Abstract
Available data suggest that estradiol exerts an inhibitory effect on food intake by modulating the actions of multiple gut- and brain-derived peptides implicated in the control of food intake. For example, recent studies have shown that estradiol decreases the orexigenic effects of ghrelin and melanin-concentrating hormone. In the present study, we examined estradiol’s ability to decrease the actions of two additional orexigenic peptides, neuropeptide Y (NPY) and agouti-related protein (AgRP). Food intake was monitored following lateral ventricular infusions of 5 µg NPY, 10 µg AgRP, or saline vehicle in ovariectomized rats treated with either 1 µg estradiol or sesame oil vehicle. NPY increased food intake for 2 h in both oil-and estradiol-treated ovariectomized rats. During this interval, the orexigenic effect of NPY was significantly greater in oil-treated rats, relative to estradiol-treated rats. In contrast to the short-term action of NPY, a single injection of AgRP increased food intake for 3 days in oil- and estradiol-treated rats. Meal pattern analysis revealed that the orexigenic effect of AgRP is mediated by an increase in meal size, not meal number. Unlike that observed following NPY treatment, estradiol failed to modulate the magnitude by which AgRP increased food intake and meal size. We conclude that a physiological regimen of estradiol treatment decreases the orexigenic effect of NPY, but not AgRP, in ovariectomized rats.
Keywords: estrous cycle, food intake, meal size, NPY, AgRP
Introduction
It is well established that neuropeptide Y (NPY) plays an important role in the physiological control of food intake. The best evidence in support of this claim involves reports that acute, pharmacological antagonism of NPY Y1 and Y5 receptors inhibits basal and NPY-induced food intake in male rats [12, 21]. Other studies have also revealed that hypothalamic infusion of NPY stimulates a robust feeding response [8, 23, 36] and NPY gene expression is increased by periods of fasting [34]. Finally, NPY deficiency in obesity-prone mice attenuates the hyperphagia induced by fasting or exposure to a highly palatable diet [28]. Within the arcuate nucleus of the hypothalamus, many, but not all, NPY neurons are co-localized with another orexigenic peptide, agouti-related protein (AgRP) [18, 20]. AgRP, acting at hypothalamic MC3/4 receptors, is an endogenous antagonist of the melanocortin system [26, 32]. As such, acute ventricular administration of AgRP promotes hyperphagia that can persist for up to one week in male rats [19]. Consistent with AgRP’s role in stimulating food intake, mice deficient in AgRP are lean [40] and display decreased daily food intake, relative to wild-type litter mates [17].
The ovarian hormone estradiol exerts an inhibitory effect on food intake that is expressed in a variety of species. In rats, ovariectomy promotes hyperphagia and weight gain [39], both of which can be prevented by a physiological regimen of estradiol treatment alone [2]. In cycling rats, the pre-ovulatory increase in estradiol secretion decreases food intake throughout behavioral estrus [15]. This action of estradiol appears to be mediated by its ability to interact with multiple orexigenic and anorexigenic neuropeptides implicated in the control of meal size [13]. It is possible that NPY and AgRP may be added to this growing list based on previous studies in which estradiol was shown to decrease NPY/AgRP signaling. For example, estradiol decreases expression of immunoreactive NPY in the arcuate nucleus [11], and release of NPY in the paraventricular nucleus of the hypothalamus of ovariectomized rats [6]. In addition, the decline in estradiol secretion following ovariectomy is associated with increased hypothalamic NPY and AgRP mRNA expression [10, 35]. Taken together, these studies raise the possibility that the anorexigenic effect of estradiol may involve decreased NPY and/or AgRP signaling. To test this hypothesis we examined whether estradiol treatment decreases NPY- and/or AgRP-induced feeding in ovariectomized rats.
Methods
Animals and housing
Eleven female Long-Evans rats (Charles River Breeding Laboratory, Raleigh, NC), weighing 200–225 g at study onset, were housed individually in custom-designed cages. The cages were equipped with feeding niches that provided access to powdered chow (Purina 5001) in spill-resistant food cups mounted on weight-sensitive load beams. Infrared beams, located on either side of the feeding niches and centered above the feeding cups, were also used to detect bouts of feeding behavior. Any food spillage was collected on a platform surrounding the food cup. Water bottles were located ~ 20 cm from the feeding niches. Rats were given free access to chow and water, except as otherwise noted. Throughout the study, the testing room was maintained at 20 ± 2°C with a 12:12 h light-dark cycle (dark onset = 1700 h). Animal usage and all procedures were approved by the Florida State University Institutional Animal Care and Use Committee.
Behavioral measures
Outputs from the load beams and photo beams were fed via an interface into a computer located in an adjacent room. Custom-designed software (ESP 500; R. Henderson; Florida State University) recorded the weights of load beams (± 0.001 g) and the activity of photo beams at 30 s intervals. Additional software (Meal Weight Analysis; R. Henderson, Tallahassee FL) was used to assess food intake at particular intervals and to convert individual feeding bouts into discrete meals. A meal was defined as any feeding bout of at least 0.35 g that was separated from other feeding bouts by at least 15 min. In previous studies, these criteria accounted for 97–99 % of daily food intake (e.g., [15]).
Surgery
Rats were anesthetized with intraperitoneal injections of a mixture of ketamine (50 mg/ml; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (4.5 mg/ml; Rompun, Mobay, Shawnee, KS) and then bilaterally ovariectomized using an intra-abdominal approach. Immediately following ovariectomized, rats were implanted with stainless-steel guide cannulas (26 gauge, Plastics One, Roanoke, VA) aimed at the right lateral ventricle. Stereotaxic coordinates (AP: −0.6 mm relative to bregma; ML: −1.7 mm from the midline; DV: −3.5 mm from the surface of the skull) were based on the atlas of Paxinos and Watson [29]. Following surgery, each rat received an intraperitoneal injection of butorphanol (0.5 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) and a subcutaneous injection of gentamicin (10 mg/ml; Pro Labs Ltd, St. Joseph, MO) to minimize post-surgical pain and the risk of infection, respectively.
Following 7 days of postoperative recovery, a behavioral assay was used to verify each rat’s cannula placement. Light-phase water intake was monitored in individual rats following ventricular infusion of 50 ng of angiotensin II (Sigma-Aldrich, St. Louis, MO) delivered in 5 µl of saline vehicle over a period of 1 min. Only those rats that consumed at least 5 ml of water in 20 min were included in the study. Nine of the eleven rats passed this criterion (mean consumption = 10.1 ± 1.9 ml).
General procedure
Prior to data collection, rats were given 1 week to adapt to the custom cages. Following adaptation, the computerized system monitoring food intake was stopped for 1 h (from 0900 – 1000 h) during the light phase. At this time, the rats’ body weights were recorded, food cups and water bottles were refilled, and behavioral data were downloaded from the computer. For 12 consecutive weeks, ovariectomized rats received acute, subcutaneous injections of either 1 µg β-estradiol-3-benzoate (estradiol, Sigma, Boston, MA) delivered in 0.1 ml sesame oil vehicle or oil vehicle alone each Wednesday between 0930 and 0945 h. Hormone treatment was reversed weekly such that all rats received alternating estradiol/oil treatment throughout the duration of the study. This acute regimen of estradiol replacement was used because it mimics, from Monday to Thursday, the changes in estradiol secretion observed across the 4-day estrous cycle in ovarian-intact rats [2]. That is, plasma estradiol is low on Monday and Tuesday, peaks on Wednesday, and then falls rapidly to basal levels on Thursday, the day of this hormone treatment protocol that models behavioral estrus [4].
Effect of estradiol treatment on NPY-induced feeding
Feeding tests were conducted in oil- and estradiol-treated rats on 4 consecutive Thursdays following ventricular infusions of either 0 or 5 µg NPY (Peninsula Labs, San Carlos, CA), dissolved in 2.5 µl saline vehicle. The dose of NPY was chosen because it produces a reliable increase in light-phase food intake in rats [8, 37]. Prior to feeding tests, food and water were removed from the cages in order to prevent rats from consuming a meal during the 30 min interval preceding NPY/saline infusions. Our concern was that any food intake during this time could influence the feeding test. At 1130 h, rats received ventricular infusions of either saline or 5 µg NPY, delivered over a period of 1 min. At 1200 h, food and water were returned to the rats’ cages and the computerized system was used to monitor feeding behavior for 24 h following drug treatment.
Effect of estradiol on AgRP-induced feeding
Feeding tests were conducted in oil- and estradiol-treated rats over an 8 week period, with feeding tests occurring every other week on Thursday following ventricular infusions of either 0 or 10 µg AgRP (Phoenix Pharmaceuticals, Belmont, CA) dissolved in 5 µl saline vehicle. The feeding tests were conducted bi-weekly based on a report that AgRP can increase food intake for up to 7 days in male rats [19]. The dose of AgRP was chosen because it produces a reliable increase in 24-h food intake in male rats [9, 37]. Every other Thursday at 1130 h, rats received ventricular infusions of either saline or 10 µg AgRP, delivered over a 1-min period. The computerized system was used to monitor feeding behavior for 7 days following drug treatment.
Data analyses
Data are presented as means + SEM throughout. Two-factor, repeated measures ANOVAs (drug treatment × hormone treatment) were used to assess the orexigenic effect of NPY at 2, 4, 6, and 24 h following drug treatment. These analyses revealed that the orexigenic effect of NPY was limited to 2 h in oil- and estradiol-treated rats. Examination of meal pattern data during the first 2 h following drug treatment revealed that most rats consumed a single meal during this interval. Because the amount of total food consumed was virtually identical to the average meal size during this 2 h interval, we did not conduct a separate analysis of the effects of NPY on meal patterns.
A two-factor, repeated-measures ANOVA (hormone treatment × day) was used to assess the long-term effects of AgRP on daily food intake in oil- and estradiol-treated rats for 8 days (i.e., following saline treatment and the first 7 days following AgRP treatment). This analysis revealed that AgRP induced a similar increase in food intake in oil- and estradiol-treated rats that persisted for 3 days. Additional 2-factor, repeated-measures ANOVAs (drug treatment × hormone treatment) were used to assess the impact of estradiol treatment on AgRP’s ability to modulate food intake, average meal size and meal number on the first day following drug treatment. Meal pattern analysis was limited to 8 rats due to equipment problems that led to a loss of meal pattern data for 1 rat. Newman Keuls post-hoc tests were used to investigate differences between groups following significant ANOVA effects (P < 0.05).
Results
Effect of NPY on food intake in oil- and estradiol-treated rats
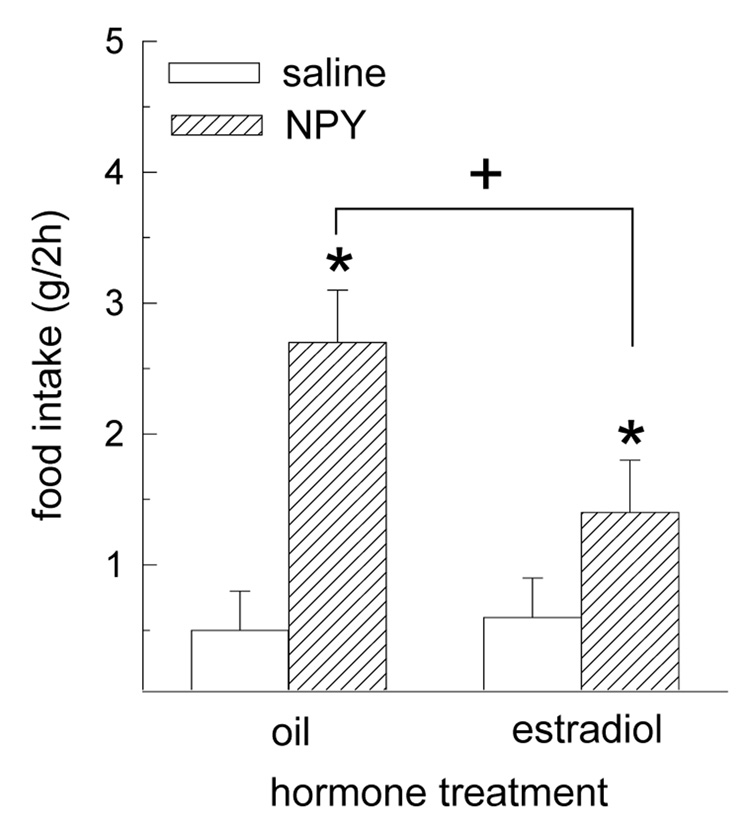
The orexigenic effect of NPY was limited to the first 2 h following drug treatment (i.e., during the mid-light phase from 1200 – 1400 h). The effect of NPY on food intake during this 2-h interval was differentially influenced by hormone treatment F(1,8) = 16.93, P < 0.01 (Fig. 1). While NPY increased 2-h food intake in both oil- and estradiol-treated rats (Ps < 0.05) at a time of day when food intake was minimal in both groups, the magnitude of NPY’s orexigenic effect was greater in oil-treated rats, relative to estradiol-treated rats, P < 0.05.
Fig. 1.
The orexigenic effect of NPY is decreased by estradiol treatment in OVX rats. Oil- and estradiol-treated rats received intracerebroventricular infusions of 5 µg NPY or saline vehicle during the mid-light phase. Analysis of food intake during the following 24 h interval revealed that the orexigenic effect of NPY was limited to the first 2 h following drug treatment. During this interval, the orexigenic effect of NPY was greater in oil-treated rats, relative to estradiol-treated rats. *Greater than saline-treated rats, P < 0.05. +Oil/NPY group greater than estradiol/NPY group, P < 0.05.
Effects of AgRP on food intake and meal patterns in oil- and estradiol-treated rats
Analysis of daily food intake following saline infusion and during the 7-days following AgRP infusion revealed a long-term, orexigenic effect of AgRP, F(7,56) = 5.65, P < 0.01. Post-hoc analyses revealed that AgRP increased food intake for 3 days in oil- and estradiol-treated rats, P < 0.05 (data not shown). Our analysis failed to reveal either a main effect of hormone treatment, F(7,56) = 0.9, P > 0.05, or an interactive effect of drug and hormone treatment, F(7,56) = 1.66, P > 0.05. Thus, the long-term, orexigenic effect of AgRP did not differ in oil- and estradiol-treated rats.
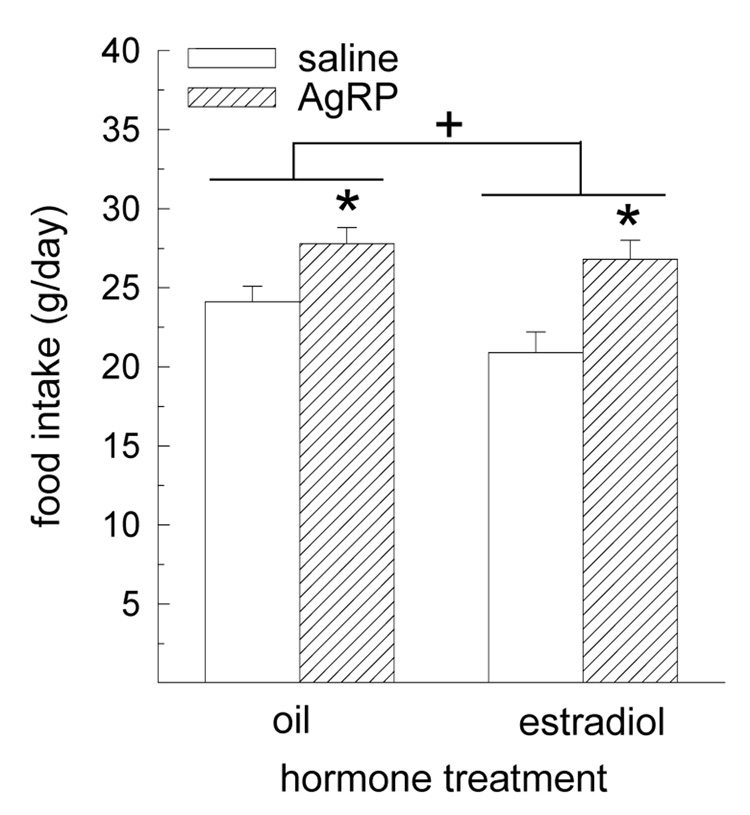
A robust, orexigenic effect of AgRP was detected during the first day following infusion, F(1,8) = 17.92, P < 0.01 (Fig. 2). While AgRP increased food intake in both oil- and estradiol-treated rats, P < 0.05, the magnitude of this effect did not differ between groups. Our analysis also revealed a main effect of hormone treatment, F(1,8) = 5.63, P < 0.05. Collapsing data across the drug treatment factor revealed that estradiol-treated rats consumed less food than oil-treated rats, (23.9 ± 1.1 g vs. 25.9 ± 0.8 g, respectively, P < 0.05). An interactive effect of drug and hormone treatment was not detected, F(1,8) = 1.01, P > 0.05.
Fig. 2.
The orexigenic effect of AgRP is not influenced by estradiol treatment in OVX rats. Oil-and estradiol-treated rats received intracerebroventricular infusions of 10 µg AgRP or saline vehicle during the mid-light phase. During the first day following drug treatment, AgRP produced a similar increase in food intake in oil- and estradiol-treated rats. A main effect of hormone treatment also revealed that food intake was reduced in estradiol-treated rats, relative to oil-treated rats. *Greater than saline-treated rats, P < 0.01. +Estradiol-treated rats less than oil-treated rats, P < 0.05.
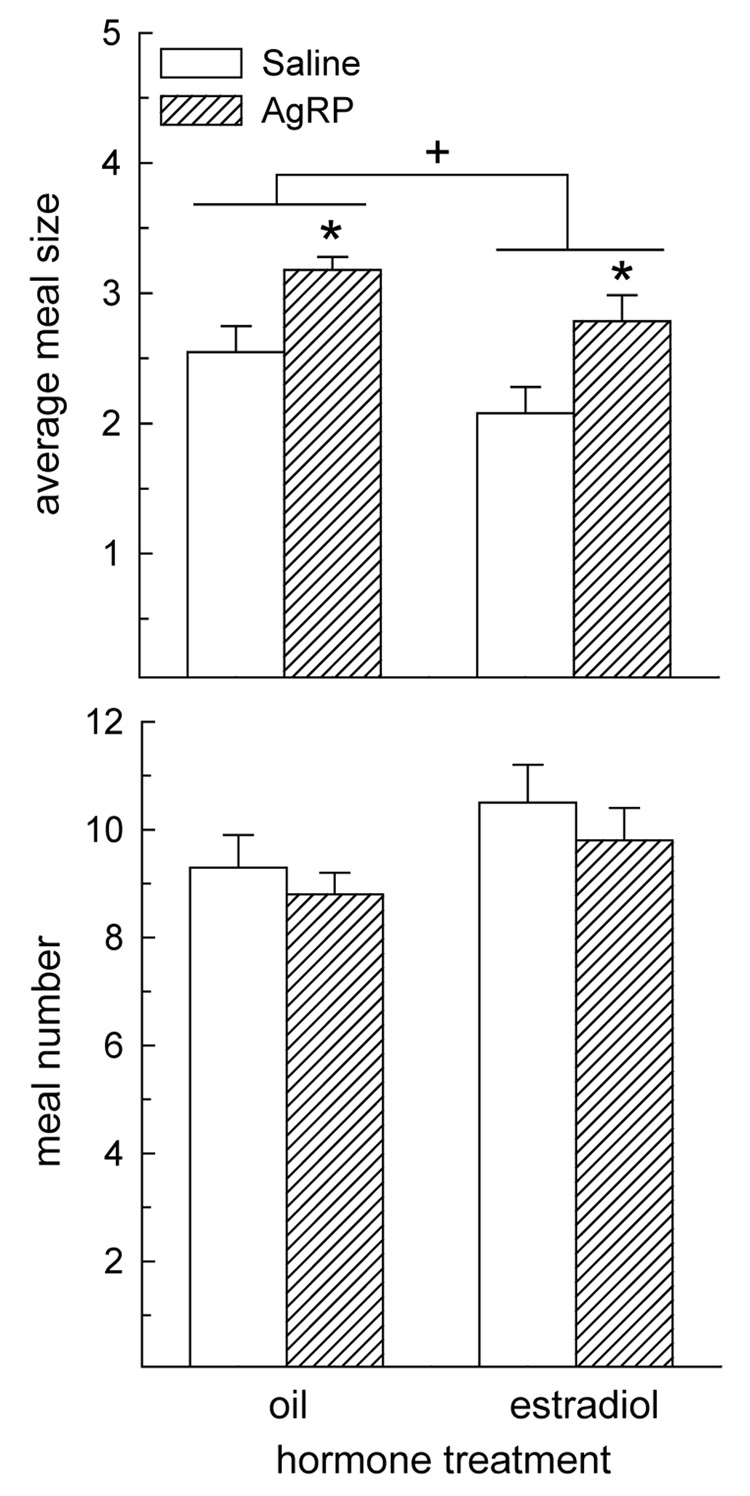
Meal pattern analysis revealed that AgRP influenced average meal size during the first day following drug treatment, F(1,7) = 9.43, P < 0.05 (Fig. 3A). AgRP increased average meal size, relative to that observed following saline treatment, in oil- and estradiol-treated rats, Ps < 0.05. Our analysis also revealed a main effect of hormone treatment, F(1,7) = 8.05, P < 0.05. Collapsing data across the drug treatment factor revealed that estradiol-treated rats consumed smaller meals than oil-treated rats, (2.4 ± 0.2 vs. 2.9 ± 0.1 g, respectively, P < 0.05). An interactive effect of drug and hormone treatment was not detected, F(1,7) = 0.03, P > 0.05, indicating that AgRP produced similar increases in meal size in oil- and estradiol-treated rats. Meal number analysis revealed neither main effects of hormone or drug treatment nor an interactive effect of hormone and drug treatment, F(1,7) = 0.04 – 1.70, Ps > 0.05 (Fig. 3B).
Fig. 3.
The orexigenic effect of AgRP is mediated by an increase in meal size, not meal number, in oil- and estradiol-treated OVX rats. (A) During the first day following drug treatment, AgRP induced a similar increase in average meal size in oil- and estradiol-treated rats. A main effect of hormone treatment also revealed that average meal size was reduced in estradiol-treated rats, relative to oil-treated rats. (B) AgRP failed to alter meal number in either oil- or estradiol-treated rats. *Greater than saline-treated rats, P < 0.05. +Estradiol-treated rats less than oil-treated rats, P < 0.05.
Discussion
Here, we tested the hypothesis that decreased sensitivity to the orexigenic effects of NPY and/or AgRP contributes to the anorexigenic effect of estradiol. As a first step in investigating this hypothesis, we examined whether a physiological dose of estradiol decreases the orexigenic effect of NPY and/or AgRP in the ovariectomized rat. While estradiol decreased the magnitude of NPY-induced feeding, it failed to modulate AgRP-induced feeding. This suggests that a decrease in NPY, but not AgRP, signaling contributes to the anorexigenic effect of estradiol.
Consistent with previous studies [8, 23, 36, 37], NPY elicited a short-term increase in food intake in oil- and estradiol-treated ovariectomized rats. Our findings extend these studies by providing the first demonstration that an acute, physiological dose of estradiol attenuates the magnitude of NPY-induced feeding in ovariectomized rats. Previous studies involving hypothalamic, site-specific infusions of NPY, which stimulated food intake for a longer duration than that observed here following ventricular infusions of NPY, suggest that NPY increases food intake by a selective increase in meal size, not meal number [22, 24]. In the present study, most rats consumed a single meal during the 2-h interval over which NPY increased food intake. As such, the 2-h feeding data presented in Fig. 1, which approximate meal size, suggest that estradiol inhibited the orexigenic effect of NPY by decreasing its ability to increase the size of meals. This is entirely consistent with previous reports that estradiol influences food intake by selectively modulating other neuropeptides involved in the direct control of meal size [5, 13, 15].
The regimen of estradiol treatment used in the present study was chosen because it mimics the fluctuation in endogenous estradiol secretion across the estrous cycle [2]. As such, our data suggest that the phasic inhibition of food intake during estrus involves decreased NPY signaling. In addition to this phasic (estrous-related) effect, estradiol exerts a tonic inhibition of food intake that is best revealed by the chronic hyperphagia observed in ovariectomized rats. Although not examined here, decreased NPY signaling may also contribute to the tonic inhibition of food intake by estradiol since a sustained increase in hypothalamic NPY gene expression is observed in hyperphagic, ovariectomized rats [1, 10].
Since estrogen receptors can function as ligand-inducible transcription factors capable of modulating target gene expression, and tritiated estradiol has been localized on NPY neurons in the arcuate nucleus [33], there are multiple ways by which estradiol may decrease NPY signaling. First, estradiol decreases the expression of NPY in the arcuate nucleus of ovariectomized rats [3, 11, 35]. Second, estradiol decreases NPY release in the paraventricular nucleus of ovariectomized rats [6]. Third, estradiol influences the number and binding affinity of NPY receptors implicated in regulating the release of gonadotropin releasing hormone and luteinizing hormone [27, 41]. It should be noted that all of these studies demonstrating estradiol’s ability to decrease NPY signaling involved chronic, pharmacological estradiol replacement paradigms in ovariectomized rats. Thus, it will be important to determine whether changes in endogenous estradiol are capable of altering NPY signaling in cycling, female rats.
In the present study, acute administration of AgRP increased food intake in oil- and estradiol-treated rats. Consistent with previous studies [19, 30, 37], this action of AgRP persisted for several days. An examination of spontaneous feeding patterns further revealed that the orexigenic effect of AgRP was mediated by an increase in meal size, not meal number. The current findings, together with a similar report in male rats [37], suggest that AgRP stimulates food intake by selectively affecting the controls of meal size. However, unlike that observed following NPY infusion, the orexigenic effect of AgRP was similar in oil- and estradiol-treated rats. Previously, Geary et al [30] reported that a similar regimen of estradiol replacement failed to modulate the orexigenic effect of AgRP in ovariectomized rats. Our findings extend this report by examining meal patterns to determine whether estradiol may selectively suppress AgRP’s ability to increase meal size, an effect that may not be revealed when examining daily food intake since it could be masked by a compensatory increase in meal number. We saw no evidence; however, that estradiol attenuates the increase in meal size following AgRP treatment. It is interesting that in our study and in the study by Geary et al [30], AgRP increased food intake for 3–4 days in female rats whereas in male rats, the orexigenic response following a smaller dose of AgRP than that used in these two studies persisted for 7 days [19]. This suggests that females may be less sensitive to the long-term, orexigenic effect of AgRP. Since there are data to suggest that the long-term orexigenic effect of AgRP is mediated by a mechanism other than competitive antagonism of MC3/4 receptors [19], it is possible that the higher circulating levels of estradiol in the female rat may influence this other mechanism. It will be interesting to examine potential sex differences in the long-term effects of AgRP, particularly since estradiol decreased AgRP gene expression in a hypothalamic cell line [38], and ovariectomy is associated with increased hypothalamic AgRP mRNA expression [10]. Taken together, these data suggest that estradiol does not modulate the initial response of AgRP to antagonize the melanocortin system. Rather, estradiol may mediate differences in long-term sensitivity to AgRP.
In summary, the current study provides the first evidence that a physiological regimen of estradiol treatment decreases the orexigenic effect of NPY in ovariectomized rats. This adds to the growing evidence that estradiol’s anorexigenic effect is mediated by its ability to modulate the strength of multiple anorexigenic and orexigenic compounds [7, 10, 14, 16, 25, 31]. It is interesting that estradiol failed to decrease the magnitude of AgRP-induced feeding since NPY and AgRP neurons are co-localized in the arcuate nucleus. Because these peptides exert their effects via independent receptors, this could suggest that estradiol acts postsynaptically, at NPY Y1 and/or Y5 receptors, to selectively decrease the orexigenic effect of NPY. Alternatively, because there are populations of NPY neurons in the arcuate nucleus and the nucleus of the solitary tract that do not express AgRP [18], one cannot rule out the possibility that estradiol acts presynaptically to decrease the orexigenic effect of NPY.
Acknowledgments
This work was supported by a grant from the NIH (DK-073936) and an NIH Joint Neuroscience Predoctoral Training Grant (NIH, NIDCR, NIGMS, NIMH, NINDS, NINR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thornburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25:1688. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- 2.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 3.Baskin DG, Norwood BJ, Schwartz MW, Koerker DJ. Estradiol inhibits the increase of hypothalamic neuropeptide Y messenger ribonucleic acid expression induced by weight loss in ovariectomized rats. Endocrinology. 1995;136:5547–5554. doi: 10.1210/endo.136.12.7588307. [DOI] [PubMed] [Google Scholar]
- 4.Becker JB, Arnold AP, Berkley KB, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 5.Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 6.Bonavera JJ, Dube MG, Kalra PS, Kalra SP. Anorectic effects of estrogen may be mediated by decreased neuropeptide Y release in the hypothalamic paraventricular nucleus. Endocrinology. 1994;134:2367–2370. doi: 10.1210/endo.134.6.8194462. [DOI] [PubMed] [Google Scholar]
- 7.Butera PC, Bradway DM, Cataldo NJ. Modulation of the satiety effect of cholecystokinin by estradiol. Physiol Behav. 1993;53:1235–1238. doi: 10.1016/0031-9384(93)90387-u. [DOI] [PubMed] [Google Scholar]
- 8.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 9.Clegg DJ, Air EL, Benoit SC, Sakai RS, Seeley RJ, Woods SC. Intraventricular melanin-concentrating hormone stimulates water intake independent of food intake. Am J Physiol Regul Integr Comp Physiol. 2003;284:R494–R499. doi: 10.1152/ajpregu.00399.2002. [DOI] [PubMed] [Google Scholar]
- 10.Clegg DJ, Brown LM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decreases in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 11.Crowley WR, Tessel RE, O'Donohue TL, Adler BA, Kalra SP. Effects of ovarian hormones on the concentrations of immunoreactive neuropeptide Y in discrete brain regions of the female rat: correlations with serum luteinizing hormone (LH) and median eminence LH-releasing hormone. Endocrinology. 1985;117:1151–1155. doi: 10.1210/endo-117-3-1151. [DOI] [PubMed] [Google Scholar]
- 12.Daniels AJ, Grizzle MK, Wiard RP, Matthews JE, Heyer D. Food intake inhibition and reduction in body weight gain in lean and obese rodents treated with GW438014A, a potent and selective NPY-Y5 receptor antagonist. Regul Pept. 2002;106:47–54. doi: 10.1016/s0167-0115(02)00034-4. [DOI] [PubMed] [Google Scholar]
- 13.Eckel LA. Estradiol: an indirect control of meal size. Physiol Behav. 2004;82:35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Eckel LA, Geary N. Endogenous cholecystokinin's satiating action increases during estrus in female rats. Peptides. 1999;20:451–456. doi: 10.1016/s0196-9781(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 15.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- 16.Geary N, Asarian L. Estradiol increases glucagon's satiating potency in ovariectomized rats. Am J Physiol (Regulatory, Integrative, & Comp Physiol) 2001;281:R1290–R1294. doi: 10.1152/ajpregu.2001.281.4.R1290. [DOI] [PubMed] [Google Scholar]
- 17.Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Bruning JC. Agouti-related peptide-expressing neurons are mandatory for feeding. Nature Neuroscience. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 18.Grove KL, Smith MS. Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav. 2003;79:47–63. doi: 10.1016/s0031-9384(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 19.Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van der Ploeg LHT, Woods SC, Seeley RJ. Long-term orexigenic effects of AgRP-(83---132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol. 2000;279:R47–R52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- 20.Hahn TM, Breninger JF, Baskin DG, Schwartz MW. Coexpression of AgRP and NPY in fasting-activated hypothalamic neurons. Nature Neuroscience. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 21.Kanatani A, Ishihara A, Asahi S, Tanaka T, Ozaki S, Ihara M. Potent neuropeptide Y Y1 receptor antagonist, 1229U91; blockade of neuropeptide Y-induced and physiological food intake. Endocrinology. 1996;137:3177–3182. doi: 10.1210/endo.137.8.8754736. [DOI] [PubMed] [Google Scholar]
- 22.Leibowitz SF, Alexander JT. Analysis of Neuropeptide Y-Induced Feeding: Dissociation of Y1 and Y2 Receptor Effects on Natural Meal Patterns. Peptides. 1991;12:1251–1260. doi: 10.1016/0196-9781(91)90203-2. [DOI] [PubMed] [Google Scholar]
- 23.Levine AS, Morley JE. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1030. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- 24.Marin-Bivens CL, Thomas WJ, Stanley BG. Similar feeding patterns are induced by perifornical neuropeptide Y injection and by food deprivation. Brain Res. 1998;782:271–280. doi: 10.1016/s0006-8993(97)01289-4. [DOI] [PubMed] [Google Scholar]
- 25.Messina MM, Boersma G, Overton JM, Eckel LA. Estradiol decreases the orexigenic effect of melanin-concentrating hormone in ovariectomized rats. Physiol Behav. 2006;88:523–528. doi: 10.1016/j.physbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Ollmann MM, Wilson BD, Yang Y-K, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 27.Parker SL, Carroll BL, Kalra SP, St-Pierre S, Fournier A, Crowley WR. Neuropeptide Y Y2 receptors in hypothalamic neuroendocrine areas are up-regulated by estradiol and decreased by progeterone cotreatment in the ovariectomized rat. Endocrinology. 1996;137:2896–2900. doi: 10.1210/endo.137.7.8770911. [DOI] [PubMed] [Google Scholar]
- 28.Patel HR, Hawkins EJ, Hileman SM, Elmquist JK, Imai Y, Ahima RS. Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesity-prone mice. Diabetes. 2006;55:3091–3098. doi: 10.2337/db05-0624. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 30.Polidori C, Geary N. Estradiol treatment fails to affect the feeding responses to melanocortin-3/4 receptor agonism or antagonism in ovariectomized rats. Peptides. 2002;23:1697–1700. doi: 10.1016/s0196-9781(02)00112-2. [DOI] [PubMed] [Google Scholar]
- 31.Rivera HM, Eckel LA. The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiol Behav. 2005;86:331–337. doi: 10.1016/j.physbeh.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Rossi M, Kim MS, Morgan DGA, Small CJ, Edwards CM, Sunter D, Abusnana S, Golstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff KA, Ghatei MA, Bloom SR. A C-terminal fragment of agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 33.Sar M, Sahu A, Crowley WR, Kalra SP. Localization of neuropeptide-Y immunoreactivity in estradiol-concentrating cells in the hypothalamus. Endocrinology. 1990;127:2752–2756. doi: 10.1210/endo-127-6-2752. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz MW, Sipols AJ, Grubin CE, Baskin DG. Differential effect of fasting on hypothalamic expression of genes encoding neuropeptide Y, galanin, and glutamic acid decarboxylase. Brain Res Bull. 1993;31:361–367. doi: 10.1016/0361-9230(93)90228-4. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu H, Ohtani K, Kato Y, Tanaka Y, Mori M. Withdrawl of [corrected] estrogen increases hypothalamic neuropeptide Y (NPY) mRNA expression in ovariectomized obese rat. Neurosci Lett. 1996;204:81–84. doi: 10.1016/0304-3940(96)12322-3. [DOI] [PubMed] [Google Scholar]
- 36.Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;33:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- 37.Tang-Christensen M, Vrang N, Ortmann S, Bidlingmaier M, Horvath TL, Tschop M. Central administration of ghrelin and agouti-related protein (83–132) increases food intake and decreases spontaneous locomotor activity in rats. Endocrinology. 2004;145:4645–4652. doi: 10.1210/en.2004-0529. [DOI] [PubMed] [Google Scholar]
- 38.Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) alpha to ERbeta in clonal hypothalamic neurons. Molecular Endocrinology. 2006;20:2080–2092. doi: 10.1210/me.2006-0027. [DOI] [PubMed] [Google Scholar]
- 39.Wade GN, Gray JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav. 1979;22:583–593. doi: 10.1016/0031-9384(79)90028-3. [DOI] [PubMed] [Google Scholar]
- 40.Wortley KE, Anderson KD, Yasenchak J, Murphy A, Valenzuela D, Diano S, Yancopoulos GD, Wiegand SJ, Sleeman MW. Agouti-related protein-deficient mice display an age-related lean phenotype. Cell Metab. 2005;2:421–427. doi: 10.1016/j.cmet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Xu M, Urban JH, Hill JW, Levine JE. Regulation of hypothalamic neuropeptide Y Y1 receptor gene expression during the estrous cycle: role of progesterone receptors. Endocrinology. 2000;141:3319–3327. doi: 10.1210/endo.141.9.7642. [DOI] [PubMed] [Google Scholar]