Abstract
Although backward folding of the epiglottis is one of the signal events of the mammalian adult swallow, the epiglottis does not fold during the infant swallow. How this functional change occurs is unknown, but we hypothesize that a change in swallow mechanism occurs with maturation, prior to weaning. Using videofluoroscopy, we found three characteristic patterns of swallowing movement at different ages in the pig: an infant swallow, a transitional swallow and a post-weaning (juvenile or adult) swallow. In animals of all ages, the dorsal region of the epiglottis and larynx was held in an intranarial position by a muscular sphincter formed by the palatopharyngeal arch. In the infant swallow, increasing pressure in the oropharynx forced a liquid bolus through the piriform recesses on either side of a relatively stationary epiglottis into the esophagus. As the infant matured, the palatopharyngeal arch and the soft palate elevated at the beginning of the swallow, so exposing a larger area of the epiglottis to bolus pressure. In transitional swallows, the epiglottis was tilted backward relatively slowly by a combination of bolus pressure and squeezing of the epiglottis by closure of the palatopharyngeal sphincter. The bolus, however, traveled alongside but never over the tip of the epiglottis. In the juvenile swallow, the bolus always passed over the tip of the epiglottis. The tilting of the epiglottis resulted from several factors, including the action of the palatopharyngeal sphincter, higher bolus pressure exerted on the epiglottis and the allometry of increased size. In both transitional and juvenile swallows, the subsequent relaxation of the palatopharyngeal sphincter released the epiglottis, which sprang back to its original intranarial position.
Keywords: Swallow mechanism, Esophagus, Soft palate, Palatopharyngeal sphincter
Introduction
The epiglottis, the most superior anterior laryngeal cartilage, folds backward during swallowing in most adult mammals, including adult humans (Fink and Demarest, 1978; Fink et al., 1979; Ekberg and Sigurjonsson, 1982; Van Daele et al., 1995), pigs and ferrets (Larson and Herring, 1996), opossums (Thexton and Crompton, 1998), dogs (Biewener et al., 1985), rabbits (Ardran et al., 1957), sheep (Amri et al., 1989) and cats (Kobara-Mates et al., 1995). Two known exceptions to this pattern of epiglottal movement are found in adult opossums when swallowing liquids (Thexton and Crompton, 1998) and in toothed whales (Reidenberg and Laitman, 1987), the latter being the only mammal known to swallow solid food by sending it around rather than over the epiglottis. Negus (1942, 1949) claims that, when ungulates swallow, both solids and liquids likewise travel around, rather than over, the epiglottis. However, this has never been confirmed radiologically.
In human infants but not human adults, and in both infants and adults of all other mammals, the larynx is intranarial, with the superior cartilages (epiglottis, corniculate) projecting into the nasopharynx (Harding, 1984). According to Laitman et al. (1977), young and maturing stump-tailed macaques keep “the larynx locked in the nasopharynx” when drinking water; however, when swallowing milk, the larynx momentarily unlocks from the nasopharynx and milk passes via the piriform sinuses to the esophagus while the soft palate elevates to close off the nasopharynx. Similarly, the human newborn infant swallows saliva around rather than over the larynx (Ardran and Kemp, 1952; Ardran et al., 1958; Laitman et al., 1977) although, as in the macaque, a momentary separation of larynx and soft palate occurs when swallowing milk (Laitman et al., 1977). It is, however, not known whether the epiglottis folds down in infant humans and macaques when momentary separation of the larynx and soft palate occurs. Conversely, when suckling on a bottle, infant pigs collect milk in the oropharynx and in the expanded piriform recesses which then empty during the swallow without any downfolding of the epiglottis (Crompton et al., 1997).
Several mechanisms for epiglottal folding in adult humans have been proposed (Fink and Demarest, 1978; Fink et al., 1979; Ekberg and Sigurjonsson, 1982; Van Daele et al., 1995), the epiglottal movement being considered as having two stages:
movement of the epiglottis to a horizontal position – suggested to occur either as a result of tongue pressure, as a result of elevation of the larynx or as a result of shortening of the thyroid-hyoid distance, which causes the thyroid attachment of the base of the epiglottis to be elevated relative to the hyoid attachment of the hyoepiglottic ligament;
further downturning of the upper third of the epiglottis to cover the laryngeal opening – suggested to occur either because of the action of laryngeal muscles (Ekberg and Sigurjonsson, 1982) or because of increased tension in the hyoepiglottic ligaments created by movement of the thyroid cartilage relative to the hyoid bone (Fink and Demarest, 1978; Fink et al., 1979; Van Daele et al., 1995).
The fact that, in adult humans, epiglottal movement can occur in the absence of a bolus, as in so-called “dry swallows”, implies an intrinsic mechanism that is not dependent on the weight or momentum of the swallowed bolus.
In non-human mammals, where the epiglottis flexes during the swallow, it stays in the flexed position after the bolus has passed over the epiglottis (or when a small bolus remains on the superior surface of the epiglottis), again suggesting that more than bolus pressure is involved in retaining the epiglottis in a flexed position. Larson and Herring (1996) also show that, during the swallow in non-humans, the epiglottis is forced against the soft palate when the larynx is elevated. They hypothesize that this initiates epiglottal flexion but further suggest that an additional mechanism, similar to that reported for human adults, might also be involved in the complete downturning of the epiglottis. A relationship between changing anatomy in the pharynx and larynx and maturation of the central nervous system has been considered by several researchers (Wood Jones, 1940; Bosma, 1985; Crelin, 1987; Laitman and Reidenberg, 1993) but has never been fully documented. Specifically, little is known about changes in epiglottal movement as the infant mammal matures, although previous work (Crompton et al., 1997) suggests that there is a change in the path taken by a liquid bolus during the swallow, just prior to weaning. We wished to test the hypothesis that there are maturational changes in epiglottal movement as the mammal approaches weaning and that these changes produce alterations in the path taken by liquids. In addition, we wanted to determine whether any of the various mechanisms proposed for epiglottal movement in humans were valid for any other infant or juvenile mammals.
Materials and methods
Harvard University IACUC (#23-05) approved all the work performed in this project. The experiments were carried out on eight infant domestic pigs (mini-Hanford strain), each ten to thirty days old, and six juvenile minipigs (three Göttingen strain and three mini-Hanford strain), each two to three months old. Infant pigs wean between 14 and 28 days old (Dritz et al., 1996; Main et al., 2004; Davis et al., 2006).
Radio-opaque markers were attached to, or inserted in, the following structures: palatopharyngeal arch, epiglottal tip, thyroid cartilage, hyoid cartilage and, in some animals, posterior tongue. All surgical procedures for the insertion or attachment of radio-opaque markers were carried out under isoflurane anesthesia (3–5 % in oxygen) administered by face mask. Several types of markers were used for different purposes. The first, used to mark the epiglottis, consisted of a tantalum micro-hematological clip (WECK Closure Systems™, Research Triangle Park, NC), attached using a custom-designed hemostat. The shape of these clips allowed the rotational orientation of the marker to be identified. The second type of marker, used to mark soft tissue (German et al., 1992), consisted of 5 mm lengths of 0.5 mm diameter wire (96 % tin, 4 % silver). These wire markers were delivered from the cavity of a 20 gauge 3” spinal needle (Terumo Corporation, Tokyo, Japan). The needle was inserted into the posterior palatopharyngeal arch under direct vision, using a rigid pediatric laryngoscope. Both pharyngeal arch and epiglottal tip markers were used in the mini-Hanford pigs but only the epiglottal marker was used in the juvenile Göttingen minipigs. Careful postmortem studies of markers indicated that no infection or inflammation was associated with any of them. Epiglottal markers had no impact on the kinematics of swallowing, as measured radiographically before and after marker implantation. Similar markers have previously been used by other investigators without adverse effects (Laitman et al., 1977). Finally, small circles of radio-opaque wire were surgically attached to the connective tissue covering the thyroid cartilage and hyoid bone. This procedure was carried out while the animals were deeply anesthetized. The complete surgical techniques were identical to those discussed in previously published work (Thexton et al., 1998).
At intervals of approximately four hours after full recovery from anesthesia, the animals were fed from a standard baby bottle fitted with a pig nipple (NASCO, Fort Atkinson, WI). The feed for the infant mini-Hanford pigs consisted of a milk replacement formula (Old Mother Hubbard all-purpose milk replacer, Chelmsford, MA) mixed with a small amount of radio-opaque barium (approximately 0.25 cup per gallon milk, E-X-EM, Westbury, NY) for contrast visualization. The juvenile minipigs were fed a homogenized mixture of pig chow pellets for minipigs with barium and water (1 cup pellets, 2 cups water, 1 tbs. barium). All animals fed unrestrained, standing in a Plexiglas box in front of the radiographic apparatus (Tridoros 150G3, Siemens) at the Museum of Comparative Zoology, Harvard University. This permitted visualization of the swallows as clear periodic movements of the accumulated milk or homogenized pig chow from the oropharynx to the esophagus. Video images (lateral and dorso-ventral) were recorded at 29.97 fps on a Sony DCR-VX1000 digital video camera. Digital images were then captured from mini-DV cassettes to the hard drive of an Apple G5 computer, using Apple’s Final Cut Pro, and saved as MPEG video files and JPEG image sequences. Movement of the markers was analyzed by digitizing the marker positions in sequential video frames using NIH ImageJ. The entire head in each frame was visibly aligned with the others. Each feeding session (recorded in either dorsoventral or lateral views) lasted 60–480 seconds and contained 30–500 swallows. Because of the depth and density of the skulls of the juvenile minipigs it was only possible to visualize the larynx in lateral view. At the conclusion of the study, all animals were euthanized using intracardiac injections of pentobarbital. Postmortem radiographs and dissections confirmed the location of the markers, as well as the anatomy of the musculature.
Results
Anatomy
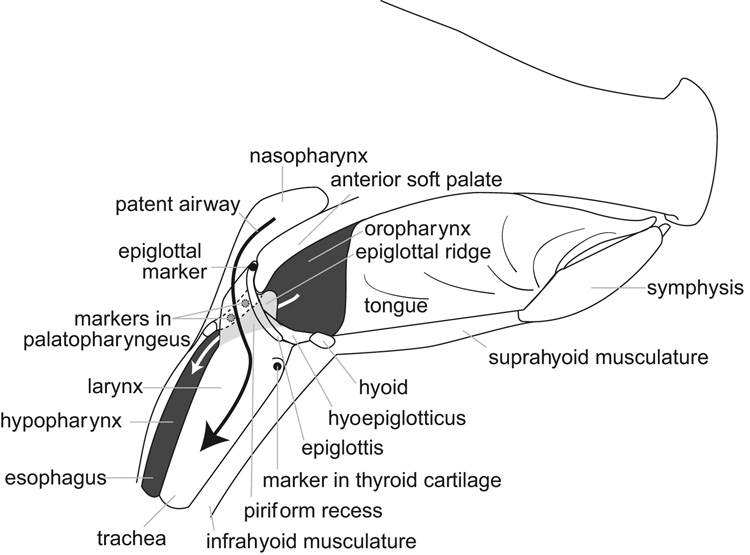
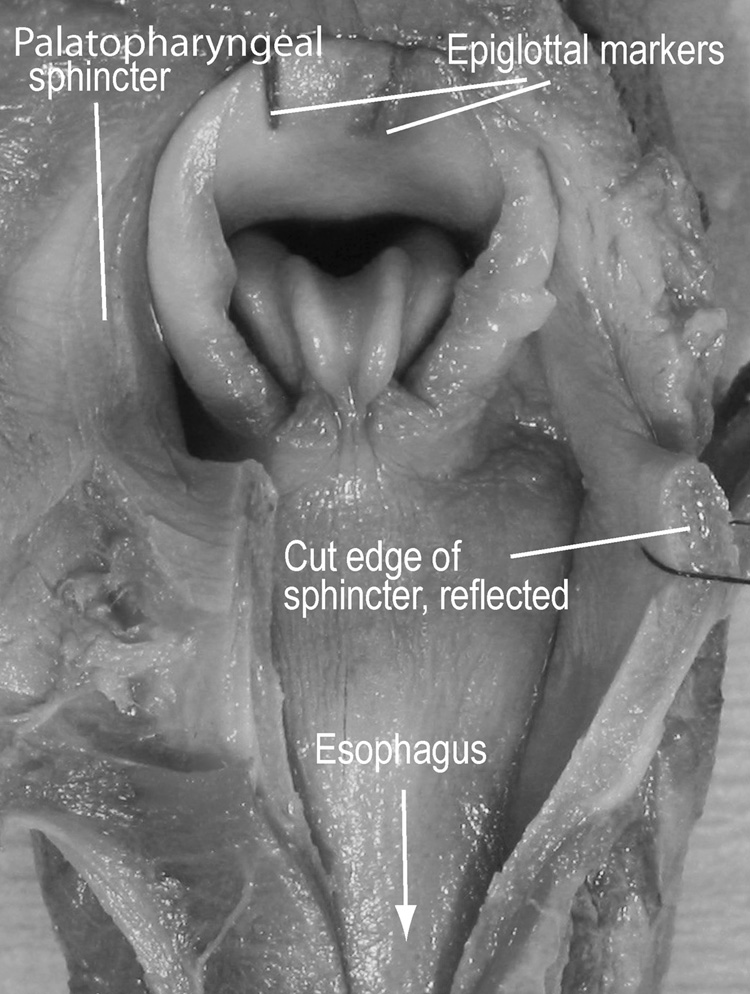
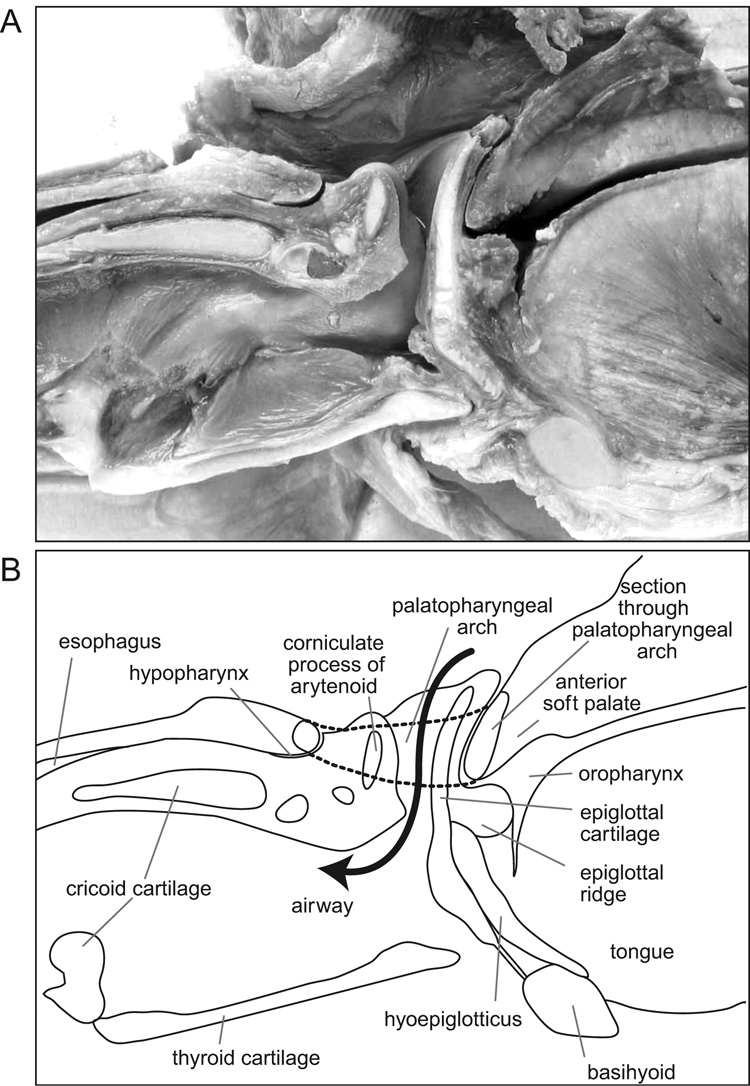
The literature that defines and describes the anatomy of the oropharyngeal region is inconsistent. Therefore, we have included three figures (Fig. 1–Fig. 3) and some description of the relevant structures. Figure 1 is a schematic sagittal section through the head of an infant pig at rest to illustrate the essential anatomical features, visible in lateral videofluoroscopy, that are also referred to in figure 4 through figure 8. In all lateral view figures, the animal faces toward the right and the ‘horizontal’ plane is defined by the hard palate. Figure 1 is based upon dissections of the head of infant pigs and it illustrates the position of two radio-opaque markers within the palatopharyngeal arch. Dark shading indicates the oropharynx, the hypopharynx and the esophagus. The piriform recess (lightly shaded) that lies lateral to the larynx connects the oropharynx to the hypopharynx and esophagus (white arrow). The patent airway (solid black arrow) is separate from the oropharynx-hypopharynx connection. In the infant pig the larynx is intranarial and the upper half of the epiglottis meets the dorsal and posterior surface of the anterior part of the soft palate, when seen in lateral view (see Fig. 1 for general orientation and Fig. 2 for detail of the contact). Figure 3 is a photograph from the dorsal view, showing the laryngeal opening and epiglottis relative to the palatopharyngeal muscle.
Fig. 1.
Schematic sagittal section through the head of a juvenile pig to aid in the interpretation of the video frames included in figure 2, figure 3, figure 4 and figure 7. See Results for description of this figure.
Fig. 3.
Photograph from a dorsal perspective. The palatopharyngeus sphincter has been cut open, and the passageway through the pharynx into the esophagus is visible. When intact, this muscle grips the laryngeal cartilages.
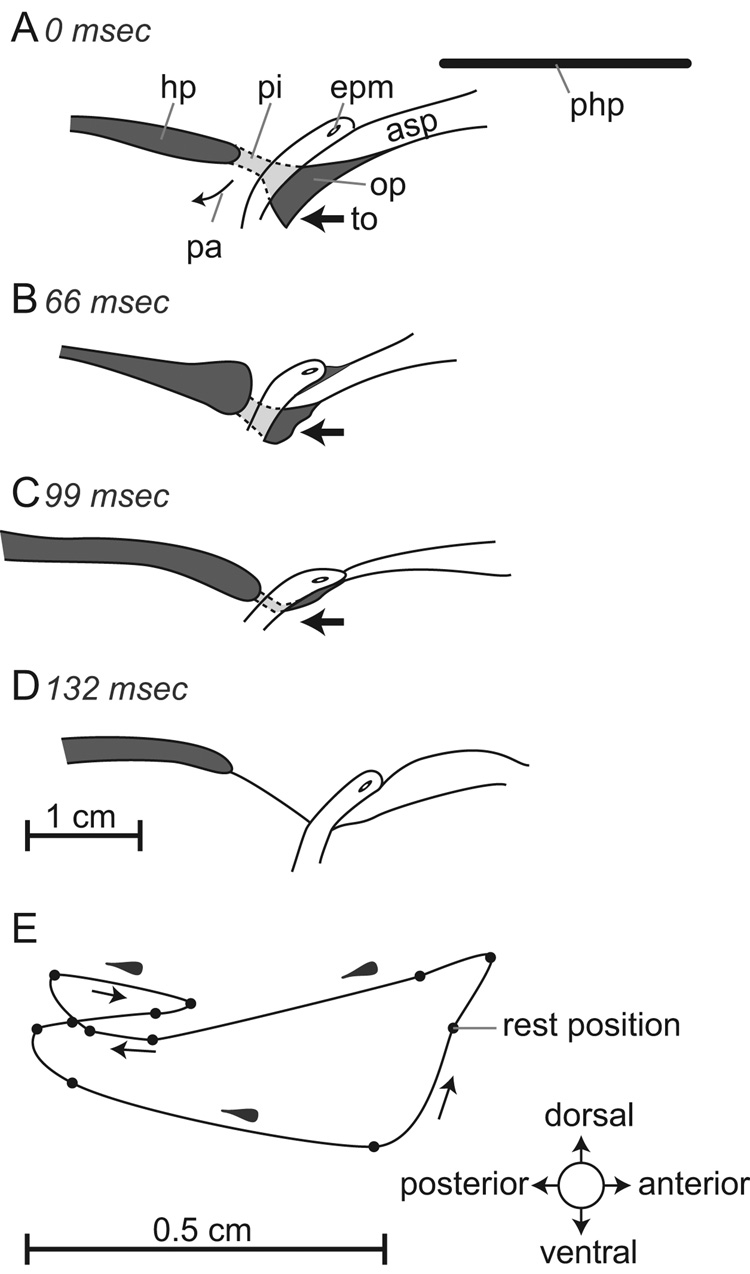
Fig. 4.
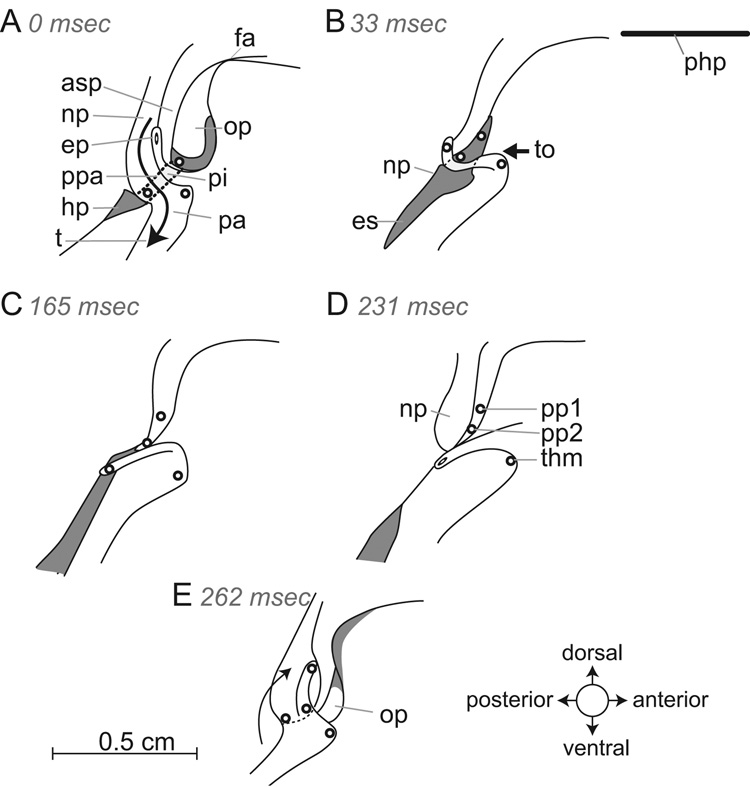
(A–D) Infant swallow. Tracings from radiographs of a swallow (during suckling from a bottle) in which the milk is forced by tongue pressure to flow back laterally to the larynx via the piriform recess, to the hypopharynx. The larynx was in an intranarial position with the epiglottis lying above the anterior part of the soft palate. The patent airway was visible. Each tracing was obtained at the intervals shown in milliseconds after the onset of the swallow; these are based on recording at 29.97 frames per second, so that each videographic frame took approximately 33 ms. (E) Orbit of movement of a marker on the tip of the epiglottis during a single swallow. The teardrop shape of the marker indicates the orientation of the epiglottal tip (thick part of marker was anterior at rest). Abbrev.: asp = anterior part of soft palate; epm = marker in the tip of epiglottis; hp = hypopharynx; op = oropharynx; pa = patent airway; php = plane of the hard palate; pi = piriform recess; to = tongue.
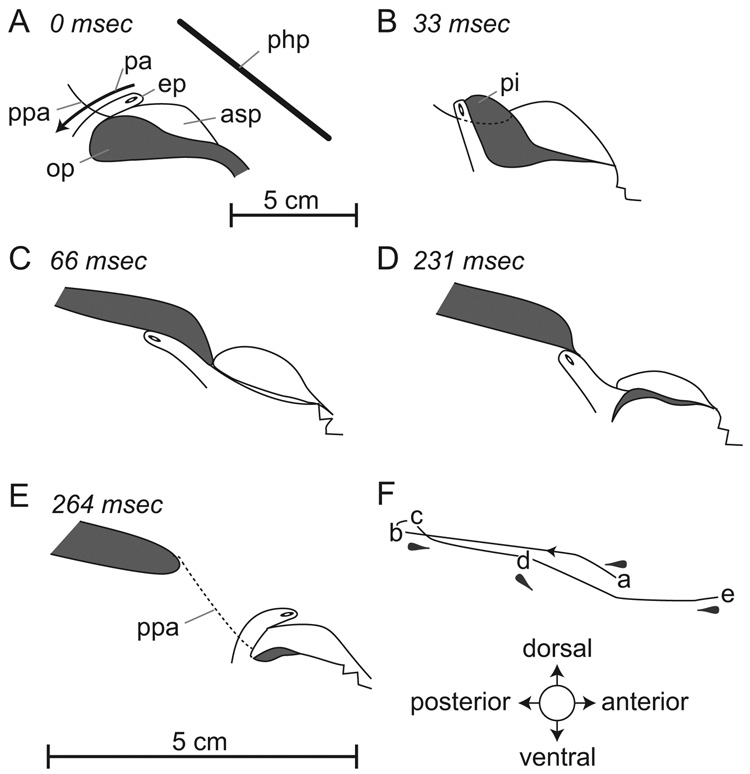
Fig. 8.
Juvenile swallow. Tracings are taken from radiographs of a swallow in a juvenile pig during drinking from a bowl (A). Bolus movement was associated with displacement of the epiglottis to an upright position (B). The epiglottis flexed rapidly below the palatopharyngeal sphincter as the milk passed over the downturned epiglottis (C, D) and then returned to its resting position (E). The orbit of movement of the epiglottal marker during the swallow is shown in (F). The scale for (A–E) is shown in the upper right corner, the scale for (F) directly below the figure. Note that the orientation of the head (plane of the hard palate, php) differs from that shown in figure 4 and figure 5 where the head was horizontal during suckling from a bottle. The head was tilted down at approximately 45° from the horizontal while drinking from a bowl. Abbrev.: asp = anterior part of soft palate; ep = epiglottis; op = oropharynx; pa = patent airway; pi = piriform recess; ppa = palatopharyngeal arch.
Fig. 2.
(A) Photograph of a mid-line sagittal section through the larynx and posterior oral region. (B) Key to the structures in A. The dotted line indicates the palatopharyngeal muscle that runs lateral to the epiglottis from the anterior part of the soft palate to the roof of the hypopharynx.
Figure 2A is a photograph and figure 2B the key to the significant features of a midline sagittal section through the larynx. These features are critical to an understanding of non-human swallowing. A terminological problem exists when describing the non-human soft palate and pharynx because of a tendency in the literature to restrict the term “soft palate” to the region lying between the hard palate and the contact with the epiglottis in non-human mammals or to the region lying between the hard palate and the uvula in humans. This probably arises because this segment of the soft palate is the only part that stands out clearly in lateral view radiographs. However, as described by Wood Jones (1940), this region is only the anterior portion of the soft palate and the actual soft palate extends to the roof of the esophagus by way of a posterior part that is perforated by the hiatus nasopharyngeus. The palatopharyngeus muscle forms a thickened edge, referred to as the palatopharyngeal arch, within the tissue surrounding the hiatus nasopharyngeus. The terminology of Wood Jones (1940) is adopted in this paper.
It is evident from figure 2 that the anterior part of the palatopharyngeal sphincter is closely adjoined to the anterior surface of the upper one third of the epiglottis and to the dorsal surface of the epiglottal ridge. The anterior region of the palatopharyngeus is thick anteriorly and thin posteriorly. Figure 2B indicates, with dotted lines, the dorsal and ventral margins of the palatopharyngeal sphincter lateral to the larynx; the sphincter itself surrounds the epiglottis and the corniculate processes of the arytenoids. During postmortem dissections of infant pigs, no macroscopically evident muscle fibers could be found within the aryepiglottic fold. However, when the palatopharyngeus muscle was cut, the component parts of the larynx sprang apart horizontally, indicating that the palatopharyngeal arch normally constrained and held the laryngeal cartilages together (Fig. 3). A ridge of tissue, the epiglottal ridge, extended around the outside surface of the epiglottis and, throughout its circumference, contacted the ventral edge of the palatopharyngeal arch, forming a seal. The piriform recesses occupied positions lateral to this seal and connected the oropharynx to the hypopharynx. The hyoepiglotticus muscle inserted on the anterior surface of the epiglottis below the epiglottal ridge and originated on the dorsal surface of the basihyoid (Fig. 2). Consequently, at rest, the epiglottis did not contact the oropharynx directly; its lower half was covered by the hyoepiglotticus muscle and its upper half was covered by the epiglottal ridge and the anterior part of the soft palate.
Kinematics
Suckling pigs exhibited three types of liquid swallow that can be broadly characterized as follows: (1) the infant swallow (seen in animals up to 20 days of age), in which the epiglottis did not tilt backward during the swallow but remained upright in an intranarial position within the palatopharyngeal arc; (2) the transitional swallow (seen as early as 10 days of age through to 30 days of age), in which the epiglottis tilted backward relatively slowly as milk passed lateral to but never over the tip of the epiglottis; (3) the juvenile swallow (seen in older animals either nearing weaning or after weaning) in which the epiglottis tilted backward more rapidly than in the transitional swallow and allowed liquids and solids to pass directly over its tip.
The infant swallow
When the youngest infant pigs suckled on a bottle, most of the swallows were observed to be of the first type: the larynx remained in an intranarial position with the tip of the epiglottis directed forward (Fig. 4) with the head held in an approximately horizontal position. The results are shown with the plane of the hard palate (php) horizontal. At the beginning of the swallow (Fig. 4A), milk had already accumulated in the oropharynx (op). The epiglottis was held behind the anterior part of the soft palate (asp) and the palatopharyngeal arch was in contact with the epiglottal ridge. Liquid reached the hypopharynx by way of the piriform recesses (pi). Four stages (A–D) of the infant swallow are shown in lateral view in figure 4: the posterior tongue (to) moved backward (bold arrow in A–C) and milk left the oropharynx through distended piriform recesses and entered the hypopharynx (D). During the infant swallow the larynx and soft palate moved as a unit. During an infant swallow the marker on the tip of the epiglottis (epm) moved in a roughly triangular orbit as seen in lateral view in figure 4E. The orientation of the epiglottal tip marker did not change through time, as indicated by the teardrop-shaped mark (broad end = front). At the beginning of the swallow the whole larynx/soft palate complex moved backward but then reversed direction momentarily as the milk passed through the piriform recesses.
The speed and extent of epiglottal movement during the infant swallow was modest. As the epiglottis remained in the intranarial position during the swallow, the antero-posterior excursion was limited to about 0.63 cm and its vertical excursion to 0.3 cm (Fig. 4E). The duration of the swallow, measured from the beginning of the backward movement of the epiglottis to the return to its original position, varied from 4 to 9 video frames (132–297 ms based on 33 ms per video frame). The backward movement took 5 frames (Fig. 4E) so that the average velocity of the anterior to posterior excursion was consequently of the order of 1 mm/frame or 3 cm/s.
The transitional swallow
In 10–15 day old infants, transitional swallows were periodically observed in suckling sequences that also included infant swallows; while in slightly older pigs (20 days and older), only transitional swallows occurred. However, this finding was variable both within and among individuals. The total number of swallows in three sibling pigs, all 7 days old, contained 60 %, 80 % and 100 % of the transitional type, respectively. In three other siblings (10 days old), one exhibited only infantile swallows and one exhibited only transitional swallows. In the third sibling, transitional swallows occurred with different frequencies in 3 different feeding sessions, i.e. 19 %, 25 % and 52 %, respectively. Figure 5 shows five stages (A–E) of a transitional swallow. In figure 5A the ventral part of the oropharynx (op) contained a bolus of milk (dark shaded area). As milk accumulated in the oropharynx it moved into the piriform recesses before the swallow. The faucial opening (fa), between the pillars of the fauces and between the tongue and palate, was closed. A patent airway (pa, illustrated by an ‘S’-shaped arrow) was clearly visible, extending from the nasopharynx (np) to the trachea (t). The epiglottis overlapped the dorsal surface of the anterior portion of the soft palate, and the palatopharyngeal arch, indicated by a dotted line and small open circles, contacted the epiglottal ridge. In figure 5B (33 ms later) the tongue had moved backward, forcing most of the milk out of the oropharynx into the piriform recesses, hypopharynx and esophagus. Both the anterior and posterior parts of the soft palate were elevated, bringing its ventral surface closer to the tip of the epiglottis and consequently exposing the anterior face of the epiglottis to the milk within the oropharynx. The lower three quarters of the epiglottis bent backward, but the tip of the epiglottis was still directed forward above the palatopharyngeal arch and was still positioned within the nasopharynx. Elevation of the soft palate broke the seal between the palatopharyngeal arch and the epiglottal ridge so that milk extended up the anterior surface of the backwardly flexed epiglottis as more milk moved into the piriform recesses. At this stage in the recordings the patent airway was no longer visible. Because no milk entered into the nasopharynx it was assumed that the palatopharyngeal sphincter closed tightly and progressively from front to back. As the epiglottal marker moved backward, the palatopharyngeal markers and thyroid cartilage (thm) markers moved forward (Fig. 5B, C). When the epiglottal marker reached its most posterior position (Fig. 5C, 165 ms after the onset of the swallow), it rotated from pointing slightly forward to pointing backward. Up until this point the tip of the epiglottis lay above the palatopharyngeal sphincter but, as the epiglottis rotated, its tip moved to below the level of the palatopharyngeal arch and the palatopharyngeal sphincter closed. In figure 5C the bolus extended from the hypopharynx into the esophagus and a small amount of milk was still visible on the anterior surface of the epiglottis. As the epiglottis was drawn forward after the bolus had passed (Fig. 5D), its tip still pointed backward, consistent with the tip being trapped below a closed palatopharyngeal sphincter. At the end of the swallow (Fig. 5E) the whole soft palate and thyroid cartilage was depressed. With the depression of the palatopharyngeal arch the nasopharynx again became visible and the epiglottis rapidly sprang upright into the nasopharynx through the relaxed palatopharyngeal sphincter. The tip of the epiglottis was again directed forward, overlapping the anterior part of the soft palate, from which it was now separated by a narrow space. A fresh aliquot of milk then entered the oropharynx.
Fig. 5.
Transitional swallow. Milk suckled from a bottle reaches the hypopharynx and esophagus via both the piriform recesses and by traveling on either side of a flexed epiglottis (A and B). Dotted lines indicate the position of the palatopharyngeal arch (ppa) and radio-opaque markers within the arch (pp1 and pp2). The tip of the epiglottis lies above the palatophayngeal arch in A and B and only flips below the palatopharyngeal sphincter when the most flexed position is reached (C). The flexed epiglottis is initially drawn forward below the palatopharyngeal arch (D), and flips forward (arrow) when the sphincter relaxes (E). Abbrev.: asp = anterior part of soft palate; ep = epiglottis; es = esophagus; fa = faucial opening; hp = hypopharynx; np = nasopharynx; op = oropharynx; pa = patent airway; php = plane of the hard palate; pi = piriform recess; pp1 = anterior marker in palatopharyngeal arch; pp2 = posterior marker in palatopharyngeal arch; ppa = palatopharyngeal arch; t = trachea; thm = marker in antero-ventral region of the thyroid cartilage; to = tongue.
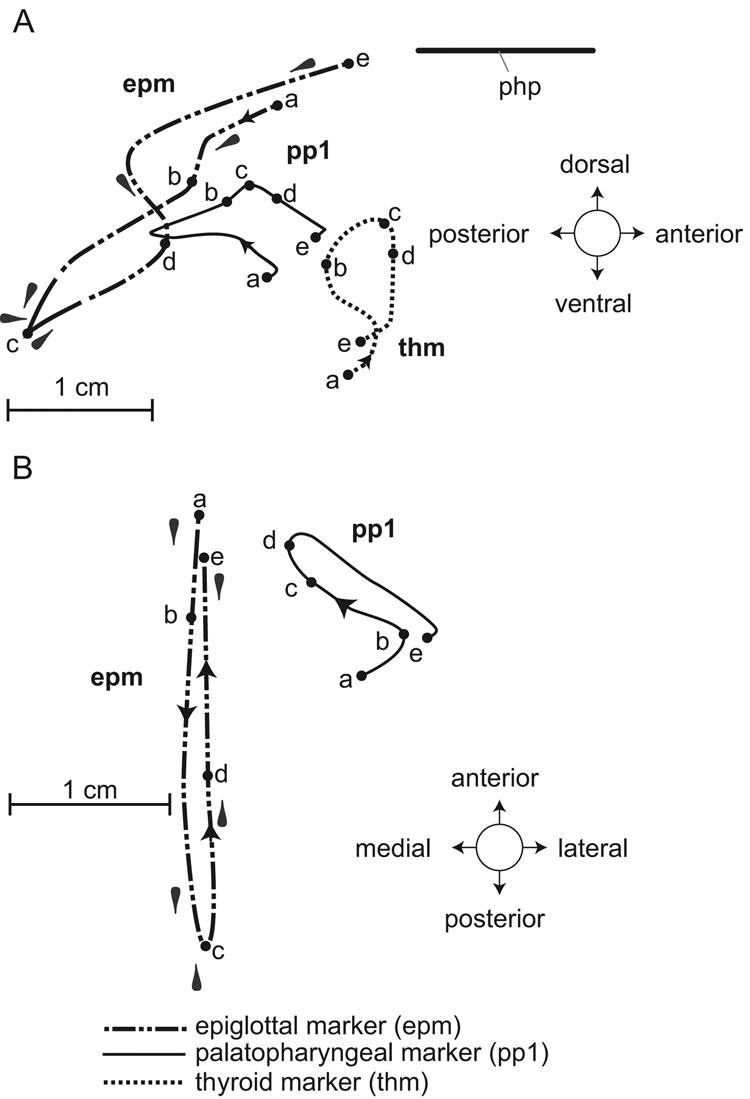
The orbits of movement of the markers in the epiglottis, palatopharyngeal arch and thyroid cartilage during a transitional swallow indicate large excursions of movement in lateral view (Fig. 6A). As the epiglottal marker (epm, shown as a dashed and dotted line) moved progressively backward (from positions a to b to c), the anterior palatopharyngeal marker (pp1, shown as a solid line) initially moved up and briefly back but then changed direction to move forward and upward (positions a to c). At the same time, the anterior base of the thyroid cartilage (thm, shown as a dotted line) moved upward and then briefly backward so that, as the epiglottis tip moved to its most posterior position (epm – c), the markers in the palatopharyngeal arch and on the thyroid cartilage reached their most dorsal positions (pp1 – c, thm – c). Only when the epiglottal marker (epm) reached position c, did the marker change from pointing forward to pointing backward. As the thyroid cartilage descended (thm – c to e), the epiglottal marker moved slowly forward (epm – c to d), then suddenly upward and forward again (d to e) as the palatopharyngeal marker moved antero-ventrally (pp1 – c to e).
Fig. 6.
Transitional swallow. Orbits of movement of markers in the tip of the epiglottis (epm), anterior part of the palatopharyngeal arch (pp1) and base of the thyroid cartilage (thm) as seen in lateral (A) and dorso-ventral (B) view. The orbit of movement of the thyroid marker is shown only in A. Plane of the hard palate (php) is indicated as a horizontal line.
The speed and amount of movement when the epiglottis flexed and sank below the palatopharyngeal sphincter was significant. The tip of the epiglottis required between 5 and 6 video frames (165–198 ms) to reach its most posterior position after the onset of the swallow. It remained below the soft palate for 2 frames (66 ms) and then took 1 frame (33 ms) to flip back upright. On this basis, the total duration of the transitional swallow was between 264 and 297 ms. The tip of the epiglottis moved 2.8 cm antero-posteriorly and 1.9 cm vertically (Fig. 6A). Because the full extent of the backward movement of the tip, when the epiglottis folded, took at least 5 frames (Fig. 4E), the average velocity was of the order of 5.5 mm/frame or 16.5 cm/s.
As milk entered the piriform recesses the palatopharyngeal marker moved laterally (Fig. 6B, pp1 – a to b), and the epiglottal marker began to move backward (epm – a to b). As the palatopharyngeal marker then moved forward and medially (Fig. 6B, pp1 – b to c), the epiglottal marker moved rapidly back (from position b to c). As the epiglottal marker reversed from a forward to a backward direction and slipped below the soft palate (epm – c to d), the palatopharyngeal marker continued to move medially (pp1 – c to d). The subsequent rapid forward movement of the tip of the epiglottal marker (epm – d to e) was associated with posterolateral movement of the palatopharyngeal marker (pp1 – d to e). As the epiglottal marker moved from position d to e it reversed orientation from pointing backward to pointing forward.
When epiglottal and palatopharyngeal marker movements were viewed dorso-ventrally (Fig. 6B), the epiglottal marker moved 2.6 cm antero-posteriorly, while the anterior palatal marker moved 1.05 cm antero-posteriorly with a medial movement of 0.9 cm. The medial movement of the palatopharyngeal marker (pp1 – b to c) reflected closing of the sphincter and this corresponded to the major part of the posterior movement of the epiglottal tip (epm – b to c).
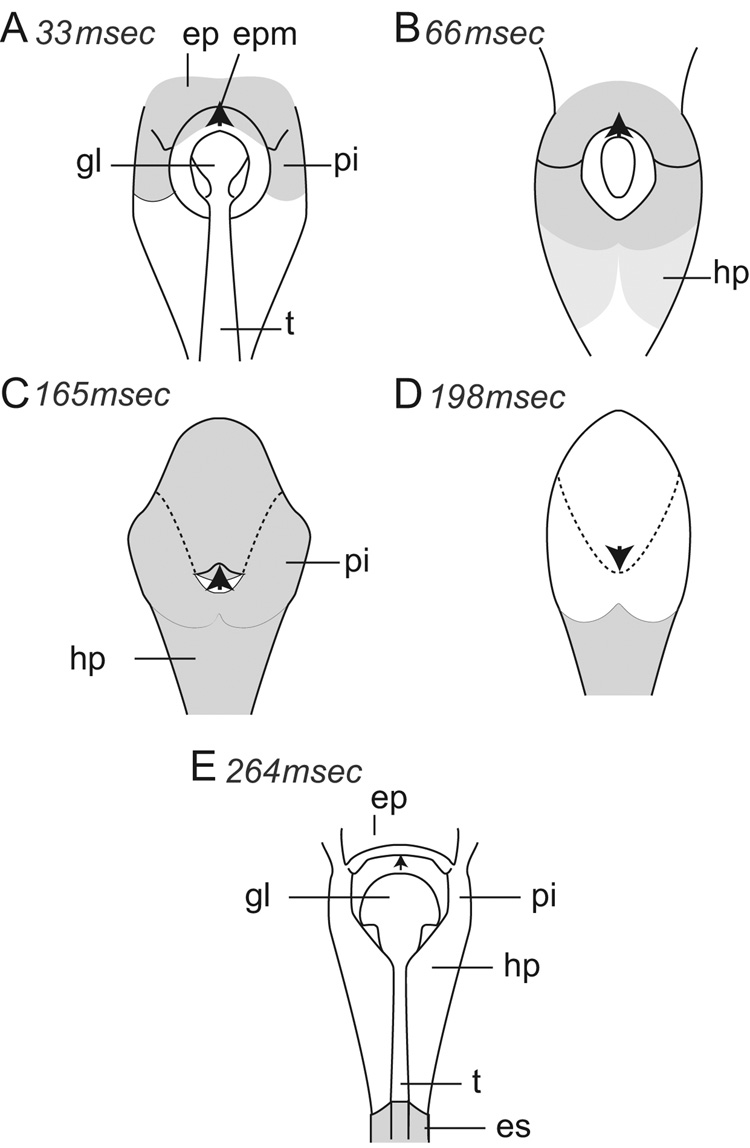
In the dorso-ventral radiographic images it was difficult to recognize all of the anatomical landmarks clearly or to recognize a clear outline of the bolus, because the bolus tended to be wide and shallow and thus insufficiently dense for videoradiographic recordings. At the initiation of the transitional swallow (Fig. 7A), the glottis (gl), the trachea (t) and the tip of the epiglottis (epm, marked with a black arrow pointing forward) were clearly visible in dorso-ventral view. Milk (shaded in the figure) abutted the anterior surface of the epiglottis (ep) and extended backward into the piriform recesses (pi) on either side of the glottis. The glottis started to close at 66 ms (1 video frame later, Fig. 7B), with the tip of the epiglottis still pointing forward. Milk extended deeply into the piriform recesses and reached the hypopharynx (hp). Further (165 ms or 5 video frames) into the swallow (Fig. 7C), the tip of the epiglottis reached its most posterior position but still pointed forward. Milk was present in the piriform recesses, hypopharynx and esophagus and covered all but the tip of the downturned epiglottis. Later (198 ms) into the swallow (Fig. 7D), the tip of the epiglottis pointed backward as it slipped below the closed sphincter. Subsequently (264 ms or 8 video frames into the swallow, Fig. 7E), the epiglottis flipped back once again, to overlap the anterior part of the soft palate. At this time, a thin film of barium remained on the walls faintly outlining the piriform recesses and hypopharynx. The glottis and trachea were again visible.
Fig. 7.
Dorsoventral view of five stages (A–E) during a transitional swallow. Many structures could not be clearly visualized in this view. A marker on the tip of the epiglottis (epm) is indicated by a black arrowhead anterior to a wide open glottis and trachea. At the beginning of the swallow (A), milk, shaded in grey, was visible against the anterior surface of the epiglottis and extended into the piriform recess. As the swallow commenced (B), milk was forced into the hypopharynx around the open glottis, obscuring the view of structures previously visible. In (C), the epiglottis was tilted backward, but its tip still pointed forward. Milk covered the entire surface of the epiglottis except the tip, which (from lateral view data) was still above the palatopharyngeal sphincter. The glottis was no longer visible and was presumably closed. In (D), milk had been cleared from the hypopharynx, the tip of the epiglottis pointed backward and (from lateral view data) was positioned below the closed palatopharyngeal sphincter. In E, the epiglottis had returned to an intranarial position and the glottis was open. Abbrev.: ep = epiglottis; es = esophagus; gl = glottis; hp = hypopharynx; pi = piriform recess; t = trachea.
The juvenile swallow
In juvenile pigs that had weaned, both liquid and solid boluses passed over a downturned epiglottis (Fig. 8), as described for adult pigs (Larson and Herring, 1996). Prior to the swallow, a bolus accumulated in the oropharynx (shaded area in Fig. 8A). As the swallow commenced, the rising tongue moved the bolus back and within one video frame the epiglottis moved to a vertical position (Fig. 8B) while bending slightly backward; however, the tip of the epiglottis remained above the palatopharyngeal arch. As the bolus continued to move back, the epiglottis flexed (within 1 video frame) to below the level of the palatopharyngeal arch and nearly all of the bolus moved over the downturned epiglottis (Fig. 8C). A fully closed palatopharyngeal sphincter prevented food from entering the nasopharynx. The epiglottis remained in that position for between four and five video frames until the bolus had passed (Fig. 8D). The epiglottis then returned to an upright position in one video frame (Fig. 8E). The orbit of movement of the epiglottal marker, viewed in lateral view (Fig. 8F), was markedly flattened when compared to that of a transitional swallow (Fig. 6A).
The timing of the swallow was different in the transitional and juvenile swallows. In the latter, the epiglottis moved from a vertical to a backwardly flexed position below the bolus in 33 ms. It remained in that position for between 132 to 165 ms and then returned to the original position in 33 ms. Conversely, in the transitional swallow, the flexing of the epiglottis was relatively slow (165 ms) although its return to an upright position occurred within the same time interval (33 ms) as in the juvenile swallow.
Discussion
The swallow is characterized by movement of an accumulated bolus of food from the oropharynx to the esophagus, fluid flow indicating the existence of a pressure gradient. This bolus movement is correlated with an elevation of pressure in the oropharynx as the posterior tongue elevates and sweeps backward (Thexton et al., 2004). The moving bolus must exert some force upon the epiglottis, the magnitude of which depends upon the muscle mass generating the pressure and the surface area of the epiglottis. We found that in infants, the liquid bolus passed lateral to the larynx while the epiglottis remained in an intranarial position. However, in both the post-weaning juvenile and the adult, liquids and solids passed over a rapidly downturned epiglottis as described by Larson and Herring (1996). We found an intermediate mechanism between the infant and the juvenile/adult stages, the transitional swallow, where the epiglottis turned down relatively slowly but the bolus never passed over the tip of the epiglottis. During the infant swallow the upper part of the epiglottis did not make direct contact with the oropharynx. As the animals matured, the soft palate was elevated at the beginning of a swallow, exposing the upper part of the epiglottis. The speed of backward movement of the epiglottal tip also changed, being more rapid in the juvenile or adult swallow than in the transitional swallow. A number of potential explanations exist for this mechanism, several of which have been previously proposed for human epiglottal movement. During growth, muscle mass scales broadly with the cube of the linear dimension while surface areas scale with the square. This suggests that the surface of the epiglottis is negatively allometric relative to the tongue and other muscles. The bolus pressure, then, would be likely to exert a progressively larger force as the animals matured, which could account for the faster backward tilting of the epiglottis in the juvenile or adult swallow than in the transitional swallow.
Epiglottal flexion in adult humans (Fink and Demarest, 1978; Fink et al., 1979; Van Daele et al., 1995) involves tightening of the lateral ligaments around the larynx. In the pigs, the vertical movement of the thyroid cartilage occurred out of phase with the vertical movement of the epiglottal tip; thyroid cartilage elevation was associated with slow epiglottal flexion. This thyroid movement could perhaps produce a tightening of the lateral ligaments. However, a small initial movement of the thyroid and a disproportionately large associated epiglottal movement suggested that other factors were involved in the flexion.
The differences in the extent of movement of the epiglottal tip between the infant swallow and the transitional swallow suggest a link between the extent of antero-posterior movement of the epiglottis and its flexion. That is, the anterior-posterior movement of the epiglottal tip occurred because of the flexion, and not because the entire laryngeal apparatus moved in a posterior direction. Compared to the excursion of the flexing epiglottal tip, the antero-posterior movement of the thyroid cartilage was trivial, i.e., about 0.2 cm, suggesting that the movement of the base of the epiglottis must similarly be limited.
The final mechanism, considered for humans, is the epiglottal folding mechanism suggested by Ekberg and Sigurjonsson (1982), which depends on aryepiglottal and thyroepiglottal muscle action. This cannot hold for our animals because we found no macroscopically obvious muscle tissue within the aryepiglottic fold.
Problems exist with each of the mechanisms outlined above. However, our results suggest a mechanism for epiglottal flexion that is consistent with the anatomy. The posterior movement of the epiglottis correlated with the closing of the palatopharyngeal sphincter during the forward and dorsal movement of the arch. The timing of this reduction in the internal diameter of the sphincter corresponded to most of the epiglottal marker’s posteriorly directed movement. Conversely, the increase in sphincter diameter corresponded to the rapid return of the epiglottis.
In immediate postmortem dissections, we cut the palatopharyngeal sphincter which produced an immediate “release” and horizontal expansion of the elements of the larynx. Thus, this sphincter was well situated to squeeze the epiglottis and displace it posteriorly and inferiorly as it closed. We propose that, firstly the epiglottis is displaced posteriorly by the contracting sphincter and then, following the relaxation of the sphincter, an ‘elastic’ recoil returns the epiglottis to its original position.
This mechanism may be facilitated by activity in the hyoepiglotticus muscle. Although we did not include this muscle in our studies, the hyoepiglotticus muscle reduces resistance to airflow in dogs (Amis et al., 1996) and horses (Holcombe et al., 2002). This suggests that airway dilation occurs by forward positioning of the epiglottis. The role of the hyoepiglotticus muscle during feeding requires further study but it seems possible that, at least in the infant, the hyoepiglotticus muscle retains the epiglottis in its upright intranarial position. This would consequently maintain the seal between the palatopharyngeal arch and the epiglottic ridge and permit milk to reach the esophagus via the piriform recesses alone. The action of the sphincter may also account for the observation by Larson and Herring (1996) that, in pigs and ferrets, “… the epiglottis stayed in the downward position until very little if any of the bolus remained on its superior surface. If it were simply bolus pressure that maintained the epiglottis in its depressed position, then the epiglottis should begin ascending earlier”.
The maturation of the swallow follows the changes in palatopharyngeal arch function and the resulting interaction between epiglottis and the changing content of the food bolus. The firm seal between the palatopharyngeal arch and the epiglottal ridge in young infants nevertheless permitted liquid to move around the larynx in the piriform recesses. It is not clear why infants of both humans and all other animals swallow around rather than over the epiglottis. It may be related to a delay in the maturation of reflexes that are designed to protect the upper airway (Harding et al., 1977). Dog pups, for example (Sasaki and Suzuki, 1976), do not develop a glottal adductor response to laryngeal stimulation by liquids until weeks postnatally.
As the infant matured, elevation of the palatopharyngeal arch and the anterior part of the soft palate at the beginning of a swallow resulted in the exposure of a larger area of the anterior surface of the epiglottis to the oropharynx and hence to bolus pressure. In the transitional swallow, where the bolus covered the anterior surface but never moved over the tip of the epiglottis, a combination of limited bolus pressure on a relatively small area of the anterior face of the epiglottis and the squeezing action of the palatopharyngeal arch may account for the relatively slow tilting of the epiglottis. In the juvenile/adult swallow, where the bolus always passed over the tip of the epiglottis, the rapid tilting of the epiglottis could have resulted from both closure of the palatopharyngeal sphincter and a higher bolus pressure exerted on a relatively large anterior surface area of the epiglottis. These functional changes are clearly related to the dietary changes towards the consumption of semi-solid and solid food.
Acknowledgement
This research was funded by NIH grant DC03604.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amis TC, O'Neill N, Van der Touw T, Brancatisano A. Electromyographic activity of the hyoepiglotticus muscle in dogs. Respir. Physiol. 1996;104:159–167. doi: 10.1016/0034-5687(96)00028-x. [DOI] [PubMed] [Google Scholar]
- Amri M, Lamkadem M, Car A. Activity of extrinsic tongue muscles during swallowing in sheep. Brain Research. 1989;503:141–143. doi: 10.1016/0006-8993(89)91714-9. [DOI] [PubMed] [Google Scholar]
- Ardran GM, Kemp FH. The protection of the laryngeal airway during swallowing. Br. J. Radiol. 1952;25:406–416. doi: 10.1259/0007-1285-25-296-406. [DOI] [PubMed] [Google Scholar]
- Ardran GM, Kemp FH, Ride W. A radiographic analysis of mastication and swallowing in the domestic rabbit: Oryctolagus cuniculus. Proc. Zool. Soc. London. 1957;130:257–274. [Google Scholar]
- Ardran GM, Kemp FH, Lind J. A cineradiographic study of bottle feeding. Br. J. Radiol. 1958;31:11–22. doi: 10.1259/0007-1285-31-361-11. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Soghikian GW, Crompton AW. Regulation of respiratory airflow during panting and feeding in the dog. Respir. Physiol. 1985;61:185–195. doi: 10.1016/0034-5687(85)90125-2. [DOI] [PubMed] [Google Scholar]
- Bosma JF. Postnatal ontogeny of performances of the pharynx, larynx, and mouth. Am. Rev. Respir. Dis. 1985;131:10–15. doi: 10.1164/arrd.1985.131.S5.S10. [DOI] [PubMed] [Google Scholar]
- Crelin ES. The Human Vocal Tract. New York: Vantage Press; 1987. [Google Scholar]
- Crompton AW, German RZ, Thexton AJ. Mechanisms of swallowing and airway protection in infant mammals (Sus domesticus and Macaca fascicularis) J. Zool. Lond. 1997;241:89–102. [Google Scholar]
- Davis ME, Sears SC, Apple JK, Maxwell CV, Johnson ZB. Effect of weaning age and commingling after the nursery phase of pigs in a wean-to-finish facility on growth, and humoral and behavioral indicators of well-being. J. Anim. Sci. 2006;84:743–756. doi: 10.2527/2006.843743x. [DOI] [PubMed] [Google Scholar]
- Dritz SS, Owen KQ, Nelssen JL, Goodband RD, Tokach MD. Influence of weaning age and nursery diet complexity on growth performance and carcass characteristics and composition of high-health status pigs from weaning to 109 kilograms. J. Anim. Sci. 1996;74:2975–2984. doi: 10.2527/1996.74122975x. [DOI] [PubMed] [Google Scholar]
- Ekberg O, Sigurjonsson SV. Movement of the epiglottis during deglutition. A cineradiographic study. Gastrointest. Radiol. 1982;7:101–107. doi: 10.1007/BF01887619. [DOI] [PubMed] [Google Scholar]
- Fink BR, Demarest RJ. Laryngeal Biomechanics. Cambridge: Harvard University Press; 1978. [Google Scholar]
- Fink BR, Martin RW, Rohrmann CA. Biomechanics of the human epiglottis. Acta Otolaryngol. 1979;87:554–559. doi: 10.3109/00016487909126464. [DOI] [PubMed] [Google Scholar]
- German RZ, Crompton AW, Levitch LC, Thexton AJ. The mechanism of suckling in two species of infant mammal: miniature pigs and long-tailed macaques. J. Exp. Zool. 1992;261:322–330. doi: 10.1002/jez.1402610311. [DOI] [PubMed] [Google Scholar]
- Harding R. Function of the larynx in the fetus and newborn. Ann. Rev. Physiol. 1984;46:645–659. doi: 10.1146/annurev.ph.46.030184.003241. [DOI] [PubMed] [Google Scholar]
- Harding R, Johnson P, McClelland ME, McLeod CN, Whyte PL. Laryngeal function during breathing and swallowing in foetal and new-born lambs. J. Physiol. 1977;272:14P–15P. [PubMed] [Google Scholar]
- Holcombe SJ, Cornelisse CJ, Berney C, Robinson NE. Electromyographic activity of the hyoepiglotticus muscle and control of epiglottis position in horses. Am. J. Vet. Res. 2002;63:1617–1621. doi: 10.2460/ajvr.2002.63.1617. [DOI] [PubMed] [Google Scholar]
- Kobara-Mates M, Logemann JA, Larson C, Kahrilas PJ. Physiology of oropharyngeal swallow in the cat: a videofluoroscopic and electromyographic study. Am. J. Physiol. 1995;268:G232–G241. doi: 10.1152/ajpgi.1995.268.2.G232. [DOI] [PubMed] [Google Scholar]
- Laitman JT, Reidenberg JS. Specializations of the human upper respiratory and upper digestive systems as seen through comparative and developmental anatomy. Dysphagia. 1993;8:318–325. doi: 10.1007/BF01321770. [DOI] [PubMed] [Google Scholar]
- Laitman JT, Crelin ES, Conlogue GJ. The function of the epiglottis in monkey and man. Yale J. Biol. Med. 1977;50:43–48. [PMC free article] [PubMed] [Google Scholar]
- Larson JE, Herring SW. Movement of the epiglottis in mammals. Am. J. Phys. Anthropol. 1996;100:71–82. doi: 10.1002/(SICI)1096-8644(199605)100:1<71::AID-AJPA7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Main RG, Dritz SS, Tokach MD, Goodband RD, Nelssen JL. Increasing weaning age improves pig performance in a multisite production system. J. Anim. Sci. 2004;82:1499–1507. doi: 10.2527/2004.8251499x. [DOI] [PubMed] [Google Scholar]
- Negus VE. The mechanism of swallowing. Proc. Roy. Soc. Med. 1942;36:85–92. doi: 10.1177/003591574203600212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus VE. The Comparative Anatomy and Physiology of the Larynx. London: William Heinemann Medical Books; 1949. [Google Scholar]
- Reidenberg JS, Laitman JT. Position of the larynx in odontoceti (toothed whales) Anat. Rec. 1987;218:98–106. doi: 10.1002/ar.1092180115. [DOI] [PubMed] [Google Scholar]
- Sasaki CT, Suzuki M. Laryngeal reflexes in cat, dog and man. Arch. Otolaryngol. 1976;102:400–402. doi: 10.1001/archotol.1976.00780120048004. [DOI] [PubMed] [Google Scholar]
- Thexton AJ, Crompton AW. The control of swallowing. In: Linden RWA, editor. The Scientific Basis of Eating. Frontiers of Oral Biology. Vol. 9. Basel: Karger; 1998. pp. 168–222. [Google Scholar]
- Thexton AJ, Crompton AW, German RZ. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. J. Exp. Zool. 1998;280:327–343. doi: 10.1002/(sici)1097-010x(19980401)280:5<327::aid-jez2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Correlation between intraoral pressures and tongue movements in the suckling pig. Arch. Oral Biol. 2004;49:567–575. doi: 10.1016/j.archoralbio.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Van Daele D, Perlman A, Cassell M. Intrinsic fibre architecture and attachments of the human epiglottis and their contributions to the mechanism of deglutition. J. Anat. 1995;186:1–15. [PMC free article] [PubMed] [Google Scholar]
- Wood Jones F. The nature of the soft palate. J. Anat. 1940;77:147–170. [PMC free article] [PubMed] [Google Scholar]