Abstract
Axin is a scaffold protein for the β-catenin destruction complex, and a negative regulator of canonical Wnt signaling. Previous studies implicated the six C-terminal amino acids (C6 motif) in the ability of Axin to activate c-Jun N-terminal kinase, and identified them as a SUMOylation target. Deletion of the C6 motif of mouse Axin in vivo reduced the steady-state protein level, which caused embryonic lethality. Here, we report that this deletion (Axin-ΔC6) causes a reduced half-life in mouse embryonic fibroblasts and an increased susceptibility to ubiquitination in HEK 293T cells. We confirmed the C6 motif as a SUMOylation target in vitro, and found that mutating the C-terminal SUMOylation target residues increased the susceptibility of Axin to polyubiquitination and reduced its steady-state level. Heterologous SUMOylation target sites could replace C6 in providing this protective effect. These findings suggest that SUMOylation of the C6 motif may prevent polyubiquitination, thus increasing the stability of Axin. Although C6 deletion also caused increased association of Axin with Dvl-1, this interaction was not altered by mutating the lysine residues in C6, nor could heterologous SUMOylation motifs replace the C6 motif in this assay. Therefore, some other specific property of the C6 motif seems to reduce the interaction of Axin with Dvl-1.—Kim, M. J., Chia, I. V., Costantini, F. SUMOylation target sites at the C terminus protect Axin from ubiquitination and confer protein stability.
Keywords: Wnt signaling, disheveled, beta-catenin
Axin is a scaffold protein for the β-catenin destruction complex, which, following a Wnt signal, promotes the degradation of β-catenin, the major effector in the canonical Wnt signaling pathway. Axin binds directly to many of the components of this complex, including APC, glycogen synthase kinase 3 (GSK3), and β-catenin, and stimulates the phosphorylation of β-catenin by the serine/threonine kinase GSK3, which leads to degradation of β-catenin via the ubiquitin-proteasome pathway (1,2,3). Thus, Axin serves as a key negative regulator of canonical Wnt signaling, and its activity is essential for normal mouse embryogenesis (4). The level of Axin in the cell is believed to be extremely low compared to other components of the Wnt signaling pathway (5) and, therefore, may be present in close to a limiting quantity. Consistent with this observation, we found a mutation that reduced the steady-state level of Axin by 3- to 4-fold (AxinΔC6, described below), but did not seem to disrupt its inherent activity in the Wnt pathway (6), caused an embryonic lethal phenotype in mice (unpublished results) similar to an Axin null allele (4).
In the canonical pathway, Wnts binds to a cell-surface receptor consisting of low-density lipoprotein receptor-related proteins 5 and 6 (LRP5/6) and a member of the Frizzled family, and this promotes the phosphorylation, by casein kinase 1γ (CK1γ) and GSK3, of several Ser/Thr residues in the cytoplasmic domain of LRP6 (7, 8). The phosphorylation of LRP6 generates a docking site for Axin and recruits it to the plasma membrane, where Axin is inactivated and/or targeted for degradation by an unknown mechanism. A second mechanism that stabilizes β-catenin in response to Wnt is the Frizzled-dependent phosphorylation of Dishevelled. Activated Dishevelled is also thought to recruit Axin to the plasma membrane and to inhibit the APC-Axin-GSK3 complex, by a poorly understood mechanism (3, 9). Thus, two separate mechanisms are thought to recruit Axin to the plasma membrane and reduce its level and/or activity.
It has been observed that the level of Axin decreases on exposure of the cell to Wnt (10, 11), and this step is thought to be an important one in the stabilization of β-catenin and propagation of the Wnt signal (2, 3, 12). One event that contributes to the destabilization of Axin may be the loss of phosphorylation by GSK3, whose activity is inhibited following a Wnt signal. However, the overall mechanisms that determine the stability of Axin, and whether the ubiquitination of Axin plays a role in this process, remain to be determined.
One domain of Axin that appears to influence its steady-state level is the 6-amino acid sequence (KVEKVD) at the extreme C terminus of Axin, the “C6 motif” (unpublished results). Previously, Lin and colleagues (13) had described several interesting features of this evolutionarily conserved motif. Although the overexpression of Axin in human embryonic kidney (HEK) 293T cells could activate the c-Jun N-terminal kinase (JNK) (13), deletion of the Axin C6 motif greatly impaired this activity (6). However, the C6 motif was not required for the ability of Axin to function as a negative regulator of canonical Wnt signaling, at least when overexpressed. In addition, the C6 motif was required for the interaction of Axin with three small ubiquitin-related modifier (SUMO) -1 conjugating E3 enzymes, PIAS1, PIASxβ, and PIASy, and two lysine residues in this motif were the main target sites for the SUMOylation of Axin, when Axin was cotransfected with hemagglutinin (HA) -tagged SUMO-1 (6). Ubiquitin and SUMO are structurally related post-translational polypeptide modifiers. Ubiquitination plays an important role in targeting proteins for proteolytic degradation by the proteasome, although covalent attachment of ubiquitin to substrate proteins can regulate localization and/or activity independent of proteolysis (14). Similar to the nonproteolytic roles of ubiquitin, SUMO modification regulates protein subcellular localization, protein stability, and activity and many SUMO modified proteins function in the regulation of transcription, chromatin structure, maintenance of the genome, and signal transduction. Several proteins can be modified by both SUMO and ubiquitin, but with distinct functional consequences (15, 16).
To examine the importance of the C6 motif for the functions of Axin in vivo, we recently generated a strain of mice carrying a mutant allele of Axin lacking this motif. We found that the steady-state expression level of the mutant Axin-ΔC6 protein was severalfold lower than wild-type Axin, which apparently caused the embryonic lethality in homozygotes for the AxinΔC6 allele. In the present work, we examine whether the low steady-state level of Axin-ΔC6 protein is due to a reduced stability and whether this mutation affects the modification of Axin by ubiquitin, as well as the potential role of SUMO-1 modification in this process. First, we show that Axin-ΔC6 protein has a reduced half-life, which largely accounts for its lower steady-state level in mutant cells and mouse embryos. We then demonstrate that Axin is a target of ubiquitination and that the C6 motif stabilizes Axin by protecting it from ubiquitination. We provide evidence that the Axin C6 motif is a substrate for SUMO-1 modification in vitro and that SUMOylation at the C terminus may protect Axin from ubiquitination in other regions of the protein.
MATERIALS AND METHODS
Mice, cell cultures, and transfections
Mouse embryonic fibroblasts (MEFs) were prepared from heterozygous AxinAx/+ or AxinΔC6/+ E13.5 embryos. AxinAx is a knock-in allele of Axin in which a wild-type mouse Axin cDNA with an N-terminal Myc epitope tag was targeted to the Axin locus, replacing the endogenous Axin coding sequences (17). AxinΔC6 is identical to AxinAx except that it encodes a form of Axin lacking the C-terminal C6 motif (unpublished results). In both cases, the Axin protein initiates at amino acid Met125 according to the original amino acid numbering (4), which is the preferred initiation site, and both proteins contain the same N-terminal Myc epitope tag. The MEFs were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma, St. Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, HyClone Laboratories, Inc., Logan, UT, USA) and 50 μg/ml penicillin and streptomycin (Gibco, Grand Island, NY, USA) in 5% CO2 at 37°C. HEK 293T cells were grown in the same medium. For transfection experiments, each plasmid DNA was applied by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol.
Plasmid constructs
pCS2-MT and Myc-tagged Axin and deletion constructs have been described previously (18). The HA-tagged ubiquitin plasmid was provided by Dr. Sunghoon Kim (Seoul National University, Seoul, South Korea) (19). The Axin constructs with point mutations in lysine residues were generated with a QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) and verified by DNA sequencing. The Axin-ΔC6 plasmids with consensus SUMOylation motif from p53, PML, or both and with alanine substitution for the lysine residue in p53, PML, or both were generated by polymerase chain reaction (PCR) using pairs of primers containing the SUMOylation sequences. The sequences for primers are summarized in Supplemental Table 1.
In vitro SUMOylation assay
[35S]Methionine-labeled substrates for SUMO modification reactions were generated by an in vitro TNT SP6 coupled transcription/translation system according to the manufacturer’s instructions (Promega, Madison, WI, USA). In vitro SUMOylation was performed with SUMOylation kits, including purified SAE I, SAE II, UBC9, and SUMO1 recombinant proteins following the manufacturer’s manuals (LAE Biotech International, Rockville, MD, USA). Reaction products were resolved by 12% SDS-PAGE gel and detected by phosphorimage analyzer.
Ubiquitination assay
HEK 293T cells were transiently transfected with 1 μg of pCS2MT, Myc-tagged Axin, or the indicated Axin mutant plasmids and with 1 μg of HA-tagged ubiquitin plasmid. After 20 h, the cells were treated with 50 μM of the proteasome inhibitor ALLN (Calbiochem, Gibbstown, NJ, USA) for 3 h to allow ubiquitinated Axin proteins to accumulate. The cells were lysed with RIPA buffer (10 mM Tris, pH 7.5; 0.1% SDS; 1 mM EDTA; 1% Nonidet P-40; 0.5% deoxycholic acid; 45 mM β-glycerophosphate; 50 mM NaF) supplemented with 1 mM dithiothreitol, 0.1 mM phenylmethysulfonyl fluoride, and 2 mM aprotinin, and the lysates were incubated with anti-Myc antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C for 3 h. After addition of 30 μl of protein G/A agarose (Santa Cruz Biotechnology), the mixtures were incubated at 4°C for an additional 1 h, and the beads were washed 3 times with RIPA buffer. The ubiquitinated Axins were resolved on 10% SDS-PAGE gel and detected by Western blotting with anti-HA antibody (Santa Cruz Biotechnology).
Coimmunoprecipitation assays
HEK 293T cells were cotransfected with the 1 μg of myc-tagged Axin or AxinΔC6 and with 1 μg of CMV-HA+LRP5 (from Dr. Dianqing Wu, University of Connecticut, Farmington, CT, USA), pcDNA3-HA+GSK3β (from Dr. James R. Woodgett, Mount Sinai Hospital, Toronto, ON, Canada), pQNCX-FLAG+mDvl1 (from Dr. Jan Kitajewski, Columbia University Medical Center, New York, NY, USA), J. R. or pCl-neo+β-catenin (from Dr. Sunghoon Kim, Seoul National University, Seoul, South Korea). The cells were lysed with RIPA buffer after 20 h and immunoprecipitated with anti-Myc antibody. The coimmunoprecipitated Wnt components were detected by Western blotting with anti-HA (Santa Cruz Biotechnology), anti-FLAG (Sigma-Aldrich), or anti-β-catenin antibody (BD Transduction Laboratories, Franklin Lakes, NJ, USA), respectively.
Primers used for PCR
K951A-F: ATCATCGGCGCGGTGGAAAAGGTGGACTGAGCACTG
K951A-B: CAGTGCTCAGTCCACCTTTTCCACCGCGCCGATGAT
K954A-F: ATCATCGGCAAGGTGGAAGCGGTGGACTGAGCACTG
K954A-B: CAGTGCTCAGTCCACCGCTTCCACCTTGCCGATGAT
K951/954A-F: ATCATCGGCGCGGTGGAAGCGGTGGACTGAGCACTG
K951/954A-B: CAGTGCTCAGTCCACCGCTTCCACCGCGCCGATGAT
SUMO-p53F: TTCAAGACAGAGTGAGCACTGGGCAGCACACCCGGA
SUMO-p53B: TCACTCTGTCTTGAAGCCGATGATCTTTTCTTCAAAGAC
SUMO-PMLF: CTCAAGCACGAGTGAGCACTGGGCAGCACACCCGGA
SUMO-PMLB: TCACTCGTGCTTGAGGCCGATGATCTTTTCTTCAAAGAC
SUMO-p53mtF: TTCCGGACAGAGTGAGCACTGGGCAGCACACCCGGA
SUMO-p53mtB: TCACTCTGTCCGGAAGCCGATGATCTTTTCTTCAAAGAC
SUMO-PMLmtF: CTCCGGCACGAGTGAGCACTGGGCAGCACACCCGGA
SUMO-PMLmtB: TCACTCGTGCCGGAGGCCGATGATCTTTTCTTCAAAGAC
SUMO-DualF: TTCAAGACAGAGCTCAAGCACGAGTGAGCACTGGGCAGCACACCCGGA
SUMO-DualB: TCACTCGTGCTTGAGCTCTGTCTTGAAGCCGATGATCTTTTCTTCAAAGAC
Dual-p53mtF: ATCATCGGCTTCCGGACAGAGCTCAAGCACGAGTGAGCACTG
Dual-p53mtB: CAGTGCTCACTCGTGCTTGAGCTCTGTCCGGAAGCCGATGAT
Dual-PMLmtF: ATCATCGGCTTCAAGACAGAGCTCCGGCACGAGTGAGCACTG
Dual-PMLmtB: CAGTGCTCACTCGTGCCGGAGCTCTGTCTTGAAGCCGATGAT
Dual-mt2F: ATCATCGGCTTCCGGACAGAGCTCCGGCACGAGTGAGCACTG
Dual-mt2B: CAGTGCTCACTCGTGCCGGAGCTCTGTCCGGAAGCCGATGAT
RESULTS
Axin-ΔC6 has shorter half-life than full-length Axin
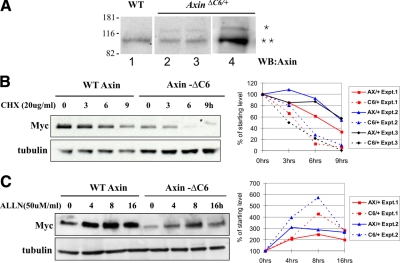
In mouse embryos heterozygous for the AxinΔC6 allele, we found that the steady-state level of Axin-ΔC6 protein was severalfold lower than that of full-length endogenous Axin (Fig. 1A). The same was true in mouse embryonic fibroblasts (MEFs) isolated from E13.5 embryos heterozygous for AxinΔC6 (Fig. 1A). To determine whether this low steady-state level might reflect a decreased stability of the mutant protein, we examined the turnover rate of Axin-ΔC6 compared to wild-type Axin. To perform these measurements under conditions where Axin is expressed at physiological levels, we used MEFs isolated from embryos heterozygous for either the AxinAx and AxinΔC6 alleles (AxinAx encodes a Myc-tagged form of wild-type Axin) (17). The MEFs were treated with cycloheximide (CHX) to block protein synthesis, and the level of Myc-Axin or Myc-Axin-ΔC6 was determined by Western blotting with anti-Myc antibody at various times thereafter. As shown in Fig. 1B, when the Axin C6 motif was removed, the half-life in the presence of CHX was reduced from approximately 8 to 4 h. The half-life of wild-type Axin was similar to the value of 8 h reported for Myc-tagged rat Axin in COS cells (10).
Figure 1.
Axin-ΔC6 has a shorter half-life than full-length Axin. A) In AxinΔC6/+ E13.5 embryos (lanes 2 and 3) or MEFs derived from such embryos (lane 4), Axin-ΔC6 (*) is present at a much lower level than endogenous Axin (**) when both are detected with anti-Axin antibody. Lane 1 shows analysis of a wild-type (WT) embryo. Axin-ΔC6 is larger than WT, endogenous Axin because it contains 6 Myc tags. B) Myc-Axin-ΔC6 (in AxinΔC6/+ MEFs) turns over more rapidly than WT Myc-Axin (in AxinAx/+ MEFs). AxinAx/+ and AxinΔC6/+ MEFs were cultured with 20 μg/ml of cycloheximide (CHX) for the indicated number of hours. The level of WT or mutant Myc-Axin encoded by the modified Axin locus was measured by Western blotting using anti-Myc antibody at different time points. The band in the Axin-ΔC6, 9 h lane, above the position of Axin, is a background band (*). Graph shows the results of three independent experiments. C) Proteasome-specific inhibitor ALLN stabilizes Axin-ΔC6 more than WT Axin. AxinAx/+ and AxinΔC6/+ MEFs were incubated with 50 μM of ALLN for the indicated times, and anti-Myc Western blotting was performed. The results show that the level Axin-ΔC6 protein increases more than WT Axin when proteasome degradation is inhibited. Graph shows the results of two independent experiments. Graphs plot independent experiments in different colors: solid lines, WT Axin; dotted lines, Axin-ΔC6. The levels of Axin or Axin-ΔC6 protein at each time point were normalized to the starting level (100%).
To examine the possibility that the accelerated turnover of Axin-ΔC6 occurred through the 26S proteasome-mediated degradation of ubiquitinated proteins, MEFs heterozygous for AxinAx or AxinΔC6 were treated with the proteasome inhibitor ALLN, and the Axin levels were compared by Western blotting with anti-Myc antibody. The level of wild-type Axin increased approximately 2- to 3-fold after treatment with ALLN, suggesting that degradation of Axin is mediated by the proteasome, whereas Axin-ΔC6 was stabilized to a greater extent, 4- to 5-fold (Fig. 1C). Together, these data show that the deletion of the C6 motif of Axin causes an enhanced protein turnover rate, due to degradation by the proteasome.
The C6 motif and the sites for binding and phosphorylation by GSK3 protect Axin from ubiquitination
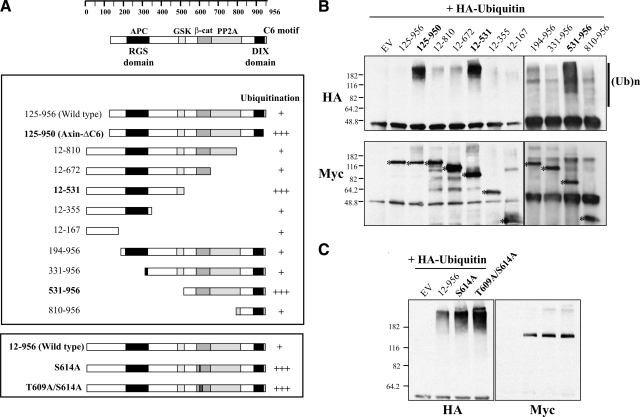
If the level of Axin protein were regulated by proteasome-mediated degradation, Axin should be susceptible to ubiquitination. To examine this, we cotransfected Myc-tagged full-length (125–956) and ΔC6 (125–950) forms of Axin into HEK 293T cells together with HA-tagged ubiquitin and then treated them with ALLN for 3 h to allow the ubiquitinated Axin proteins to accumulate. Axin was immunoprecipitated using anti-Myc, and ubiquitinated proteins were detected by Western blotting with anti-HA antibody. A very small fraction of the full-length Axin (125–956) was polyubiquitinated, whereas Axin-ΔC6 (125–950) showed a large increase in polyubiquitination (Fig. 2A, B), consistent with its reduced stability. The role of the C6 motif in protection from polyubiquitination is further examined below (Figs. 3and 4).
Figure 2.
The C6 motif and the GSK3 binding and phosphorylation sites are important to protect Axin from polyubiquitination. A) Diagrams of Axin constructs used and summary of the polyubiquitination levels in the assay. Top: diagram indicates homology domains and some known binding sites for other proteins. The amino acid numbering from 1 to 956 is based on the open reading frame described by Zeng et al. (4). Axin 125–956 is the predominant form of Axin initiating at Met125, whereas Axin 12–956 includes an additional 5′ open reading frame, which encodes a longer form of the protein (4, 18). The extent of polyubiquitination observed with each construct, relative to WT Axin, from three independent experiments is indicated by plus signs. B) Ubiquitination assay with different forms of Axin. HA-tagged ubiquitin and the indicated full-length and deletion or mutant Myc-tagged Axin constructs were transfected into HEK 293T cells, and the cells were treated with 50 μM of ALLN for 3 h to prevent degradation and allow accumulation of ubiquitinated forms of Axin. Top panel: cell lysates were immunoprecipitated with anti-Myc antibody, and the resulting immunoprecipitates were subjected to Western blotting with anti-HA antibody to reveal polyubiquitinated forms of Axin, as indicated by (UB)n in the top panel. Lanes labeled in bold showed consistently increased ubiquitination compared to WT Axin (125–956, or 12–956 in C). Bottom panel: Western blot with anti-Myc to reveal the Axin bands (asterisks). C) Increased ubiquitination of mutant forms of Axin in which T609 and S614, phosphorylation sites for GSK3, were replaced with Alanine. EV, empty vector.
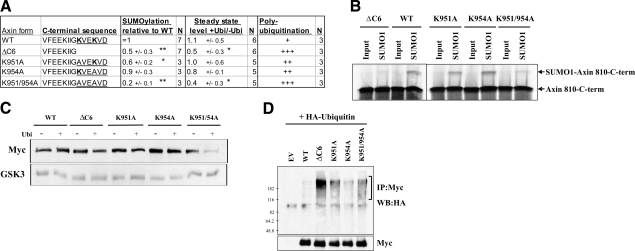
Figure 3.
The Axin C6 motif is a target of SUMOylation in vitro and protects Axin from ubiquitination in HEK 293T cells. A) Sequence of the C-terminal end of WT and mutant forms of Axin, and average values obtained in the assays shown in B–D. Conserved lysine residues in the C6 domain of WT Axin are shown in bold; conserved SUMOylation motifs (KVE/D) are underlined. Values are averages ± sd; N independent measurements. *P < 0.05, **P < 0.01 vs. WT Axin; t test. Level of polyubiquitination is estimated from three experiments. B) In vitro SUMOylation assay with Myc-tagged wild-type or mutant Axin fragments (from amino acid 810 to the C terminus). The Axin fragments were translated and labeled in vitro with [35S]methionine and subsequently incubated with an active SUMO-1 modification mixture containing or lacking (input) SUMO-1, then separated by SDS-PAGE and detected by autoradiography. Values listed in A indicate the average amounts of SUMOylated protein, relative to WT Axin 810–956 (set to 1). Deletion of the C6 motif (ΔC6), or substitution of alanine for lysine 951 (K951A) or both lysine residues in the C6 motif (K951/954A), significantly reduced the SUMOylation of Axin. C) Effects of ubiquitin on the stability of WT and mutant Axin. The indicated Myc-tagged Axin constructs (1 μg) were cotransfected with empty vector (EV) or HA-ubiquitin (1 μg), and the steady-state levels of Axin were measured by Western blotting with anti-Myc. GSK3 was detected as a loading control. Numbers in A indicate the average ratio of the steady-state Axin levels with or without ubiquitin for each construct. Axin-ΔC6 showed a decreased stability compared to WT Axin in the presence of ubiquitin, and substitution of A for both K951 and K954 also significantly reduced the stability of Axin. D) Ubiquitination assay with Myc-tagged full-length Axin (WT) or mutant Axin. HEK 293T cells were cotransfected with the indicated Axin constructs and HA-ubiquitin. After 20 h, the cells were treated with ALLN for 3 h, then lysed, immunoprecipitated with anti-Myc, and analyzed by Western blotting with anti-HA antibody. The higher-molecular-weight species indicated by the bracket are the polyubiquitinated forms of Axin. Like deletion of C6, replacement of K951 and K954 with alanine caused strongly increased ubiquitination, whereas replacement of either lysine reside with alanine caused moderately increased ubiquitination.
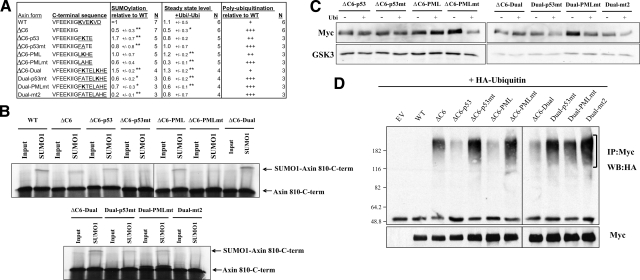
Figure 4.
The addition of exogenous SUMOylation sites restores the stability of Axin-ΔC6 and its resistance to ubiquitination. A) Sequences of the C-terminal regions of WT and mutant Axin. ΔC6-p53, ΔC6-PML, and ΔC6-Dual are constructs in which SUMOylation sites from p53 (FKTE including K386) PML (LKHE including K160), or both, were added to Axin-ΔC6; “mt” indicates site-directed mutants in which the lysine resides in the SUMOylation sites were replaced with alanine. SUMOylation motifs are underlined; lysine residues are shown in bold. Values are averages ± sd. *P < 0.05, **P < 0.01; t test: ΔC6 vs. WT, ΔC6-p53 or ΔC6-PML vs. ΔC6, ΔC6-p53mt vs. ΔC6-p53, ΔC6-PML-mt vs. ΔC6-PML, ΔC6-Dual vs. ΔC6, and D-p53mt, D-PMLmt and Dual-mt2 vs. Dual. Polyubiquitination levels are averages of three different experiments. B) In vitro SUMOylation assay with the indicated WT or mutant Axin fragments (amino acid 180 to the indicated C terminus). Average levels of SUMOylated Axin, normalized to wild-type Axin (set to 1), are indicated in A. Addition of one or both artificial SUMOylation sites to AxinΔC6 increased its ability to be SUMOylated, whereas mutation of the lysine residues reduced or eliminated this effect. C) Effects of ubiquitin on the stability of WT and mutant Axin. The indicated Myc-tagged Axin constructs (1 μg) were cotransfected with empty vector (–Ubi) or 1 μg of HA-ubiquitin vector (+Ubi), and the levels of Axin protein were analyzed by Western blotting with anti-Myc. The protein level in the presence of ubiquitin was normalized to that without ubiquitin, for each construct. In comparison to AxinΔC6, which showed decreased stability with ubiquitin (Fig. 3C), the addition of the p53, PML, or both SUMOylation sites partially or completely restored its stability in the presence of ubiquitin. Replacement of the lysine residues within the SUMOylation sites with alanine reduced or abolished their effects on stability (with the exception of the lysine in the p53 site in construct ΔC6-p53mt, which showed highly variable results in different experiments). GSK3 was used as a loading control. D) Ubiquitination of AxinΔC6 variants, after cotransfection with HA-ubiquitin (as in Fig. 3D), was detected by Western blotting with anti-HA antibody. Polyubiquitinated forms of Axin are indicated by brackets. Average levels of polyubiquitinated Axin, from three experiments, are indicated in A. Addition of SUMOylation sequences from p53, PML, or both protected Axin-ΔC6 from excess ubiquitination, whereas mutation of the target lysine residues reduced or eliminated this protective effect, resulting in increased polyubiquitination.
To identify the domains of Axin that are either targets for ubiquitination, or otherwise required for its ubiquitination, we examined several mutant forms of Axin in the same assay. All the deletion mutants we tested were ubiquitinated to some extent, and two nonoverlapping Axin fragments (12–531 and 531–956) were highly ubiquitinated, likely indicating multiple ubiquitin target sites in Axin (Fig. 2A, B). The first of these two deletion mutants (Axin531–956) is missing the GSK3 binding domain, whereas the second (Axin12–531) lacks the major phosphorylation sites for GSK3, T609, and S614 (20). This observation is consistent with previous reports that the dephosphorylation of Axin following a Wnt signal leads to its destabilization (10, 11). As Axin residues threonine 609 and serine 614 are known to be physiological target sites for phosphorylation by GSK3, we examined ubiquitination using full-length Axin constructs with substitutions of alanine for S614, or both T609 and S614, to ascertain further whether the phosphorylation by GSK3 is necessary for Axin’s stability. Consistent with our expectations, the S614A and T609A/S614A mutants displayed high levels of polyubiquitination (Fig. 2A, C). Together, these experiments suggest that the binding and phosphorylation sites for GSK3, as well as the C6 motif, are important to protect Axin from ubiquitination.
SUMOylation sites in the C6 motif protect Axin from ubiquitination
As the two lysine residues in the C6 motif have been implicated as major sites for the SUMOylation of Axin (6), and the C6 motif is also required for the normal stability of Axin (Fig. 1) and its protection from ubiquitination (Fig. 2), we investigated the potential role of SUMOylation in these processes. First, we reexamined the ability of Axin to be SUMOylated on the C6 motif. We were unable to detect the SUMOylation of Axin after cotransfection into HEK 293T cells with HA-tagged SUMO-1 (data not shown). However, we found that Axin could be SUMOylated in vitro and therefore employed the in vitro assay to test the role of the C6 motif. Full-length Axin lacking only the C6 motif was SUMOylated at a comparable level to the wild type (data not shown), perhaps because there are eight other potential SUMOylation sites (ψKxE, where ψ is a hydrophobic residue and x is any amino acid) (15) throughout the Axin protein sequence (6). However, when we used an ∼140 amino acid C-terminal fragment of Axin as the substrate, to exclude some of the other potential SUMOylation sites from the analysis, Axin (810–950) lacking the C6 motif was 2-fold less SUMOylated than Axin (810–956) (Fig. 3A, B). Although replacement of lysine 954 with alanine (K954A) had no effect, replacement of lysine 951 with alanine (K951A) in the C6 motif caused a significant reduction in SUMOylation of Axin (810–956), and the double lysine mutant K951A/K954A displayed a greater reduction in SUMOylation (Fig. 3A). Although these two lysine residues are located in nonconsensus binding sites lacking the usual hydrophobic residue preceding the lysine, several other SUMO-1-modified proteins, including proliferating cell nuclear antigen (PCNA), death domain-associated protein (Daxx), and cAMP-responsive element binding protein (CREB) also have similar nonconsensus binding sites (21,22,23).
To examine the effects of the C-terminal SUMOylation target motifs on protein stability, the various forms of full-length Myc-Axin were transfected into HEK 293T cells with or without HA-tagged ubiquitin. After 20 h the cells were lysed, and the relative steady-state levels of Myc-Axin were determined by Western blotting (Fig. 3A, C). In this assay, the double substitution of alanine for both lysine 951 and lysine 954 (K951A/K954A) reduced the level of Axin (i.e., increased its sensitivity to ubiquitin) similarly to deletion of the C6 domain (Fig. 3A).
To test the role of C-terminal SUMOylation target sites in the protection of Axin from ubiquitination, we performed a ubiquitination assay using Myc-tagged wild-type or mutant full-length Axin (Fig. 3D). When cotransfected with HA-ubiquitin, Axin K951A and K954A mutants were more heavily ubiquitinated than wild-type Axin (but less so than Axin-ΔC6), whereas the double mutant K951A/K954A was ubiquitinated at levels similar to those of Axin-ΔC6 (Fig. 3A). Together, these results show that two lysine residues in the C6 motif, which are potential targets for SUMOylation, also protect Axin from ubiquitination. Although no strict correlation was found among the level of SUMOylation, ubiquitination, and stability for all the mutant constructs tested, the two constructs with the lowest SUMOylation (Axin-ΔC6 and K951/954A) also showed the lowest stability and the highest ubiquitination (Fig. 3A), suggesting that these three phenomena are related. Thus, the results suggest a model in which SUMOylation of Axin in the C6 motif interferes with ubiquitination elsewhere in the protein, protecting it from degradation.
The addition of exogenous SUMOylation target sites restores the protein stability of Axin-ΔC6
To test further the hypothesis that the SUMOylation of Axin at its C terminus protects it from ubiquitination and degradation, we added consensus SUMOylation motifs from two different SUMO substrate proteins, p53 and PML, in place of the C6 motif. We examined the ability of p53 and PML to serve as SUMOylation targets in vitro, as well as their effects on the ubiquitination and stability of the protein in transfected cells (Fig. 4). Addition of the SUMOylation site from p53 (FKTE, construct ΔC6-p53) (24), the SUMOylation site from PML (LKHE, construct ΔC6-PML) (25), or both of these sequences (ΔC6-Dual) increased the ability of the Axin C-terminal fragment (810–950) to be SUMOylated in vitro. Mutation of the lysine residues in the p53 or the PML SUMOylation sites reduced this effect, whereas mutation of both lysine residues virtually abolished SUMOylation (Fig. 4A, B).
To examine the effects of these heterologous SUMOylation sequences on stability of Axin, we expressed the various forms of full-length Myc-Axin in HEK 293T cells transfected with (or without) HA-tagged ubiquitin and examined the steady-state levels of the various forms of Myc-Axin, in the presence vs. the absence of ubiquitin (Fig. 4A, C). The addition of the PML SUMOylation site (ΔC6-PML) or both SUMOylation sites (ΔC6-Dual) to Axin-ΔC6 restored its steady-state level to the wild-type level (presumably reflecting an increased stability) when cotransfected with ubiquitin. Substitution of alanine for the lysine residue in the PML SUMOylation site eliminated its stabilizing effect on the level of Axin (ΔC6-PMLmt), as did alanine substitutions for either lysine in ΔC6-Dual (Dual-p53mt, Dual-PMLmt). Overall, these results show that the addition of heterologous SUMOylation motifs to the C terminus of Axin can substitute for the normal C6 motif in protecting Axin from ubiquitin-mediated instability.
Measurements of the susceptibility of the same Axin mutants to polyubiquitination revealed that the stabilization of Axin by the addition of heterologous SUMOylation sites can be largely explained by protection from ubiquitination (Fig. 4A, D). Axin-ΔC6-p53, Axin-ΔC6-PML, and Axin-ΔC6-Dual showed lower levels of polyubiquitination than Axin-ΔC6, whereas mutation of the lysine residue in these SUMOylation sites eliminated their protective effect. Therefore, in general, an inverse correlation can be found between the stability (as reflected in the steady-state level) and the susceptibility to ubiquitination of the different Axin variants. Furthermore, these parameters are strongly affected by the presence or absence of SUMOylation target sites, either natural or heterologous, at the C terminus.
Dvl-1 shows a stronger association with Axin-ΔC6 than with wild-type Axin but is independent of the presence of SUMOylation sites in the C6 motif
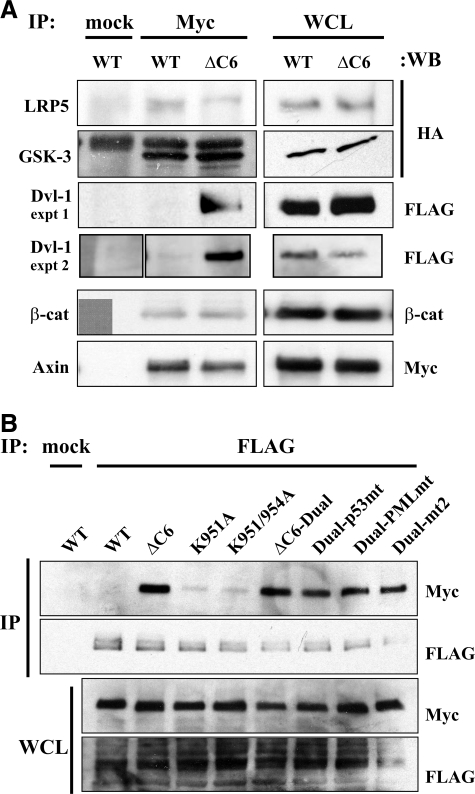
To investigate why the C6 motif, or heterologous SUMOylation sites, might protect Axin from ubiquitination and degradation, we examined the interaction of wild-type Axin or Axin-ΔC6 with different components of the canonical Wnt signaling pathway. Myc-Axin was coexpressed in HEK 293T cells with FLAG-Axin (full-length), HA-LRP5, FLAG-Dvl-1, HA-GSK3β, or β-catenin, and the cell lysates were immunoprecipitated with anti-Myc antibody and blotted with anti-HA, anti-FLAG, or anti-β-catenin antibodies. Equal amounts of FLAG-Axin coimmunoprecipitated with Myc-Axin or Myc-Axin-ΔC6 (data not shown), confirming that the C6 motif is not required for the dimerization of Axin (6). The amounts of LRP5, GSK3β, and β-catenin coimmunoprecipitated with Axin-ΔC6 vs. wild-type Axin were also similar, but an increased amount of Dvl-1 coimmunoprecipitated with Axin-ΔC6 compared to wild-type Axin (Fig. 5). This finding was intriguing because it suggested a possible link between binding of Axin to Dvl and the destabilization of Axin, both of which are thought to occur following exposure of the cell to a Wnt signal (3, 9,10,11, 26,27,28).
Figure 5.
Increased association of Axin-ΔC6 with Dvl-1. A) Axin-ΔC6 displays an increased association with Dvl1 compared to WT Axin, but not compared to the other proteins examined. Cells were cotransfected with Myc-tagged WT or ΔC6 Axin and HA-LRP5, HA-GSK3, FLAG-Dvl-1, or β-catenin, then immunoprecipitated (IP) with anti-Myc or mouse IgG (mock), and blotted with the indicated antibodies. Expression of transfected Myc-Axin constructs and the cotransfected proteins in whole cell lysates (WCL, right) shows that the increased coimmunoprecipitation of Dvl-1 with Axin-ΔC6 is not due to increased expression of Dvl-1. B) Myc-tagged WT Axin (125–956), Axin-ΔC6 (125–950), Axin lysine mutants (K951A, K951/954A), and Axin-ΔC6 with WT (ΔC6-Dual) or mutant heterologous SUMOylation sites (Dual-p53mt, Dual-PMLmt, Dual-mt2) were coexpressed with FLAG-Dvl-1. Top panels: the cell lysates were immunoprecipitated with anti-FLAG, and immunoprecipitates were detected with anti-Myc or anti-FLAG antibodies. Bottom panels: expression of transfected Myc-Axin constructs and FLAG-Dvl-1 in whole cell lysates.
To ask whether the presence of SUMOylation target lysine residues in the C6 motif affected its ability to bind to Dvl-1, we examined the coimmunoprecipitation of several additional Axin mutants with Dvl-1. The mutants K951A and K951A/K954A displayed little or no binding to Dvl-1 under the conditions of the assay; thus, they behaved like wild-type Axin, not Axin-ΔC6. Furthermore, the addition of heterologous SUMOylation sites, with or without lysine mutations, to Axin-ΔC6 did not affect its increased binding to Dvl-1. Therefore, we conclude that the decreased association of Dvl-1 with wild-type Axin, compared to Axin-ΔC6, is due to some specific inhibitory feature of the C6 motif, other than its ability to be SUMOylated. Thus, no apparent correlation exists between the ubiquitination and instability of different C-terminal forms of Axin and their ability to associate with Dvl-1 in this assay.
DISCUSSION
Axin is a scaffold protein for the β-catenin destruction complex and a negative regulator of canonical Wnt signaling. The steady-state level of Axin is very low, suggesting that it is the limiting component of the destruction complex and that modulation of its stability may be important for Wnt signaling. In the present study, we sought to explain why the deletion of the six C-terminal amino acids of Axin (the C6 motif) reduces its steady-state level by severalfold and therefore generates an embryonic lethal allele in mice (unpublished results). We first showed that Axin–ΔC6 has a reduced half-life compared to the wild-type protein. We then showed that Axin is susceptible to ubiquitination in multiple regions of the protein and that the C6 motif as well as the GSK3 binding and target sites are necessary to protect Axin from ubiquitination. As the C6 motif had been described as a target for SUMOylation, we confirmed that this sequence is a potential SUMOylation target in vitro and found a correlation among 1) the presence of the SUMOylation target motifs, 2) the steady-state level of Axin when cotransfected with ubiquitin, and 3) the resistance of Axin to ubiquitination. In addition, we showed that SUMOylation target sites from the heterologous proteins p53 or PML can replace the Axin C6 motif in providing this protective effect. Together, these findings suggest a model in which the SUMOylation of Axin on the C6 motif may play a role in regulating its ubiquitination and thus its stability and consequent abundance in the cell.
Although Axin plays an important role in the degradation of β-catenin through the ubiquitin-proteasome pathway and is itself subject to degradation following a Wnt signal (10, 11, 27, 28), the ubiquitination of Axin has not been previously reported. Here, we show that Axin is susceptible to polyubiquitination when it is coexpressed with ubiquitin in cultured cells, strongly suggesting that Axin also may be ubiquitinated in vivo and that this is likely to be important to control its stability. Axin can be ubiquitinated at multiple regions of the protein, as shown by deletion analysis, although the specific target lysine residues have not been identified. In addition to the C6 motif, discussed below, another sequence that appeared to protect Axin from ubiquitination was the GSK3 phosphorylation target residue S614 (and possibly also T609), as shown by mutagenesis of one or both residues (Fig. 2). Furthermore, a fragment of Axin that contained T609 and S614 but lacked the GSK3 binding site (Axin 531–956) was also heavily ubiquitinated. These findings are consistent with the observation that phosphorylation by GSK3 stabilizes Axin (10, 11), and they indicate that the mechanism of stabilization involves protection from ubiquitination. How the phosphorylation of these residues may prevent ubiquitination of Axin is unclear, but it might act either by altering the conformation of the protein or by altering its association with other proteins. The ubiquitin ligases responsible for ubiquitination of Axin are not yet known, but it is interesting that Axin can bind to the E3 ubiquitin ligase Arkadia (29,30,31) and promote the ubiquitination and degradation of Smad7 (32); therefore, Arkadia is also a candidate for an Axin ubiquitin ligase.
The Axin C6 motif, which was the major subject of our studies, also protects Axin from ubiquitination (Fig. 2), which probably accounts for its effects on protein stability (Fig. 1B). We found that Axin-ΔC6 has an ∼2-fold lower half-life than wild-type Axin, which accounts, at least in part, for its lower steady-state level in the mutant mouse embryo (Fig. 1A). Although the actual half-life of Axin is probably influenced by many factors (e.g., cell type, culture conditions), and the values we measured for wild-type Axin in cultured cells probably do not reflect its in vivo half-life, the relative instability of Axin-ΔC6 most likely persists under different conditions.
By mutating one or both lysines in the C6 motif (K951 and K954), we found that these residues are critical for the ability of the C6 motif to protect Axin from ubiquitination. They are unlikely to be targets of ubiquitination themselves, as deletion of the C6 motif, or replacement of one or both lysines with alanine, increased total polyubiquitination of full-length Axin, whereas the C-terminal 810–956 fragment of Axin was itself susceptible to only minimal polyubiquitination (Fig. 3). Therefore, the C6 motif perhaps influences the ability of Axin to associate with other proteins, which in turn affect its ubiquitination. One intriguing hypothesis, which we investigated, is based on the prior observation that the C6 motif is important for the interaction of Axin with the SUMO-1 conjugating enzymes PIAS1, PIASxβ, and PIASy and is itself a target for SUMOylation (6). Furthermore, it was shown that residues K951 and K954 in the C6 motifs could themselves be SUMOylated when Axin was cotransfected with SUMO-1 into HEK 293T cells, and these residues appeared to be the major targets of SUMOylation in Axin (6). Although we were unable to detect the SUMOylation of Axin in similar cotransfection experiments—perhaps because of the often transient nature of SUMO modification (15)—we found that Axin could be SUMOylated in vitro and that K951, and possibly also K954, appeared to be SUMOylation target residues (Fig. 3). Thus, we demonstrated a strong inverse correlation between the ability of Axin to be SUMOylated on the C6 motif and its susceptibility to ubiquitination and destabilization. Although it remains to be determined whether Axin is actually SUMOylated in vivo, our results raise the possibility that the SUMOylation of Axin on the C6 motif might protect it from ubiquitination at other sites, and thus control its stability.
This hypothesis received further support from the observation that the addition of heterologous SUMOylation target sites (from two other proteins that are known SUMO-1 targets, p53 and PML) in place of the C6 motif decreased Axin’s ubiquitination and increased its stability, whereas mutation of the target lysine residues in these heterologous SUMOylation sites diminished or eliminated their effects (Fig. 4). The mechanism by which the SUMOylation of Axin on the C6 motif might protect it from ubiquitination at other sites is unclear. Several proteins, including IκBα, NEMO/IKKγ, PCNA, and Huntingtin, are known to be protected from ubiquitination by SUMOylation of the same target lysine residues (15, 16). In the case of Axin, it is possible that SUMO modification of the C6 motif either alters its interactions with other proteins, which in turn affect its ubiquitination at other lysine residues, or else SUMOylation may alter Axin’s subcellular localization, as it is known to do for many other proteins (15, 16).
To test the former hypothesis, we examined the ability of Axin vs. Axin-ΔC6 to interact with several other components of the canonical Wnt pathway. Although full-length Axin immunoprecipitated only weakly with Dvl-1 under the conditions of the assay, interestingly, Axin-ΔC6 showed a greatly increased association with Dvl-1, indicating that the C6 motif diminishes this interaction. However, unlike the SUMOylation and ubiquitination of Axin, the interaction of Axin with Dvl-1 was not altered by mutating the lysine residues in the C6 motif, nor could heterologous SUMOylation motifs replace the C6 motif in this assay. Therefore, some other specific property of the C6 motif seems to reduce the interaction of Axin with Dvl-1. Because the DIX domain, which plays a role in Axin-Dvl interactions (33,34,35) is very close to the C terminus, it is possible that the C6 motif interferes with interaction between the DIX domain of Axin and Dvl.
Because the degradation of Axin may play an important role in the transmission of a Wnt signal (3, 12), it will be interesting in the future to determine whether the ubiquitination of Axin is altered by Wnt signaling and whether the SUMOylation of the C6 motif plays a regulatory role in this process.
Acknowledgments
This work was supported by grant HD44265 to F.C. from the National Institute of Child Health and Human Development. We thank Zaiqi Wu for excellent technical assistance, David Virshup (Huntsman Cancer Institute, Salt Lake City, UT, USA) for the anti-Axin antiserum, and Eek-hoon Jho (University of Seoul, Seoul, South Korea) and Wei Hsu (University of Rochester, Rochester, NY, USA) for comments on the manuscript.
References
- Luo W, Lin S C. Axin: a master scaffold for multiple signaling pathways. Neurosignals. 2004;13:99–113. doi: 10.1159/000076563. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Cadigan K M, Liu Y I. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek T J, Perry W L I, Lee J J, Tilghman S M, Gumbiner B M, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner M W. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui H L, Fan E, Zhou H M, Xu Z, Zhang Y, Lin S C. SUMO-1 modification of the C-terminal KVEKVD of Axin is required for JNK activation but has no effect on Wnt signaling. J Biol Chem. 2002;277:42981–42986. doi: 10.1074/jbc.M208099200. [DOI] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- Cliffe A, Hamada F, Bienz M. A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol. 2003;13:960–966. doi: 10.1016/s0960-9822(03)00370-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of Axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwinski N S, Wieschaus E. Rethinking WNT signaling. Trends Genet. 2004;20:177–181. doi: 10.1016/j.tig.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Neo S Y, Wang X, Han J, Lin S C. Axin forms a complex with MEKK1 and activates c-Jun NH(2)-terminal kinase/stress-activated protein kinase through domains distinct from Wnt signaling. J Biol Chem. 1999;274:35247–35254. doi: 10.1074/jbc.274.49.35247. [DOI] [PubMed] [Google Scholar]
- Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 2004;18:2046–2059. doi: 10.1101/gad.1214604. [DOI] [PubMed] [Google Scholar]
- Hay R T. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Dohmen R J. SUMO protein modification. Biochim Biophys Acta. 2004;1695:113–131. doi: 10.1016/j.bbamcr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Chia I V, Costantini F. Mouse axin and axin2/conductin proteins are functionally equivalent in vivo. Mol Cell Biol. 2005;25:4371–4376. doi: 10.1128/MCB.25.11.4371-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F. Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol. 1999;145:741–756. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M J, Park B J, Kang Y S, Kim H J, Park J H, Kang J W, Lee S W, Han J M, Lee H W, Kim S. Downregulation of FUSE-binding protein and c-myc by tRNA synthetase cofactor p38 is required for lung cell differentiation. Nat Genet. 2003;34:330–336. doi: 10.1038/ng1182. [DOI] [PubMed] [Google Scholar]
- Jho E, Lomvardas S, Costantini F. A GSK3beta phosphorylation site in axin modulates interaction with beta-catenin and Tcf-mediated gene expression. Biochem Biophys Res Commun. 1999;266:28–35. doi: 10.1006/bbrc.1999.1760. [DOI] [PubMed] [Google Scholar]
- Comerford K M, Leonard M O, Karhausen J, Carey R, Colgan S P, Taylor C T. Small ubiquitin-related modifier-1 modification mediates resolution of CREB-dependent responses to hypoxia. Proc Natl Acad Sci U S A. 2003;100:986–991. doi: 10.1073/pnas.0337412100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoege C, Pfander B, Moldovan G L, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- Jang M S, Ryu S W, Kim E. Modification of Daxx by small ubiquitin-related modifier-1. Biochem Biophys Res Commun. 2002;295:495–500. doi: 10.1016/s0006-291x(02)00699-x. [DOI] [PubMed] [Google Scholar]
- Muller S, Berger M, Lehembre F, Seeler J S, Haupt Y, Dejean A. c-Jun and p53 activity is modulated by SUMO-1 modification. J Biol Chem. 2000;275:13321–13329. doi: 10.1074/jbc.275.18.13321. [DOI] [PubMed] [Google Scholar]
- Boutell C, Orr A, Everett R D. PML residue lysine 160 is required for the degradation of PML induced by herpes simplex virus type 1 regulatory protein ICP0. J Virol. 2003;77:8686–8694. doi: 10.1128/JVI.77.16.8686-8694.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Jones K A. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr G H, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Tolwinski N S, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4:407–418. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Koinuma D, Shinozaki M, Komuro A, Goto K, Saitoh M, Hanyu A, Ebina M, Nukiwa T, Miyazawa K, Imamura T, Miyazono K. Arkadia amplifies TGF-beta superfamily signalling through degradation of Smad7. EMBO J. 2003;22:6458–6470. doi: 10.1093/emboj/cdg632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederlander C, Walsh J J, Episkopou V, Jones C M. Arkadia enhances nodal-related signalling to induce mesendoderm. Nature. 2001;410:830–834. doi: 10.1038/35071103. [DOI] [PubMed] [Google Scholar]
- Episkopou V, Arkell R, Timmons P M, Walsh J J, Andrew R L, Swan D. Induction of the mammalian node requires Arkadia function in the extraembryonic lineages. Nature. 2001;410:825–830. doi: 10.1038/35071095. [DOI] [PubMed] [Google Scholar]
- Liu W, Rui H, Wang J, Lin S, He Y, Chen M, Li Q, Ye Z, Zhang S, Chan S C, Chen Y G, Han J, Lin S C. Axin is a scaffold protein in TGF-beta signaling that promotes degradation of Smad7 by Arkadia. EMBO J. 2006;25:1646–1658. doi: 10.1038/sj.emboj.7601057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yuan H, Weaver C D, Mao J, Farr G H, 3rd, Sussman D J, Jonkers J, Kimelman D, Wu D. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 1999;18:4233–4240. doi: 10.1093/emboj/18.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M A, Schelbert B, Hsu W, Fitzpatrick E, Jho E, Fagotto F, Costantini F, Kitajewski J. Domains of axin and disheveled required for interaction and function in Wnt signaling. Biochem Biophys Res Commun. 2000;276:1162–1169. doi: 10.1006/bbrc.2000.3607. [DOI] [PubMed] [Google Scholar]