Abstract
Primary hepatocytes from several different species rapidly lose viability and phenotypic functions on isolation from their native microenvironment of the liver. Stromal cells derived from both within and outside the liver can induce phenotypic functions in primary hepatocytes in vitro; however, the molecular mediators underlying this “coculture effect” have not been fully elucidated. We have previously developed a functional genomic screen utilizing cocultures of hepatocytes and 3T3 fibroblasts to identify such candidate hepatocyte-function-inducing molecules. In particular, truncated-cadherin (T-cadherin) was identified as a potential molecule of interest in induction of hepatic functions. Here we demonstrate that liver-specific functions of primary rat hepatocytes are induced on cocultivation with Chinese hamster ovary cells engineered to express T-cadherin on their surface as compared with wild-type controls. Additionally, culture of cells on substrata presenting recombinant T-cadherin protein (acellular presentation) enhanced hepatic functions in both pure hepatocyte cultures and in hepatocyte-stromal cocultures lacking endogenous T-cadherin expression. Collectively, these data indicate that both cellular and acellular presentation of T-cadherin can be used to modulate the hepatocyte phenotype in vitro for tissue engineering applications. Our work suggests potential avenues for investigating the role of T-cadherin on hepatocellular function in vivo in settings such as embryogenesis and liver pathology.—Khetani, S. R., Chen, A. A., Ranscht, B., Bhatia, S. N. T-cadherin modulates hepatocyte functions in vitro.
Keywords: CHO, fibroblasts, coculture, cytochrome P450
Engineered liver tissue is finding utility in pharmaceutical drug development and the development of cell-based therapies for liver disease (1,2,3). Such tissue models are also useful for elucidating the mechanisms underlying liver development, regeneration, and disease pathogenesis (4, 5). Primary hepatocytes are generally considered as the “gold standard” for constructing robust in vitro liver tissues (6); however, these cells display a precipitous decline in viability and phenotypic functions on isolation from their native in vivo microenvironment (7, 8). Over the last few decades, investigators have employed a plethora of different strategies to preserve liver-specific functions in vitro and extend the lifetime of the model systems. These strategies typically include extracellular matrix (ECM) manipulations (e.g., Matrigel substratum or collagen sandwich culture), defined culture media, manipulation of cell-cell interactions by forming three-dimensional spheroidal aggregates or cocultivation with nonparenchymal cell types, and introduction of fluid flow in bioreactors (4, 6, 9,10,11,12,13,14,15).
Cocultivation of hepatocytes with stromal cells has been found to be a particularly robust method for “rescuing” the phenotype of both rodent and human hepatocytes (3, 4, 7, 10); however, for tissue engineering applications, one may seek to replace the stromal cells with acellular components (such as biomaterial coatings and media additives) that do not consume nutrients and precious space. Some molecules have been identified that play a role, such as liver-regulating protein, E-cadherin, TGF-β1, and decorin (16,17,18,19,20,21) (7); however, none of these molecules are sufficient to replace the stromal cells completely nor are they all expressed by all cell types known to rescue the hepatocyte phenotype. Using a functional genomic screen (7), we identified a novel candidate, truncated-cadherin (T-cadherin, also referred to as CDH13 or H-cadherin), as a molecule upregulated in liver-function-inducing stromal cells.
In contrast to classical cadherins (e.g., E-cadherin), which are transmembrane proteins linked to the actin cytoskeleton via signaling molecules such as catenins (22), T-cadherin lacks both transmembrane and cytoplasmic domains and is instead anchored to the cell membrane through a glycosylphosphatidylinositol (GPI) moiety (23, 24). T-cadherin can mediate calcium-dependent adhesion; however, it is not concentrated at cell-cell junctions of transfected cells in culture (23, 25). The presence of T-cadherin in membrane domains enriched in other GPI-anchored proteins as well as signaling molecules such as Src family kinases suggests that T-cadherin may be involved in intercellular signaling (26). T-cadherin has been shown to play diverse roles in physiology and pathophysiology, including negative guidance cue for motor axon projections, tumor suppressor factor in various types of cancer, an atypical lipoprotein-binding protein, and stimulator of angiogenesis (24, 27, 28). Recently T-cadherin was shown to be a receptor for the high-molecular-weight isoforms of the hormone adiponectin, which is known to synergize with insulin to increase glycogen stores and suppress gluconeogenesis in the liver (29). In normal liver samples, T-cadherin is expressed in endothelial cells of large blood vessels and in myofibroblasts, weakly expressed in sinusoidal endothelial cells, and absent in hepatocytes (30). Its role in modulating hepatocyte function (in vitro or in vivo) remains unknown. In this study we therefore explore the potential for T-cadherin to modulate differentiated functions in primary rat hepatocytes (albumin secretion, urea synthesis, and CYP1A1 activity) when presented in a cellular context or as an isolated recombinant protein presented on a polymeric surface.
MATERIALS AND METHODS
Rat hepatocyte isolation and culture
Primary rat hepatocytes were isolated from 2- to 3-month-old adult female Lewis rats (Charles River Laboratories, Wilmington, MA, USA) weighing 180–200 g by a modified procedure of Seglen’s (31). Detailed procedures for hepatocyte isolation and purification were previously described (7). Routinely, 200–300 million cells were isolated with 85–95% viability, as judged by trypan-blue exclusion. Stromal cells, as judged by their size (<10 μm diameter) and morphology (nonpolygonal), were less than 1%. Hepatocyte culture medium consisted of Dulbecco’s Modified Eagle’s medium (DMEM from Invitrogen, Carlsbad, CA, USA) with high-glucose, 10% (v/v) fetal bovine serum (FBS), 0.5 U/ml insulin, 7 ng/ml glucagon, 7.5 μg/ml hydrocortisone, and 1% (v/v) penicillin-streptomycin (pen/strep).
Stromal cell culture
Chinese hamster ovary (CHO) cells were transfected by calcium phosphate coprecipitation with pcD-Tcad (plasmid containing the coding region of T-cadherin) and pSV2neo (plasmid-carrying neomycin resistance, American Type Culture Collection, Rockville, MD, USA) as described previously (28). CHO cells were cultured at 37°C with 5% CO2 in minimal essential medium (MEM, Alpha GlutaMAX™ 1× with ribonucleosides and deoxyribonucleosides, Invitrogen) supplemented with 10% FBS, 0.1 mM sodium hypoxanthine, 0.016 mM thymidine (1× hypoxanthine-thymidine or HT supplement), 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 1% (v/v) pen/strep. Mouse embryonic fibroblasts (MEFs) were the gift of James Thomson (University of Wisconsin, Madison, WI, USA). MEF culture medium consisted of DMEM with 10% fetal bovine serum, 0.1 mM nonessential amino acids, and 1% (v/v) pen/strep.
Generation of recombinant histidine-tagged T-cadherin protein
Mouse T-cadherin cDNA was amplified by polymerase chain reaction (PCR) (using mTcad in PBS as a template) with a forward primer set at the initiator codon and a FLAG-His6-tagged reverse primer set at 100 bp upstream from a unique HindIII site. The obtained PCR product was ligated into the pCEP4 mammalian vector (Invitrogen) and transfected into 293 cells with the Polyfect transfectant reagent according to the manufacturer’s instructions (Qiagen, Valencia, CA, USA). T-cadherin fusion protein-expressing cells were selected and expanded in media containing 300 μg/ml hygromycin. Serum-free culture medium supernatant was concentrated with an Amicon concentrator, cell debris removed by ultracentrifugation, fusion protein purified over a nickel column according to the manufacturer’s instructions (Qiagen), and its purity checked by electrophoresis and silver staining (Sekisu Media Co., LTD., Tokyo, Japan).
Preparation of protein-modified substrates
Tissue culture-treated plates were coated by adsorption of 100 μg/ml collagen (type-I) in water for 1 h at 37°C. Purification of collagen from rat-tail tendons was previously described (11). Briefly, rat-tail tendons were denatured in acetic acid, salt-precipitated, dialyzed against hydrochloric acid, and sterilized with chloroform. For certain experiments, tissue culture polystyrene was first coated with Ni-NTA (nickel bound to nitrilotriacetic acid), followed by incubation with histidine-tagged T-cadherin protein dissolved in calcium supplemented (1 mM) phosphate buffered saline solution (Ca2+ PBS) for 3 h at 37°C. Excess T-cadherin solution was aspirated, and substrates were further coated with type-I collagen (1 μg/ml in Ca2+ PBS for 1 h at 37°C) to promote hepatocyte attachment.
Hepatocyte-stromal cocultures
Protein-coated culture dishes were seeded with hepatocytes (3×105 cells/10 cm2) in hepatocyte culture medium (1 ml/10 cm2). For coculture experiments, CHO cells (∼1×106cells/10 cm2) or MEFs (∼6×105 cells/10 cm2) were seeded in their respective medium 12–24 h after initiation of adherent hepatocyte cultures. The stromal culture medium was replaced to hepatocyte culture medium 24 h later and subsequently replaced daily.
RNAi-mediated knockdown of T-cadherin in CHO cells
T-cadherin-transfected CHOs were treated with 50 nM siRNA (siGENOME SMARTpool reagent M-049465; Dharmacon, Lafayette, CO, USA) targeted against the T-cadherin (also known as CDH13) mRNA sequence (accession number NM_019707). siRNA was delivered via cationic liposome transfection reagent (Lipofectamine 2000, Invitrogen) according to manufacturer’s instructions. Briefly, 100 pmol liposome reagent was diluted to 250 μl with 1× DMEM and incubated at room temperature for 15 min. Also, 50 nM siRNA, diluted to 250 μl with 1× DMEM, was then mixed with liposome dilution and incubated an additional 15 min. Cells were incubated with the liposome-siRNA complexes in 1 ml total serum-free medium. Six hours after transfection, serum-free medium was replaced with serum-supplemented CHO culture medium. After 2–3 h, CHO cells treated with siRNA were trypsinized and plated onto adherent hepatocyte cultures on collagen to create cocultures.
Hepatocellular function assays
Spent media was stored at −20°C. Urea concentration was assayed using a colorimetric end point assay utilizing diacetylmonoxime with acid and heat (Stanbio Labs, Boerne, TX, USA). Albumin content was measured using enzyme-linked immunosorbent assays (MP Biomedicals, Irvine, CA, USA) with horseradish peroxidase detection and 3,3′,5,5′-tetramethylbenzidine (TMB, Fitzgerald Industries, Concord, MA) as substrate. Cytochrome P450 1A1 (CYP1A1) activity was assessed via dealkylation of ethoxy-resorufin (ER; Sigma, St. Louis, MO, USA) into fluorescent resorufin. Briefly, cultures were incubated with 5 μM ER dissolved in DMEM without phenol red for 30–60 min. Resorufin fluorescence (excitation/emission: 530/590 nm) in collected supernatants was quantified by means of a fluorescence microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Microscopy
Specimens were observed and recorded using a Nikon Diaphot microscope equipped with a SPOT digital camera (SPOT Diagnostic Equipment, Sterling Heights, MI, USA) and MetaMorph Image Analysis System (Universal Imaging, West Chester, PA, USA) for digital image acquisition.
Statistical analysis
Experiments were repeated 2–3 times with separate rats and 3 replicate wells for each condition. For functional assays, one representative outcome is presented where similar trends were observed in multiple trials. Statistical significance was determined using Student’s t test or one-way ANOVA (analysis of variance) and Tukey’s post hoc test on Prism software (GraphPad Software, San Diego, CA, USA). All error bars represent sem with n = 3.
RESULTS
Induction of hepatocyte functions on cocultivation with T-cadherin-transfected CHO cells
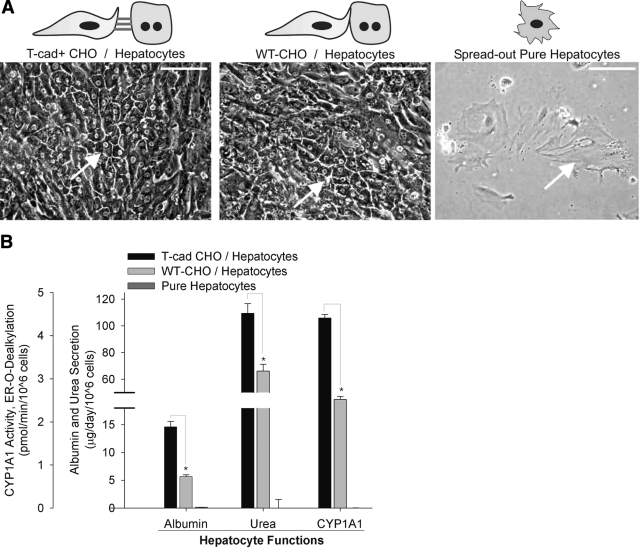
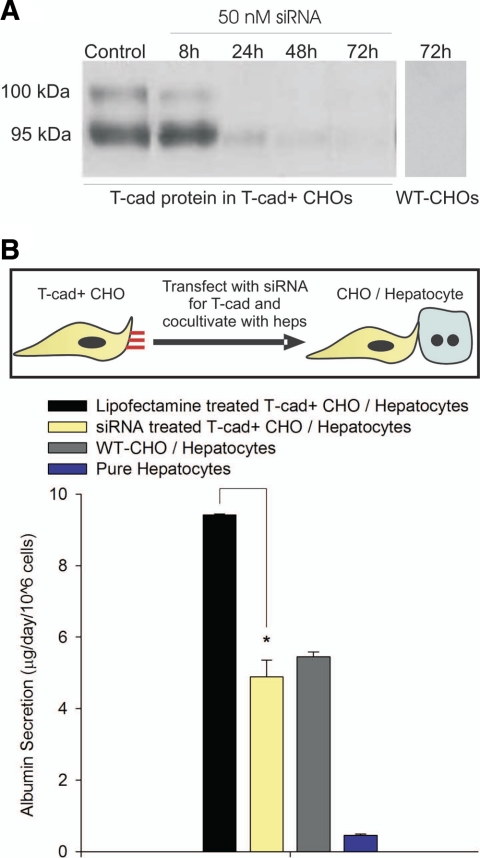
To determine whether presentation of T-cadherin to primary rat hepatocytes in a cellular context could induce liver-specific functions, we cocultivated hepatocytes with either CHO cells that overexpressed T-cadherin (T-cad+ CHO/hepatocyte) on their surface or null wild-type CHO cells (WT-CHO/hepatocyte). Consistent with our previous studies (7), we observed here that pure hepatocytes adherent on collagen-coated tissue culture polystyrene adopted a “fibroblastic” morphology (Fig. 1A) and lost phenotypic functions within a few days of in vitro culture. However, hepatocyte morphology on cocultivation with CHO cells (WT-CHO and T-cad+ CHO cells) was similar to the polygonal morphology of freshly isolated cells with distinct nuclei/nucleoli and appearance of bile canaliculi. We next measured distinct liver-specific functions in the various culture models. In particular, albumin secretion, urea synthesis, and cytochrome P450 1A1 (CYP1A1) activity were assessed as surrogate markers for liver-specific protein synthesis, nitrogen metabolism, and detoxification functions, respectively. We found that hepatic functions were ∼2–3-fold higher in the T-cad+ CHO/hepatocyte model than in the WT-CHO/hepatocyte control (Fig. 1B). Furthermore, the functional effects of T-cadherin were observed for several weeks in vitro (Supplemental Fig. 1). To demonstrate specificity of T-cadherin in induction of hepatic functions, we transfected T-cad+ CHO cells with siRNA specific to T-cadherin mRNA prior to initiation of cocultures. Our results indicated that T-cadherin protein in T-cad+ CHO cells was knocked down to negligible levels for at least 72 h posttransfection (Fig. 2A). Furthermore, resultant liver-specific functions in cocultures containing siRNA-transfected T-cad+ CHO cells were down-regulated by ∼50% as compared with mock-transfected controls (Fig. 2B).
Figure 1.
Cocultivation of primary rat hepatocytes with WT-CHO or T-cad+ CHO cells on collagen-coated substrates. A) Morphology (representative day 15) of cocultures and pure hepatocyte cultures (hepatocytes indicated by arrows). Scale bars = 100 μm. B) Hepatocyte functions (representative day 10) in pure cultures and cocultures. Similar trends were seen for at least 2 wk of culture in 3 independent biological repeat experiments. *P < 0.05; 2-tailed, unpaired Student’s t test. Error bars = sem (n=3).
Figure 2.
RNAi-mediated knockdown of T-cadherin protein in CHO cells and effects on hepatic functions. A) Western blot showing silencing of T-cadherin protein in Tcad+ CHO cells up to 72 h. Control lanes contain protein from T-cad+ CHO cells treated with lipofectamine only. The 2 bands represent the 95 kDa mature T-cadherin protein and the 100 kDa prepeptide. B) Hepatic albumin secretion (representative day 9) in cocultures containing T-cad+ CHO cells treated with either lipofectamine or lipofectamine complexed with T-cad siRNA, cocultures containing WT-CHO cells, and pure hepatocytes. Similar trends were seen for at least 2 wk of culture in 3 independent biological repeat experiments. *P < 0.05; 1-way ANOVA with Tukey’s post hoc test. Error bars = sem (n=3).
Induction of hepatocyte functions on substrates presenting recombinant T-cadherin protein
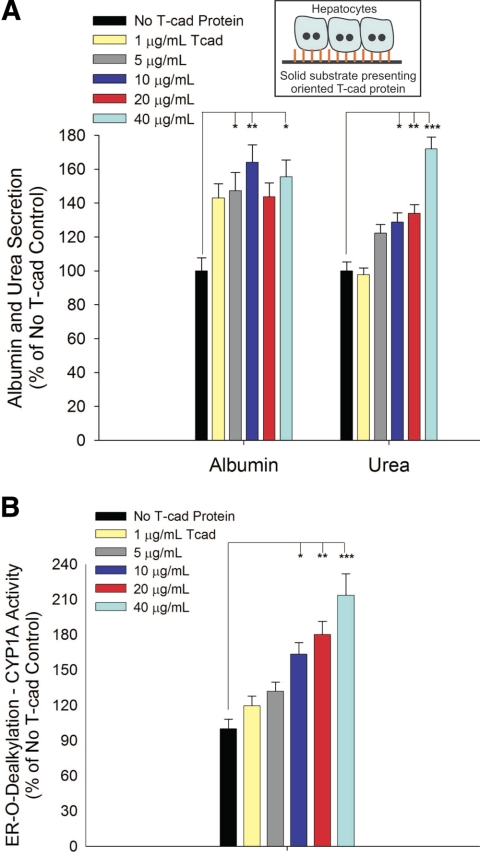
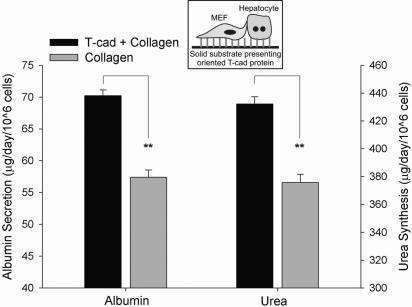
After demonstrating that cellular presentation of T-cadherin (i.e., transfected CHO cesss) can induce liver-specific functions in hepatocytes, we sought to determine whether similar responses could be obtained on utilization of purified, recombinant T-cadherin. We first oriented histidine-tagged T-cadherin onto tissue culture polystyrene using an Ni-NTA based strategy (see Methods and Materials for details). Next, we conducted hepatocyte attachment studies on substrates presenting increasing levels of T-cadherin. Our results indicated that hepatocytes did not attach to oriented T-cadherin up to 100 μg/ml, and therefore we adsorbed type-I collagen after T-cadherin coating to promote hepatocyte attachment. In Fig. 3 we show that hepatocyte functions were subsequently induced on T-cadherin/collagen substrates. Albumin secretion on T-cadherin/collagen substrates increased to 143% of collagen-only controls at the lowest T-cadherin protein concentration utilized (1 μg/ml), increasing to 164% at 10 μg/ml and then declining slightly with increasing T-cadherin coating densities. In contrast, urea synthesis and CYP1A1 activity in hepatocytes increased monotonically with T-cadherin coating densities greater than 1 μg/ml (172% urea synthesis and 213% CYP1A1 activity over collagen-only controls at 40 μg/ml T-cadherin coating density). Furthermore, dose-dependent up-regulation of hepatic functions on T-cadherin-coated substrates was observed for several weeks in culture (Supplemental Fig. 2).
Figure 3.
Phenotypic functions of hepatocytes adhered to substrates presenting recombinant T-cadherin protein. A) Albumin and urea secretion data represents average values for days 5–14 of culture. B) CYP1A activity (as evaluated by ER o-dealkylation) on a representative day 10 is shown. Similar trends were seen for at least 2 wk in 2 independent biological repeat experiments. *P < 0.05, **P < 0.01, ***P < 0.001; 1-way ANOVA and Tukey’s post hoc test. Error bars = sem (n=3).
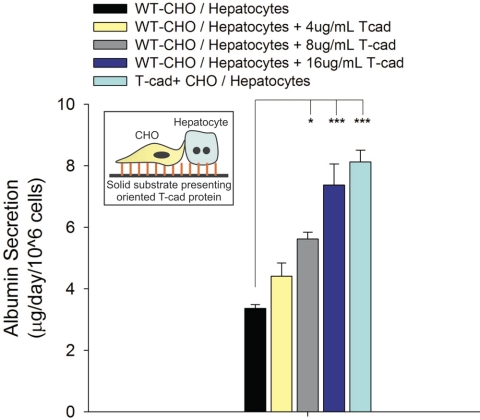
Purified T-cadherin induced hepatocyte functions in vitro; however, it was not able to prevent the eventual loss of viability and differentiated functions characteristic of pure hepatocyte monolayers on collagen. Therefore, we sought to determine whether purified T-cadherin could up-regulate hepatocyte functions in models of the liver that are relatively stable at baseline: cocultivation of hepatocytes and nonparenchymal cells. To isolate the effects of acellular T-cadherin in this model, we cocultured hepatocytes with stromal cells lacking T-cadherin expression, namely, WT-CHO cells and MEFs. In Fig. 4, we show that recombinant T-cadherin protein induced albumin secretion in WT-CHO/hepatocyte cocultures in a dose-dependent manner. Furthermore, albumin secretion in WT-CHO/hepatocyte cocultures on the highest coating density of acellular T-cadherin approached levels similar to those measured in cocultures of T-cad+ CHO cells and hepatocytes. Next, we created cocultures of hepatocytes and MEFs on T-cadherin-coated substrates to determine whether hepatocyte response to T-cadherin could be observed in coculture models utilizing stromal cells from different species. In Fig. 5 we show up-regulation of liver-specific functions (albumin secretion and urea synthesis) in MEF/hepatocyte cocultures that were seeded on substrates coated with T-cadherin (4 μg/ml) and collagen (1 μg/ml).
Figure 4.
Albumin secretion by rat hepatocytes cocultivated with WT-CHO cells on substrates presenting increasing concentrations of recombinant T-cadherin protein. Function in cocultures containing T-cad+ CHO cells (no recombinant T-cadherin protein present on substrate) is shown for reference. Data represent cumulative albumin secretion for days 8–14 of coculture. Similar trends were seen for at least 2 wk in 2 independent biological repeat experiments. *P < 0.05, **P < 0.01, ***P < 0.001; 1-way ANOVA and Tukey’s post hoc test. Error bars = sem (n=3).
Figure 5.
Albumin secretion by rat hepatocytes cocultivated with T-cadherin null MEFs on substrates presenting increasing concentrations of recombinant T-cadherin protein. Data represent cumulative albumin secretion for days 3–10 of coculture from one representative experiment, whereas similar trends were seen for at least 2 wk in 2 independent biological repeat experiments. **P < 0.01; 2-tailed, unpaired Student’s t test. Error bars = sem (n=3).
DISCUSSION
Rapid loss of phenotypic functions in isolated primary hepatocytes poses a key challenge for engineering highly functional models of liver tissues (1, 2). Thus, one of the goals of hepatic tissue engineering has been to identify bioactive factors that can interface with hepatocytes within the context of a biomaterial (synthetic or natural) and induce key liver-specific functions (16, 32). In a previous study, we utilized gene expression profiling of different subclones of 3T3 fibroblasts that induce phenotypic functions in primary hepatocytes to identify potential molecular mediators of liver-specific functions (7). T-cadherin, in particular, was one of the candidate molecules identified through this functional genomic screen, expressed close to 40-fold in highly inductive cell types as compared with low inducers. Here we demonstrate that T-cadherin alone can indeed induce a diverse set of phenotypic functions in primary rat hepatocytes in both cellular (bound to membrane of a secondary cell) and acellular (recombinant protein on a substrate) contexts.
We first developed a coculture model in which CHO cells engineered to express T-cadherin on their surface interacted with hepatocytes to stabilize their phenotypic functions. Our results showed that the synthetic (albumin secretion), metabolic (urea synthesis), and detoxification (CYP1A1 activity) functions of hepatocytes were enhanced in the T-cadherin-positive coculture model as compared to wild-type null controls. We further demonstrated specificity of the T-cadherin-mediated hepatocyte response via RNA interference. Next, we showed that purified T-cadherin protein oriented on a solid substrate induced hepatocyte functions in pure cultures. However, T-cadherin was not able to rescue pure hepatocytes from an eventual decline in viability and liver-specific functions, which is consistent with previous findings suggesting that several molecules may coordinate to produce a differentiated hepatocyte phenotype (7, 18). To evaluate the role of T-cadherin protein in a relatively stable model of the liver, we created on T-cadherin-coated substrates cocultures in which the stromal cells lacked endogenous T-cadherin expression (e.g., WT-CHO and MEFs). As with pure hepatocytes, we found that T-cadherin induced liver-specific functions in hepatocytes on cocultivation with WT-CHO cells. With increasing T-cadherin coating densities, hepatic function in WT-CHO/hepatocyte cocultures approached levels similar to those measured in cocultures containing T-cad+ CHO cells on collagen alone. Furthermore, cocultivation of rat hepatocytes with mouse embryonic fibroblasts (negative for T-cadherin; ref 7) on T-cadherin-coated substrates also caused up-regulation of hepatic functions.
In developing the CHO-hepatocyte coculture model, we discovered that WT-CHO cells had an intrinsic ability to stabilize phenotypic functions of primary rat hepatocytes. Although viability and functions in pure hepatocyte monolayers declined to negligible levels after only a few days of cultures, these parameters remained relatively stable for 2–3 wk in CHO-hepatocyte cocultures. Introduction of T-cadherin protein on the CHO cell surface caused further up-regulation (2–3-fold on average over wild-type controls) of hepatic functions for several weeks. Thus, use of a “stable” CHO-hepatocyte coculture model that is not confounded by declining hepatic viability and phenotypic functions is particularly advantageous for elucidating the role of various molecular signals on liver-specific functions. This coculture model can also be used to present cell surface molecules in their native state to hepatocytes. We specifically chose CHO cells for this study because of their lack of liver-specific functions, their ready availability, and the ease with which they can be genetically engineered to express various proteins. Last, CHO cells can be engineered to express multiple molecules with potential roles in modulating the hepatic phenotype.
Our recent studies suggest that to stabilize liver-specific functions in cocultures, heterotypic cell-cell contact between primary hepatocytes and stromal cells (e.g., murine embryonic 3T3 fibroblasts) is required for ∼18–24 h before continuous stimulation with only stromal-derived soluble signals (33). However, the use of stromal cells to induce hepatic functions in culture systems may pose several key challenges including overgrowth of stromal cells leading to nutrient and oxygen depravation, surface area limitations, and difficulty in distinguishing the hepatic molecular signals from the stromal background. Thus, it is desirable to replace stromal cells by coating biomaterials with recombinant cell contact molecules that can interact with hepatocytes to mimic initial heterotypic cell signaling, followed by continuous stimulation with stromal-derived soluble factors. In this study we wanted to evaluate whether T-cadherin could serve as a contact signal to up-regulate hepatic functions. Although supplementation of culture medium with T-cadherin did not induce hepatocyte functions to any considerable degree (data not shown), presentation of T-cadherin on a culture surface with collagen enhanced hepatocyte functions in a dose-dependent manner for at least 2 wk as compared to control cultures on collagen alone. Thus, we believe that T-cadherin may indeed serve as one of the stromal-derived cell adhesion molecules for designing robust bioartifical microenvironments for primary hepatocytes.
In this study we show a role of T-cadherin in modulating hepatic functions in vitro; however, the binding partner of stromal-derived T-cadherin on the hepatocyte surface is not clear. In freshly isolated hepatocytes, we did not detect either T-cadherin protein via Western blotting or its corresponding mRNA transcript, a finding that is consistent with the literature showing lack of T-cadherin expression in hepatocytes from normal liver samples (30). However, T-cadherin expression in sinusoidal endothelial cells and hepatocytes may increase in vitro on stimulation with soluble factors such as FGF-2 and adipocyte-derived soluble factors (Yarmush lab, unpublished observations) and in vivo in hepatocellular carcinomas (30). Thus, it is possible that stromal-derived T-cadherin in our cultures interacts with T-cadherin that is up-regulated on the hepatocyte surface over several days in culture. Such a hypothesis is consistent with the long time course for functional up-regulation we observed here (Supplemental Figs. 1 and 2). Heterophilic interactions of T-cadherin with hepatocyte-derived integrins, growth factor receptors, and other types of cadherins (e.g., E- and N-cadherin) are another possible mode of interaction (34,35,36). Indeed, T-cadherin has been shown to engage in heterophilic interactions with adiponectin and low-density lipoproteins, initiate signal transduction cascades, and influence a variety of cell fate processes (e.g., proliferation, migration, protection against oxidative stress) (27, 29, 30, 37).
In conclusion, this study presents a novel role of T-cadherin in modulation of liver-specific functions in freshly isolated primary hepatocytes from healthy rat livers. Our study is merely the first step in elucidating the role of T-cadherin as a regulator of the hepatic phenotype. We anticipate that our work will spawn future investigations of T-cadherin-mediated signaling in liver both in vitro and in vivo. Last, T-cadherin and other molecular mediators of hepatic differentiation may prove useful for engineering optimal microenvironments that keep hepatocytes and other cell types of the liver viable and highly functional for weeks to months.
Supplementary Material
Acknowledgments
We thank Jennifer Felix and Kathryn Hudson for rat hepatocyte isolation, Craig Sharp for assistance with cell culture and biochemical assays, Sandra March for critical review of the manuscript, and James Thomson for providing mouse embryonic fibroblasts. Funding was provided by National Science Foundation (NSF) Graduate Fellowships (S.R.K, A.A.C.), National Institutes of Health (NIH) grant HD 25938 (B.R), NSF CAREER, NIH-National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and the David and Lucile Packard Foundation.
References
- Allen J W, Bhatia S N. Engineering liver therapies for the future. Tissue Eng. 2002;8:725–737. doi: 10.1089/10763270260424097. [DOI] [PubMed] [Google Scholar]
- Hewitt N J, Lechon M J, Houston J B, Hallifax D, Brown H S, Maurel P, Kenna J G, Gustavsson L, Lohmann C, Skonberg C, Guillouzo A, Tuschl G, Li A P, LeCluyse E, Groothuis G M, Hengstler J G. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007;39:159–234. doi: 10.1080/03602530601093489. [DOI] [PubMed] [Google Scholar]
- Khetani S R, Bhatia S N. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2007;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- Bhatia S N, Balis U J, Yarmush M L, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- Mesnil M, Fraslin J M, Piccoli C, Yamasaki H, Guguen-Guillouzo C. Cell contact but not junctional communication (dye coupling) with biliary epithelial cells is required for hepatocytes to maintain differentiated functions. Exp Cell Res. 1987;173:524–533. doi: 10.1016/0014-4827(87)90292-8. [DOI] [PubMed] [Google Scholar]
- Guillouzo A. Liver cell models in in vitro toxicology. Environ Health Perspect. 1998;106:511–532. doi: 10.1289/ehp.98106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khetani S R, Szulgit G, Del Rio J A, Barlow C, Bhatia S N. Exploring interactions between rat hepatocytes and nonparenchymal cells using gene expression profiling. Hepatology. 2004;40:545–554. doi: 10.1002/hep.20351. [DOI] [PubMed] [Google Scholar]
- Guguen-Guillouzo C, Guillouzo A. Modulation of functional activities in cultured rat hepatocytes. Mol Cell Biochem. 1983;53–54:35–56. doi: 10.1007/BF00225245. [DOI] [PubMed] [Google Scholar]
- Bissell D M, Arenson D M, Maher J J, Roll F J. Support of cultured hepatocytes by a laminin-rich gel: evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987;79:801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlu A, Ilyin G, Cariou S, Lamy I, Loyer P, Guguen-Guillouzo C. The coculture: a system for studying the regulation of liver differentiation/proliferation activity and its control. Cell Biol Toxicol. 1997;13:235–242. doi: 10.1023/a:1007475122321. [DOI] [PubMed] [Google Scholar]
- Dunn J C, Tompkins R G, Yarmush M L. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- Gebhardt R, Hengstler J G, Muller D, Glockner R, Buenning P, Laube B, Schmelzer E, Ullrich M, Utesch D, Hewitt N, Ringel M, Hilz B R, Bader A, Langsch A, Koose T, Burger H J, Maas J, Oesch F. New hepatocyte in vitro systems for drug metabolism: metabolic capacity and recommendations for application in basic research and drug development, standard operation procedures. Drug Metab Rev. 2003;35:145–213. doi: 10.1081/dmr-120023684. [DOI] [PubMed] [Google Scholar]
- Reid L M, Narita M, Fujita M, Murray Z, Liverpool Z, Rosenberg L. Matrix and hormonal regulation of differentiation in liver cultures. Guillouzo A, Guguen-Guillouzo C, editors. Paris: Les Editions INSERM and John Libbey Eurotext; Isolated and Cultured Hepatocytes. 1986:226–258. [Google Scholar]
- Eschbach E, Chatterjee S S, Noldner M, Gottwald E, Dertinger H, Weibezahn K F, Knedlitschek G. Microstructured scaffolds for liver tissue cultures of high cell density: morphological and biochemical characterization of tissue aggregates. J Cell Biochem. 2005;95:243–255. doi: 10.1002/jcb.20360. [DOI] [PubMed] [Google Scholar]
- Sivaraman A, Leach J K, Townsend S, Iida T, Hogan B J, Stolz D B, Fry R, Samson L D, Tannenbaum S R, Griffith L G. A microscale in vitro physiological model of the liver: predictive screens for drug metabolism and enzyme induction. Curr Drug Metab. 2005;6:569–591. doi: 10.2174/138920005774832632. [DOI] [PubMed] [Google Scholar]
- Chia S M, Lin P C, Yu H. TGF-beta1 regulation in hepatocyte-NIH3T3 co-culture is important for the enhanced hepatocyte function in 3D microenvironment. Biotechnol Bioeng. 2005;89:565–573. doi: 10.1002/bit.20372. [DOI] [PubMed] [Google Scholar]
- Corlu A, Kneip B, Lhadi C, Leray G, Glaise D, Baffet G, Bourel D, Guguen-Guillouzo C. A plasma membrane protein is involved in cell contact-mediated regulation of tissue-specific genes in adult hepatocytes. J Cell Biol. 1991;115:505–515. doi: 10.1083/jcb.115.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieva T A, Moghe P V. Functional engineering of hepatocytes via heterocellular presentation of a homoadhesive molecule, E-cadherin. Biotechnol Bioeng. 2001;76:295–302. doi: 10.1002/bit.10041. [DOI] [PubMed] [Google Scholar]
- Goulet F, Normand C, Morin O. Cellular interactions promote tissue-specific function, biomatrix deposition and junctional communication of primary cultured hepatocytes. Hepatology. 1988;8:1010–1018. doi: 10.1002/hep.1840080506. [DOI] [PubMed] [Google Scholar]
- Hamilton G A, Jolley S L, Gilbert D, Coon D J, Barros S, LeCluyse E L. Regulation of cell morphology and cytochrome P450 expression in human hepatocytes by extracellular matrix and cell-cell interactions. Cell Tissue Res. 2001;306:85–99. doi: 10.1007/s004410100429. [DOI] [PubMed] [Google Scholar]
- Rojkind M, Novikoff P M, Greenwel P, Rubin J, Rojas-Valencia L, de Carvalho A C, Stockert R, Spray D, Hertzberg E L, Wolkoff A W. Characterization and functional studies on rat liver fat-storing cell line and freshly isolated hepatocyte coculture system. Am J Pathol. 1995;146:1508–1520. [PMC free article] [PubMed] [Google Scholar]
- Marrs J A, Nelson W J. Cadherin cell adhesion molecules in differentiation and embryogenesis. Int Rev Cytol. 1996;165:159–205. doi: 10.1016/s0074-7696(08)62222-6. [DOI] [PubMed] [Google Scholar]
- Ranscht B, Dours-Zimmermann M T. T-cadherin, a novel cadherin cell adhesion molecule in the nervous system lacks the conserved cytoplasmic region. Neuron. 1991;7:391–402. doi: 10.1016/0896-6273(91)90291-7. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Ohtsuki Y. Recent progress in T-cadherin (CDH13, H-cadherin) research. Histol Histopathol. 2001;16:1287–1293. doi: 10.14670/HH-16.1287. [DOI] [PubMed] [Google Scholar]
- Vestal D J, Ranscht B. Glycosyl phosphatidylinositol–anchored T-cadherin mediates calcium-dependent, homophilic cell adhesion. J Cell Biol. 1992;119:451–461. doi: 10.1083/jcb.119.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippova M P, Bochkov V N, Stambolsky D V, Tkachuk V A, Resink T J. T-cadherin and signal-transducing molecules co-localize in caveolin-rich membrane domains of vascular smooth muscle cells. FEBS Lett. 1998;429:207–210. doi: 10.1016/s0014-5793(98)00598-5. [DOI] [PubMed] [Google Scholar]
- Joshi M B, Philippova M, Ivanov D, Allenspach R, Erne P, Resink T J. T-cadherin protects endothelial cells from oxidative stress-induced apoptosis. FASEB J. 2005;19:1737–1739. doi: 10.1096/fj.05-3834fje. [DOI] [PubMed] [Google Scholar]
- Fredette B J, Miller J, Ranscht B. Inhibition of motor axon growth by T-cadherin substrata. Development. 1996;122:3163–3171. doi: 10.1242/dev.122.10.3163. [DOI] [PubMed] [Google Scholar]
- Hug C, Wang J, Ahmad N S, Bogan J S, Tsao T S, Lodish H F. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi Y, Takeuchi T, Sonobe H, Ohtsuki Y. An adiponectin receptor, T-cadherin, was selectively expressed in intratumoral capillary endothelial cells in hepatocellular carcinoma: possible cross talk between T-cadherin and FGF-2 pathways. Virchows Arch. 2006;4481:311–318. doi: 10.1007/s00428-005-0098-9. [DOI] [PubMed] [Google Scholar]
- Seglen P O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Brieva T A, Moghe P V. Engineering the hepatocyte differentiation-proliferation balance by acellular cadherin micropresentation. Tissue Eng. 2004;10:553–564. doi: 10.1089/107632704323061915. [DOI] [PubMed] [Google Scholar]
- Hui E E, Bhatia S N. Micromechanical control of cell-cell interactions. Proc Natl Acad Sci U S A. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippova M, Ivanov D, Joshi M B, Kyriakakis E, Rupp K, Afonyushkin T, Bochkov V, Erne P, Resink T J. Identification of proteins associating with GPI-anchored T-cadherin on the surface of vascular endothelial cells: the role for Grp78/BiP in T-cadherin-dependent cell survival. Mol Cell Biol. 2008;28:4004–4017. doi: 10.1128/MCB.00157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko T, Fetisova E, Ivanova O, Bonder E M, Feder H, Vasiliev J M, Gelfand I M. Contact interactions between epitheliocytes and fibroblasts: formation of heterotypic cadherin-containing adhesion sites is accompanied by local cytoskeletal reorganization. Proc Natl Acad Sci U S A. 2001;98:8632–8637. doi: 10.1073/pnas.151247698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M S, McNutt P M. Cadherins and their connections: adhesion junctions have broader functions. Curr Opin Cell Biol. 1999;11:554–560. doi: 10.1016/s0955-0674(99)00027-7. [DOI] [PubMed] [Google Scholar]
- Tkachuk V A, Bochkov V N, Philippova M P, Stambolsky D V, Kuzmenko E S, Sidorova M V, Molokoedov A S, Spirov V G, Resink T J. Identification of an atypical lipoprotein-binding protein from human aortic smooth muscle as T-cadherin. FEBS Lett. 1998;421:208–212. doi: 10.1016/s0014-5793(97)01562-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.