnitude of MCH’s orexigenic effect was attenuated in estrous rats, relative to diestrous rats, P < 0.05.
Fig. 7.
The orexigenic effect of MCH is influenced by stage of the estrous cycle. MCH increased food intake in diestrous (D2) and estrous (E) rats. However, the magnitude of this effect was decreased in E rats, relative to D2 rats. *Greater than saline treatment, P < 0.05. +E/MCH rats less than D2/MCH rats, P < 0.05.
4. Discussion
A major goal of the present study was to determine whether the behavioral effects of MCH are sexually dimorphic. In support of this hypothesis, the increase in food intake, meal size, and water intake following MCH treatment was attenuated in female rats (i.e., EB-treated OVX rats), relative to male rats. Interestingly, MCH induced a short-term decrease in wheel running that, unlike its effect on ingestive behavior, was similar in male and female rats. A secondary goal of the present study was to determine whether stage of the estrous cycle influences the orexigenic effect of MCH in intake female rats. Consistent with this hypothesis, the orexigenic effect of MCH was attenuated in estrous rats, relative to diestrous rats.
In Experiment 1, both doses of MCH increased food intake for 4 h in male rats. This is consistent with previous studies in which similar doses of MCH induced short-term (up to 4 h) increases in food intake in male rats [4, 6, 23, 24]. In contrast, MCH increased food intake for only 2 h in female rats. Moreover, EB treatment abolished the orexigenic effect of the lower dose of MCH (Fig. 1A), and it attenuated the orexigenic effect of the higher dose of MCH (Fig. 3). These findings extend an earlier study [18] by providing the first demonstration that EB treatment induces a right-ward shift in the dose-response curve of the orexigenic effect of MCH. That is, a higher dose of MCH is necessary to induce feeding in EB-treated OVX rats, relative to oil-treated OVX rats. It is interesting that the magnitude of the orexigenic effect of MCH was similar in male and oil-treated OVX rats (Fig. 3). This suggests that an activational, rather than an organizational, effect of estradiol mediates the reduced sensitivity to the orexigenic effect of MCH in female rats. Experiment 2 provides the first demonstration that MCH-induced feeding is also influenced by the stage of the estrous cycle. That is, the orexigenic effect of MCH was attenuated in estrous rats, relative to diestrous rats. Thus, both exogenous and endogenous estradiol attenuates the orexigenic effect of MCH. Given the fact that circulating levels of estradiol are lower in males, relative to females, it is possible that such a sex difference may contribute to the male rat’s increased sensitivity to many of the behavioral effects of MCH. In order to test this hypothesis directly, the behavioral effects of MCH will need to be examined in male rats receiving physiological doses of estradiol.
There are multiple mechanisms by which estradiol, acting via nuclear estrogen receptors (ERs) capable of altering gene transcription, could decrease MCH signaling. For instance, the expression of ERs within the LH and ZI [25] suggests that estradiol could act locally in either brain region to decrease synthesis of MCH. In support of this hypothesis, physiological doses of estradiol decreased pre-pro MCH mRNA expression in the ZI of OVX rats [26] and the LH of obese male rats [27]. In addition, chronic estradiol treatment in male rats blocked the increase in lateral hypothalamic MCH mRNA expression induced by negative energy balance [27]. However, pharmacological doses of estradiol in male mice increased MCH mRNA within hypothalamic tissue punches [28]. These discrepant findings emphasize the need for additional research involving intact, cycling rats, which should reveal the role of endogenous estradiol in the regulation of MCH mRNA expression. It is also possible that estradiol, acting at nuclear ERs in brain regions that express MCH-1 receptors [29, 30], could decrease MCH signaling by decreasing the number and/or binding affinity of MCH-1 receptors. To test this hypothesis, additional studies are required to determine whether cells containing MCH-1 receptors also express ERs and, as a result, could function as targets of estradiol action.
In addition to its orexigenic effect, MCH has been reported to exert a dipsogenic effect in male rats [4, 10]. This action of MCH may be sexually dimorphic, since a similar dipsogenic effect was not observed in MCH-treated female rats [18]. To investigate this hypothesis, water and food intake were monitored in male and female rats in the present study. Although MCH increased food intake in both sexes, a concomitant increase in water intake was only observed in male rats. This finding is consistent with a previous study in which MCH increased dark-phase water intake, in the presence of food, in male rats [4]. While it is somewhat surprising that MCH increased food intake without producing a reliable increase in water intake in female rats, a similar finding was reported in male rats following acute administration of agouti-related protein [4]. Thus, under certain conditions, orexigenic peptides can stimulate feeding in the absence of a prandial-related increase in water intake.
In the present study, an increase in the ratio of water intake to food intake would provide evidence for a selective dipsogenic effect of MCH, independent of its orexigenic effect. In both sexes, MCH failed to produce a reliable increase in the ratio of water intake to food intake. While our findings are in agreement with a previous study in which two selective MCH1-R agonist and antagonist compounds influenced food intake without affecting water intake [8], they are not in agreement with other studies in which MCH stimulated water intake, independent of food intake in male rats [4, 10]. It appears unlikely that these discrepant findings reflect a sex difference since a reliable, selective dipsogenic effect of MCH was not observed in either male or female rats in the present study. Rather, it seems likely that these discrepant findings are the result of methodological differences between studies. For example, in those studies that support a dipsogenic effect, MCH was administered into the third ventricle [4, 10]. However, here, and in the other study that failed to detect a dipsogenic effect in male rats [8], MCH was administered into the lateral ventricle. Thus, a dipsogenic effect of MCH may only be apparent when MCH is infused into the third ventricle, which lies in closer proximity to brain areas implicated in the regulation of fluid balance. To test this hypothesis, additional studies involving site-specific administration of MCH are necessary.
An important first step in elucidating the mechanism by which any compound influences food intake is to determine whether it affects the controls of meal size and/or meal number [16]. Here, the size of the first meal following MCH treatment was increased in all groups. In male and oil-treated OVX rats, MCH continued to increase average dark meal size throughout the interval over which MCH increased food intake. A similar effect was not observed in EB-treated OVX rats. No differences in meal number were observed in any group. Thus, MCH appears to stimulate food intake by selectively affecting the controls of meal size. The present findings are consistent with a previous study in which the anorexigenic effect of a selective MCH-1 receptor antagonist was mediated by a decrease in meal size, not meal number [17]. The present findings are also somewhat, but not entirely, consistent with a more recent study by Scheurink and colleagues [10], who reported an increase in meal duration in rats treated with MCH, relative to rats treated concurrently with MCH and a selective MCH-1 receptor antagonist. However, in this same paper, MCH-treated rats did not display a reliable increase in meal size despite the fact that meal size is typically highly correlated with meal duration. Rather, MCH-treated rats displayed a reliable increase in meal number. Thus, while there is some agreement that MCH affects the controls of meal size/meal duration, additional studies are necessary to reconcile the discrepant findings regarding meal number.
According to Smith [31], meal size is directly controlled by food stimuli acting on preabsorptive receptors lining the alimentary canal. While orosensory stimulation during a meal elicits positive feedback, which sustains the meal, gastrointestinal stimulation elicits negative feedback, which terminates the meal [31]. The size of a given meal is determined by the relative potencies of these peripheral feedback signals, collectively known as the direct controls of meal size. According to Smith’s theory, other factors that modulate the potency of direct controls, like MCH-1 receptor stimulation, are termed indirect controls [31]. MCH’s ability to increase meal size, observed here and in a previous study [17], must be the result of an increase in positive feedback associated with the stimulation of orosensory receptors and/or a decrease in negative feedback associated with the stimulation of gastrointestinal receptors.
Mice with targeted deletion of the genes encoding either pre-pro MCH or the MCH-1 receptor display increased home-cage locomotor activity [15, 32–35] and running wheel activity [14]. This suggests that endogenous MCH exerts an inhibitory effect on locomotor energy expenditure. Here, we found that MCH decreased wheel running during the first 2 h following drug treatment in both sexes. It appears unlikely that this action of MCH is simply related to a generalized sedative effect since MCH failed to decrease the number of meals consumed by either sex during the same interval. Rather, this hypoactive effect of MCH appears to be consistent with its putative role in promoting positive energy balance. Our findings that acute i.c.v. infusion of MCH decreased running wheel activity are not consistent with previous studies in which chronic i.c.v. infusions of either MCH or an MCH-1 receptor antagonist had no effect on daily home-cage activity [12, 17, 36–38]. It should be noted, however, that in three of these studies, chronic MCH treatment failed to increase food intake [12, 36, 37]. Clearly, additional studies are required to better understand the impact of acute versus chronic administration of MCH on food intake and locomotor energy expenditure. Finally, unlike its effect on food intake, MCH’s ability to influence locomotor activity does not appear to be sexually dimorphic.
In summary, the present findings provide the first evidence that the orexigenic effect of MCH is both sexually dimorphic and suppressed by exogenous and endogenous estradiol. Thus, MCH may be added to the growing list of orexigenic (e.g., ghrelin) and anorexigenic (e.g., cholecystokinin, glucagon, serotonin, leptin) compounds that are modulated by estradiol [39–44]. An important goal for future research is to determine the relative contribution of each of these compounds to the anorexigenic effect of estradiol. With respect to MCH, Tritos and colleagues [28] reported that the anorexigenic effect of estradiol persists in MCH-deficient male mice. It should be noted, however, that in this study the anorexigenic effect of estradiol was only observed on the first day of a 7-day estradiol replacement paradigm. Thus, MCH-deficient male mice appear to maintain short-term, but not long-term, sensitivity to the anorexigenic effect of chronic estradiol treatment. Additional studies, particularly those involving female rodents, are necessary to determine the extent to which the anorexigenic effect of estradiol can be expressed independent of MCH signaling. A second key finding in the present study was that the orexigenic effect of MCH is mediated by a selective increase in meal size and that this action of MCH is attenuated by estradiol. Finally, not all of the behavioral responses to MCH were sexually dimorphic. Similar decreases in wheel running were observed in males and females following acute administration of MCH, and MCH failed to alter the ratio of water intake to food intake in either sex. We conclude that estradiol decreases the orexigenic effect of MCH and that this, in turn, may contribute to the mechanism underlying the potent, anorexigenic effect of estradiol in the female rat.
Acknowledgments
GRANTS
This work was supported by a grant from the NIH (MH-63932) and an NIH Joint Neuroscience Predoctoral Training Grant (NIH, NIDCR, NIGMS, NIMH, NINDS, NINR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Skofitsch G, Jacobowitz DM, Zamir N. Immunohistochemical localization of a melanin-concentrating-like hormone in the rat brain. Brain Res Bull. 1985;15:635–649. doi: 10.1016/0361-9230(85)90213-8. [DOI] [PubMed] [Google Scholar]
- 2.Pissios P, Bradley RL, Maratos-Flier E. Expanding the scales: The multiple role of MCH in regulating energy balance and other biological functions. Endocr Rev. 2006;27:606–620. doi: 10.1210/er.2006-0021. [DOI] [PubMed] [Google Scholar]
- 3.Westerfield DB, Pang PK, Burns JM. Some characteristics of melanophore-concentrating horomone (MCH) from teleost pituitary glands. Gen Comp Endocrinol. 1980;42:494–499. doi: 10.1016/0016-6480(80)90215-4. [DOI] [PubMed] [Google Scholar]
- 4.Clegg DJ, Air EL, Benoit SC, Sakai RS, Seeley RJ, Woods SC. Intraventricular melanin-concentrating hormone stimulates water intake independent of food intake. Am J Physiol Regul Integr Comp Physiol. 2003;284:R494–R499. doi: 10.1152/ajpregu.00399.2002. [DOI] [PubMed] [Google Scholar]
- 5.Abbott CR, Kennedy AR, Wren AM, Rossi M, Murphy KG, Seal LJ, Todd JF, Ghatei MA, Small CJ, Bloom SR. Identification of hypothalamic nuclei involved in the orexigenic effect of melanin-concentrating hormone. Endocrinology. 2003;144:3943–3949. doi: 10.1210/en.2003-0149. [DOI] [PubMed] [Google Scholar]
- 6.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 7.Della-Zuana O, Presse F, Ortola C, Duhault J, Nahon JL, Levens N. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague-Dawley rats. Int J Obes Relat Metab Disord. 2002;26:1289–1295. doi: 10.1038/sj.ijo.0802079. [DOI] [PubMed] [Google Scholar]
- 8.Shearman LP, Camacho RE, Sloan-Stribling D, Zhou D, Bednarek MA, Hreniuk DL, Feighner SD, Tan CP, Howard AD, Van der Ploeg LHT, MacIntyre DE, Hickey GJ, Strack AM. Chronic MCH-1 receptor modulation alters appetite, body weight and adiposity in rats. Eur J Pharmacol. 2003;475:37–47. doi: 10.1016/s0014-2999(03)02146-0. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morens C, Norregaard P, Receveur J, van Dijk G, Scheurink AJW. Effects of MCH and MCH1-receptor antagonist on (palatable) food and water intake. Brain Res. 2005;1062:32–38. doi: 10.1016/j.brainres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Gomori A, Ishihara A, Oda Z, Mashiko S, Matsushita H, Yumoto M, Ito M, Sano H, Tokiat S, Moriya M, Iwaasa H, Kanatani A. Characterization of MCH-mediated obesity in mice. Am J Physiol Endocrinol Metab. 2003;284:E940–E945. doi: 10.1152/ajpendo.00529.2002. [DOI] [PubMed] [Google Scholar]
- 12.Zheng H, Patterson LM, Morrison C, Banfield BW, Randall JA, Browning KN, Travagli RA, Berthoud HR. Melanin concentrating hormone innervation of caudal brainstem areas involved in gastrointestinal functions and energy balance. Neurosci. 2005;135:611–625. doi: 10.1016/j.neuroscience.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 13.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 14.Zhou D, Shen Z, Strack AM, Marsh DJ, Shearman LP. Enhanced running wheel activity of both Mch1r- and Pmch-deficient mice. Regul Pept. 2005;124:53–63. doi: 10.1016/j.regpep.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, Guan X-G, Jiang MM, Feng Y, Camacho RE, Shen Z, Frazier EG, Yu H, Metzger JM, Kuca SJ, Shearman LP, Gopal-Truter S, MacNeil DJ, Strack AM, Maclntyre DE, Van der Ploeg LHT, Qian S. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci USA. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–820. doi: 10.1016/s0899-9007(00)00457-3. [DOI] [PubMed] [Google Scholar]
- 17.Kowalski TJ, Farley C, Cohen-Williams ME, Varty G, Spar BD. Melanin-concentrating hormone-1 receptor antagonism decreases feeding by reducing meal size. Eur J Pharmacol. 2004;497:41–47. doi: 10.1016/j.ejphar.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Messina MM, Boersma G, Overton JM, Eckel LA. Estradiol decreases the orexigenic effect of melanin-concentrating hormone in ovariectomized rats. Physiol Behav. 2006;88:523–528. doi: 10.1016/j.physbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Fourth edition ed. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 20.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- 21.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 22.Becker JB, Arnold AP, Berkley KB, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 23.Rossi M, Choi SJ, O'Shea D, Miyoshi T, Ghatei MA, Bloom SR. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138:351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- 24.Tritos NA, Vicent D, Gillette J, Ludwig DS, Flier ES, Maratos-Flier E. Functional interactions between melanin-concentrating hormone, neuropeptide Y, and anorectic neuropeptides in the rat hypothalamus. Diabetes. 1998;47:1687–1692. doi: 10.2337/diabetes.47.11.1687. [DOI] [PubMed] [Google Scholar]
- 25.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Murray JF, Baker BI, Levy A, Wilson CA. The influence of gonadal steroids on pre-pro melanin-concentrating hormone mRNA in female rats. J Neuroendocrinol. 2000;12:53–59. doi: 10.1046/j.1365-2826.2000.00425.x. [DOI] [PubMed] [Google Scholar]
- 27.Morton GJ, Mystkowski P, Matsumoto AM, Schwartz MW. Increased hypothalamic melanin concentrating hormone gene expression during energy restriction involves a melanocortin-independent, estrogen sensitive mechanism. Peptides. 2004;25:667–674. doi: 10.1016/j.peptides.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Tritos NA, Segal-Lieberman G, Vezeridis PS, Maratos-Flier E. Estradiol-induced anorexia is independent of leptin and melanin-concentrating hormone. Obesity Res. 2004;12:716–724. doi: 10.1038/oby.2004.84. [DOI] [PubMed] [Google Scholar]
- 29.Osterlund M, Kuiper GGJM, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Molecular Brain Res. 1998;54:175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- 30.Hervieu GJ, Cluderay JE, Harrison D, Meakin J, Maycox P, Nasir S, Leslie RA. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. Eur J Neurosci. 2000;12:1194–1216. doi: 10.1046/j.1460-9568.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev. 1996;20:41–46. doi: 10.1016/0149-7634(95)00038-g. [DOI] [PubMed] [Google Scholar]
- 32.Kokkotou E, Jeon JY, Wang X, Marino FE, Carlson M, Trombly DJ, Maratos-Flier E. Mice with MCH ablation resist diet-induced obesity through strain-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;289:R117–R124. doi: 10.1152/ajpregu.00861.2004. [DOI] [PubMed] [Google Scholar]
- 33.Segal-Lieberman G, Bradley RL, Kokkotou E, Carlson M, Trombly DJ, Wang X, Battaglia G, Myers MG, Flier JS, Maratos-Flier E. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc Natl Acad Sci USA. 2003;100:10085–10090. doi: 10.1073/pnas.1633636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Astrand A, Bohlooly YM, Larsdotter S, Mahlapuu M, Anderson H, Tornell J, Ohlsson C, Snaith M, Morgan DGA. Mice lacking melanin-concentrating hormone receptor 1 demonstrate increased heart rate associated with altered autonomic activity. Am J Physiol Regulatory Integrative Comp Physiol. 2004;287:R749–R758. doi: 10.1152/ajpregu.00134.2004. [DOI] [PubMed] [Google Scholar]
- 35.Jeon JY, Bradley RL, Kokkotou EG, Marino FE, Wang X, Pissios P, Maratos-Flier E. MCH−/− Mice are resistant to aging-associated increases in body weight and insulin resistance. Diabetes. 2006;55:428–434. doi: 10.2337/diabetes.55.02.06.db05-0203. [DOI] [PubMed] [Google Scholar]
- 36.Messina MM, Overton JM. Cardiovascular effects of melanin-concentrating hormone. Regul Pept. 2006;139:23–30. doi: 10.1016/j.regpep.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Gomori A, Ishihara A, Ito M, Mashiko S, Matsushita H, Yumoto M, Ito M, Tanaka T, Tokita S, Moriya M, Iwaasa H, Kanatani A. Chronic intracerebroventricular infusion of MCH causes obesity in mice. Am J Physiol Endocrinol Metab. 2003;284:E583–E588. doi: 10.1152/ajpendo.00350.2002. [DOI] [PubMed] [Google Scholar]
- 38.Mashiko S, Ishihara A, Gomori A, Moriya M, Ito M, Iwaasa H, Matsuda M, Feng Y, Shen Z, Marsh DJ, Bednarek MA, MacNeil DJ, Kanatani A. Antiobesity effect of a melanin-concentrating hormone 1 receptor antagonist in diet-induced obese mice. Endocrinology. 2005;146:3080–3086. doi: 10.1210/en.2004-1150. [DOI] [PubMed] [Google Scholar]
- 39.Clegg DJ, Brown LM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decreases in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 40.Eckel LA, Geary N. Endogenous cholecystokinin's satiating action increases during estrus in female rats. Peptides. 1999;20:451–456. doi: 10.1016/s0196-9781(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 41.Eckel LA, Rivera HM, Atchley DPD. The anorectic effect of fenfluramine is influenced by sex and stage of the estrous cycle in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1486–R1491. doi: 10.1152/ajpregu.00779.2004. [DOI] [PubMed] [Google Scholar]
- 42.Geary N, Asarian L. Estradiol increases glucagon's satiating potency in ovariectomized rats. Am J Physiol Regulatory Integrative Comp Physiol. 2001;281:R1290–R1294. doi: 10.1152/ajpregu.2001.281.4.R1290. [DOI] [PubMed] [Google Scholar]
- 43.Butera PC, Bradway DM, Cataldo NJ. Modulation of the satiety effect of cholecystokinin by estradiol. Physiol Behav. 1993;53:1235–1238. doi: 10.1016/0031-9384(93)90387-u. [DOI] [PubMed] [Google Scholar]
- 44.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]