Abstract
Treatment for acute hepatitis B is recommended in order to reduce the risk of progression to fulminant hepatitis and the need of OLT. We report our experience on treatment with high dose lamivudine, in patients with severe acute HBV infection. The diagnosis was based on clinical and virological findings and exclusion of other known causes of liver damage. The decision to treat was based on the prolongation of INR together with increasing values of bilirubin and ALT. Four patients received Lamivudine 200 mg/daily until clearance of serum HBV-DNA and then 100 mg/daily until clearance of HBsAg and appearance of anti-HBs antibodies. One patient received 100 mg/daily because of chronic renal impairment. The median period of hospitalization was 13 days, and none of the patients had complications, related either to underlying disease or to therapy. The complete normalization of serum transaminases and bilirubin occurred on average after 5.5 weeks and 3 weeks respectively. All patients cleared serum HBV-DNA within three months, lost HBeAg and HBsAg and seroconverted to anti-HBe; four patients developed anti-HBs at a protective titre. Early antiviral treatment attenuates the clinical and biochemical impairment leading to fast healing and promoting complete recovery.
Keywords: acute HBV hepatitis, lamivudine, fulminant hepatitis, treatment, recovery
Introduction
The incidence of acute hepatitis B (HBV) is largely reduced during the last 20 years as a result of the use of vaccination and routine blood donor screening 1 and, nowadays, in western countries, the risk of HBV infection is limited to sexual intercourses, intravenous drug users and, in a few cases, in patients undergoing dental therapy, acupuncture, piercing and tattooing 2-4. Acute HBV may still occur in adults and may result in fatal complications. Most symptomatic patients recover and treatment is not necessary; when there are signs of severe liver failure, treatment is recommended in order to reduce the risk of progression to fulminant or subfulminant hepatitis and the need of emergency liver transplantation 5.
The experience on acute HBV treatment is limited and controversial. A randomised controlled trial on standard interferon versus placebo shows greater HBs seroconversion in patients treated with 10 MU/TIW 6.
With regard to lamivudine, studies with a limited number of patients and case reports are encouraging 7-9 while the only randomised controlled study available does not show a significant clinical and biochemical improvement compared to placebo at a dose of 100 mg/daily 10.
We report our experience on treatment with high dose lamivudine, in a series of 5 patients with severe acute HBV infection.
Patients and Methods
From November 2006 to March 2007, 5 patients with acute HBV related hepatitis were admitted to our department: 4 patients were HBeAg positive and 1 anti HBe positive. The diagnosis was based on consistent clinical and virological findings (medical history, jaundice, hypertransaminasemia, HBsAg positivity, IgM anti-HBc > 1.20 mU/mL and presence of serum HBV-DNA by PCR) and exclusion of other known causes of liver damage. Baseline characteristics of patients are shown in table 1. All patients underwent ultrasonography guided liver biopsy to confirm the diagnosis.
Table 1.
Baseline characteristics of patients.
| Characteristics | n = 5 |
|---|---|
| Sex (M:F) | 4:1 (80%) |
| Age | 45 (33 - 64) |
| Hepatic encephalopathy (I-II°) | 2/5 (40%) |
| Bilirubin (mg/dL) | 26,28 (23,4-45,1) |
| ALT (IU/L) | 2494 (1472-3260) |
| INR | 1,47 (1,4-1,51) |
| HBeAg positive | 4 (80%) |
| HBeAb positive | 1 (20%) |
| HBV-DNA (log IU/mL) | 6,7 (4,9-8,6) |
Treatment
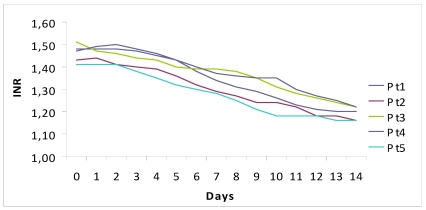
The decision to treat was based on the prolongation of INR together with increasing values of bilirubin and ALT.
Four patients received Lamivudine 200 mg/daily until clearance of serum HBV-DNA was reached and then 100 mg/daily until resolution (clearance of HBsAg and appearance of anti-HBs antibodies). One patient received 100 mg/daily because of renal impairment (creatinine clearance 32 ml/min). Patients were followed up for at least six months (range: 6-11 months) after the end of treatment.
Results are expressed as median (range).
Results
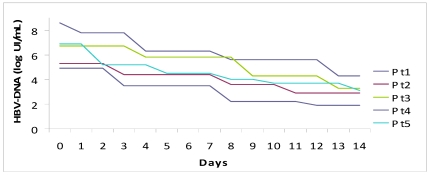
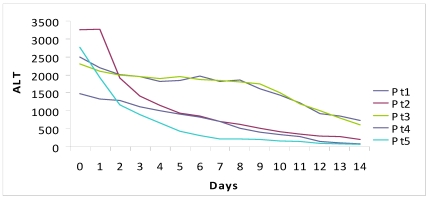
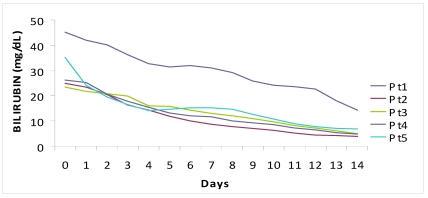
The median period of hospitalization was 13 days (12-15) and no patient had complications, both related to underlying disease or to therapy. We observed a prompt decrease of ALT (-1399 IU/L in a week, -2120 IU/L in two weeks) and serum bilirubin (-15 mg in a week, -23.9 mg in two weeks). The complete normalization of transaminases and bilirubinemia occurred on average after 5.5 weeks and 3 weeks, respectively.
All HBeAg+ patients lost e-antigen and seroconverted to anti HBe; they lost HBsAg within six months from the start of treatment and 4/5 developed anti-HBs at a protective titre (>10 mU/mL).
We observed an average drop of HBV-DNA of 1.58 logarithms in a week and 3.38 logarithms in two weeks. All patients cleared HBV-DNA (evaluated by PCR) in two months on average.
There were no adverse reactions to medication which was well tolerated.
Discussion
Differently from previous studies, we have chosen to treat acute hepatitis B with a higher dose of lamivudine (200 mg/day instead of 100mg/day) achieving a rapid viral clearance and clinical improvement. All patients were discharged by the hospital, in spite of disease severity at presentation, within 13 days from the start of oral antiviral treatment.
From our data, treatment with nucleoside analogues for severe and fulminant hepatitis B (acute or exacerbation of a chronic infection) is certainly indicated. In fact, early antiviral treatment shortens and improves the symptomatic phase of infection and allows a ready clinical and biochemical improvement. On the other hand, when patients with end stage liver disease are treated with nucleoside analogues, the dramatic improvement of hepatic function often leads to withdrawal from the liver transplantation list.
In our experience, lamivudine administration did not favour, as previously suggested by Kumar et al., the development of a chronic infection, since all patients displayed undetectable HBV-DNA and cleared HBsAg.
Severe or fulminant acute hepatitis B generally do not evolve to a chronic disease, since the immune response that causes liver damage also leads to the viral clearance. In this setting, with the use of nucleoside analogues we obtained a prompt hepatic biochemical and functional improvement and a strong suppression of viral replication, which probably enforced the ongoing process of recovery. Furthermore, we reduced the risk of fatal outcome which can occur in some of these patients.
With the use of a double dose of lamivudine (200 mg/day) we obtained a decrease of viral load even faster than observed in previous studies: in fact we observed an average decrease of serum HBV-DNA of 3.38 log IU/mL in the first two weeks of treatment, respect to 1 log decrease in one month reported by other authors 11.
To the best of our knowledge, only the studies of Tillman et al. and by Kumar et al. had a control group10,11. From those studies two major results emerge: the number of responders (HBV-DNA negative) is higher in the treated group compared to controls (91.6% against 71.6%). Even though not statistically significant, this figure outlines a trend and should not be underestimated. Furthermore, the number of adverse events (death and transplantation) was significantly lower in both studies in the treated groups (5.2% against 21.6% in controls). The lack of a control group is certainly a limitation but according to recent guidelines treatment is indicated for patients with fulminant hepatitis B and those with protracted, severe acute hepatitis B; therefore, a control group in this setting would likely not be judged ethical.
Many authors have suggested the need for a large placebo-controlled study. However, in light of our results and from data previously published, the only ethical choice in patients with severe and fulminant acute hepatitis B seems to be the treatment with oral antiviral drugs.
On the basis of our results we suggest that, with regard to lamivudine, a higher dose provides an effective and fast healing of severe acute hepatitis.
Despite the low genetic barrier of lamivudine, we chose it because at that time it was the drug with the fastest antiviral activity among those available.
It is conceivable that new available drugs for the treatment of hepatitis B (i.e. entecavir and telbivudine) might be even better in reaching a rapid decrease in viral load and a faster recovery in patients with fulminant or severe acute hepatitis.
Figure 1.
Fall of viral load and biochemical response during high dose lamivudine therapy.
Table 2.
Time(days) to virological seroconversion and biochemical normalization.
| Time to Virological Seroconversion and Biochemical Normalization | |||||||
|---|---|---|---|---|---|---|---|
| Patients | |||||||
| # 1 | # 2 | # 3 | # 4 | # 5 | Mean (days) | ||
| HBV-DNA-ve | 30 | 30 | 90 | 90 | 30 | 54±14.7 | |
| HBsAg -ve | 60 | 30 | 90 | 90 | 30 | 60±13.4 | |
| anti-HBs >10 | 60 | 30 | 90 | / | 30 | 52.5±14.4 | |
| anti-HBs >100 | 90 | 60 | 180 | / | 90 | 105±25.9 | |
| Bilirubin | 30 | 15 | 30 | 15 | 15 | 21±3.7 | |
| ALT | 45 | 45 | 45 | 30 | 30 | 39±3.7 | |
| INR | 13 | 12 | 15 | 16 | 12 | 13.6±0.8 | |
References
- 1.Zanetti AR, Romano L, Zappa A, Velati C. Changing patterns of hepatitis B infection in Italy and NAT testing for improving the safety of blood supply. J Clin Virol. 2006;36(Suppl 1):S51–5. doi: 10.1016/s1386-6532(06)80009-0. [DOI] [PubMed] [Google Scholar]
- 2.Mele A, Spada E, Sagliocca L, Ragni P, Tosti ME, Gallo G, Moiraghi A, Balocchini E, Sangalli M, Lopalco PL Stroffoli T. Risk of parenterally transmitted hepatitis following exposure to surgery or other invasive procedures: results from the hepatitis surveillance system in Italy. J Hepatol. 2001;35:284–9. doi: 10.1016/s0168-8278(01)00111-8. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber GB, Busch MP, Kleinman SH Korelitz JJ. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996;334:1685–90. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 5.Hoofnagle JH, Doo E, Liang TJ, Fleischer R Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056–75. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- 6.Tassopoulos NC, Koutelou MG, Polychronaki H, Paraloglou-Ioannides M Hadziyannis SJ. Recombinant interferon-alpha therapy for acute hepatitis B: a randomized, double-blind, placebo-controlled trial. J Viral Hepat. 1997;4:387–94. doi: 10.1046/j.1365-2893.1997.00072.x. [DOI] [PubMed] [Google Scholar]
- 7.Torii N, Hasegawa K, Ogawa M, Hashimo E Hayashi N. Effectiveness and long-term outcome of lamivudine therapy for acute hepatitis B. Hepatol Res. 2002;24:34. doi: 10.1016/s1386-6346(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 8.Kondili LA, Osman H Mutimer D. The use of lamivudine for patients with acute hepatitis B (a series of cases) J Viral Hepat. 2004;11:427–31. doi: 10.1111/j.1365-2893.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- 9.Schmilovitz-Weiss H, Ben-Ari Z, Sikuler E, Zuckerman E, Sbeit W, Ackerman Z, Safadi R, Lurie Y, Rosner G, Tur-Kaspa R Reshef R. Lamivudine treatment for acute severe hepatitis B: a pilot study. Liver Int. 2004;24:547–51. doi: 10.1111/j.1478-3231.2004.0983.x. [DOI] [PubMed] [Google Scholar]
- 10.Tillmann HL, Hadem J, Leifeld L, Zachou K, Canbay A, Eisenbach C, Graziadei I, Encke J, Schmidt H, Vogel W, Schneider A, Spengler U, Gerken G, Dalekos GN, Wedemeyer H, Manns MP. Safety and efficacy of lamivudine in patients with severe acute or fulminant hepatitis B, a multicenter experience. J Viral Hepat. 2006;13(4):256–63. doi: 10.1111/j.1365-2893.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 11.Kumar M, Satapathy S, Monga R, Das K, Hissar S, Pande C, Sharma BC Sarin SK. A randomized controlled trial of lamivudine to treat acute hepatitis B. Hepatology. 2007;45:97–101. doi: 10.1002/hep.21486. [DOI] [PubMed] [Google Scholar]