Abstract
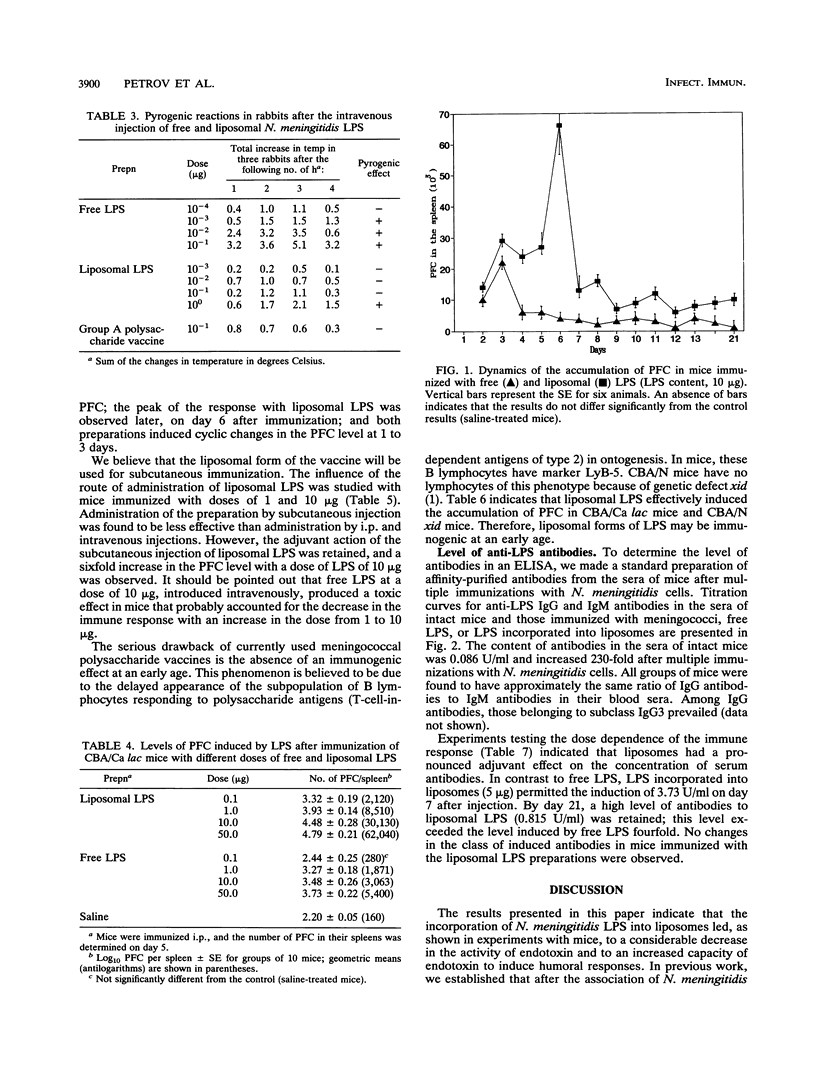
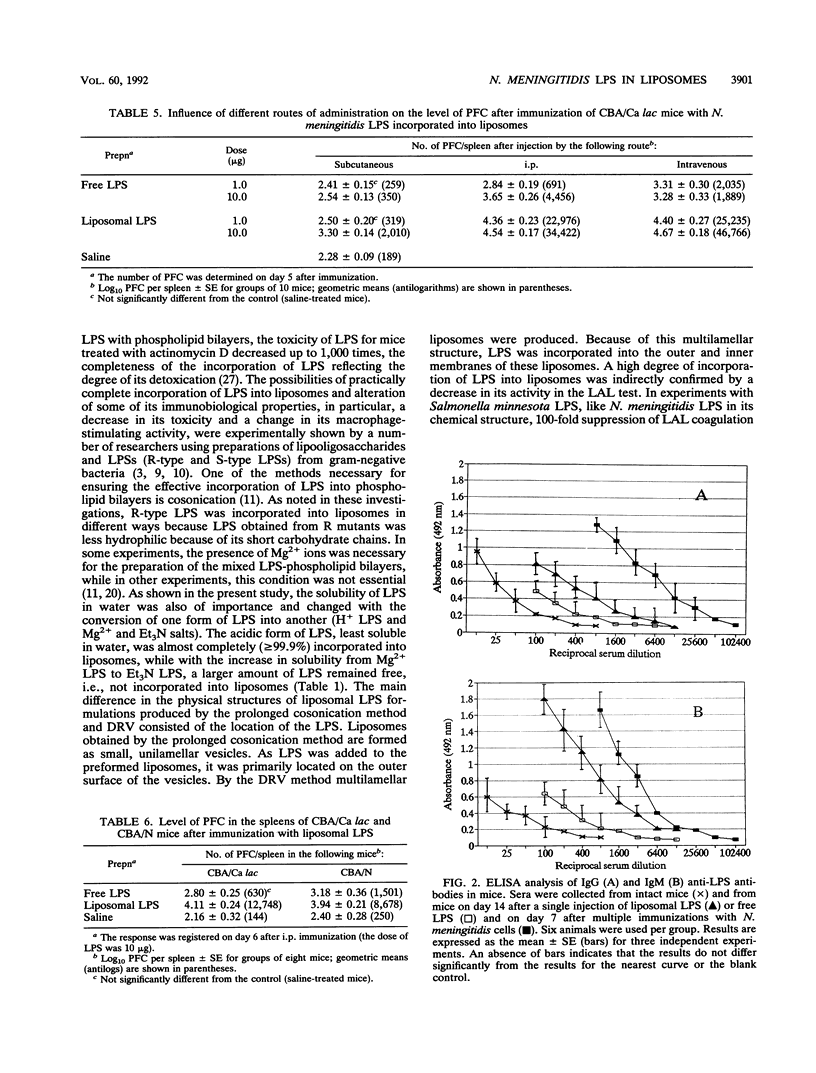
To obtain nontoxic and highly immunogenic lipopolysaccharide (LPS) for immunization, we incorporated Neisseria meningitidis LPS into liposomes. Native LPS and its salts were incorporated by the method of dehydration-rehydration of vesicles or prolonged cosonication. The most complete incorporation of LPS into liposomes and a decrease in toxicity were achieved by the method of dehydration-rehydration of vesicles. Three forms of LPS (H+ form, Mg2+ salt, and triethanolamine salt) showed different solubilities in water, the acidic form of LPS, with the most pronounced hydrophobic properties, being capable of practically complete association with liposomal membranes. An evaluation of the activity of liposomal LPS in vitro (by the Limulus amoebocyte test) and in vivo (by monitoring the pyrogenic reaction in rabbits) revealed a decrease in endotoxin activity of up to 1,000-fold. In addition, the pyrogenic activity of liposomal LPS was comparable to that of a meningococcal polysaccharide vaccine. Liposomes had a pronounced adjuvant effect on the immune response to LPS. Thus, the level of anti-LPS plaque-forming cells in the spleens of mice immunized with liposomal LPS was 1 order of magnitude higher and could be observed for a longer time (until day 21, i.e., the term of observation) than in mice immunized with free LPS. The same regularity was revealed in a study done with an enzyme-linked immunosorbent assay. This study also established that antibodies induced by immunization belonged to the immunoglobulin M and G classes, which are capable of prolonged circulation. Moreover, liposomal LPS induced a pronounced immune response in CBA/N mice (defective in B lymphocytes of the LyB-5+ subpopulation). The latter results indicate that the immunogenic action of liposomal LPS occurs at an early age.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A., Scher I., Sharrow S. O., Smith A. H., Paul W. E., Sachs D. H., Sell K. W. B-lymphocyte heterogeneity: development and characterization of an alloantiserum which distinguishes B-lymphocyte differentiation alloantigens. J Exp Med. 1977 Jan 1;145(1):101–110. doi: 10.1084/jem.145.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. W., Pichichero M. E., Insel R. A., Betts R., Eby R., Smith D. H. Vaccines consisting of periodate-cleaved oligosaccharides from the capsule of Haemophilus influenzae type b coupled to a protein carrier: structural and temporal requirements for priming in the human infant. J Immunol. 1986 Aug 15;137(4):1181–1186. [PubMed] [Google Scholar]
- Bakouche O., Koff W. C., Brown D. C., Lachman L. B. Interleukin 1 release by human monocytes treated with liposome-encapsulated lipopolysaccharide. J Immunol. 1987 Aug 15;139(4):1120–1126. [PubMed] [Google Scholar]
- Brade L., Brandenburg K., Kuhn H. M., Kusumoto S., Macher I., Rietschel E. T., Brade H. The immunogenicity and antigenicity of lipid A are influenced by its physicochemical state and environment. Infect Immun. 1987 Nov;55(11):2636–2644. doi: 10.1128/iai.55.11.2636-2644.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon J. M., Haeffner-Cavaillon N. Polymyxin-B inhibition of LPS-induced interleukin-1 secretion by human monocytes is dependent upon the LPS origin. Mol Immunol. 1986 Sep;23(9):965–969. doi: 10.1016/0161-5890(86)90127-6. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Wheeler M. W. Pertussis Vaccine Prepared with Phase-I Cultures Grown in Fluid Medium. Am J Public Health Nations Health. 1946 Apr;36(4):371–376. [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J. A method of increased sensitivity for detecting single antibody-forming cells. Nature. 1965 Sep 4;207(5001):1106–1107. doi: 10.1038/2071106a0. [DOI] [PubMed] [Google Scholar]
- Desiderio J. V., Campbell S. G. Immunization against experimental murine salmonellosis with liposome-associated O-antigen. Infect Immun. 1985 Jun;48(3):658–663. doi: 10.1128/iai.48.3.658-663.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra J., Mellors J. W., Ryan J. L. Altered in vivo activity of liposome-incorporated lipopolysaccharide and lipid A. Infect Immun. 1989 Nov;57(11):3357–3363. doi: 10.1128/iai.57.11.3357-3363.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra J., Mellors J. W., Ryan J. L., Szoka F. C. Modulation of the biological activity of bacterial endotoxin by incorporation into liposomes. J Immunol. 1987 Apr 15;138(8):2663–2670. [PubMed] [Google Scholar]
- Dijkstra J., Ryan J. L., Szoka F. C. A procedure for the efficient incorporation of wild-type lipopolysaccharide into liposomes for use in immunological studies. J Immunol Methods. 1988 Nov 10;114(1-2):197–205. doi: 10.1016/0022-1759(88)90174-3. [DOI] [PubMed] [Google Scholar]
- Elkins K. L., Stashak P. W., Baker P. J. Specific immunological unresponsiveness to bacterial lipopolysaccharides develops in a cyclic manner. Infect Immun. 1989 Jul;57(7):2253–2255. doi: 10.1128/iai.57.7.2253-2255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Reynolds H. Y. Use of Pseudomonas aeruginosa lipopolysaccharide immunoadsorbents to prepare high potency, mono-specific antibodies. J Immunol Methods. 1980;38(1-2):103–116. doi: 10.1016/0022-1759(80)90335-x. [DOI] [PubMed] [Google Scholar]
- Frasch C. E. Production and control of Neisseria meningitidis vaccines. Adv Biotechnol Processes. 1990;13:123–145. [PubMed] [Google Scholar]
- Gregoriadis G. Immunological adjuvants: a role for liposomes. Immunol Today. 1990 Mar;11(3):89–97. doi: 10.1016/0167-5699(90)90034-7. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Ashton F. E. Conjugation of meningococcal lipopolysaccharide R-type oligosaccharides to tetanus toxoid as route to a potential vaccine against group B Neisseria meningitidis. Infect Immun. 1984 Jan;43(1):407–412. doi: 10.1128/iai.43.1.407-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Mandrell R. E., Hu Z., Westerink M. A., Poolman J. T., Griffiss J. M. Electromorphic characterization and description of conserved epitopes of the lipooligosaccharides of group A Neisseria meningitidis. Infect Immun. 1988 Oct;56(10):2631–2638. doi: 10.1128/iai.56.10.2631-2638.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Human immune response to meningococcal outer membrane protein epitopes after natural infection or vaccination. Infect Immun. 1989 May;57(5):1590–1598. doi: 10.1128/iai.57.5.1590-1598.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Takeuchi Y., Ohnishi S. I., Nakae T. Outer membrane of Salmonella typhimurium. Electron spin resonance studies. Biochim Biophys Acta. 1977 Feb 14;465(1):152–164. doi: 10.1016/0005-2736(77)90363-7. [DOI] [PubMed] [Google Scholar]
- Pollack M., Chia J. K., Koles N. L., Miller M., Guelde G. Specificity and cross-reactivity of monoclonal antibodies reactive with the core and lipid A regions of bacterial lipopolysaccharide. J Infect Dis. 1989 Feb;159(2):168–188. doi: 10.1093/infdis/159.2.168. [DOI] [PubMed] [Google Scholar]
- Roeder D. J., Lei M. G., Morrison D. C. Endotoxic-lipopolysaccharide-specific binding proteins on lymphoid cells of various animal species: association with endotoxin susceptibility. Infect Immun. 1989 Apr;57(4):1054–1058. doi: 10.1128/iai.57.4.1054-1058.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L. K., Karol M. H. Production of antibody to lipopolysaccharide (LPS) after immunization with a LPS-polymyxin B-agarose immunogen. J Appl Bacteriol. 1988 Jun;64(6):487–495. doi: 10.1111/j.1365-2672.1988.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Saukkonen K., Leinonen M., Käyhty H., Abdillahi H., Poolman J. T. Monoclonal antibodies to the rough lipopolysaccharide of Neisseria meningitidis protect infant rats from meningococcal infection. J Infect Dis. 1988 Jul;158(1):209–212. doi: 10.1093/infdis/158.1.209. [DOI] [PubMed] [Google Scholar]
- Senior J., Gregoriadis G. Dehydration-rehydration vesicle methodology facilitates a novel approach to antibody binding to liposomes. Biochim Biophys Acta. 1989 May 15;1003(1):58–62. doi: 10.1016/0005-2760(89)90098-2. [DOI] [PubMed] [Google Scholar]
- Tadakuma T., Yasuda T., Tamauchi H., Saito K., Tsumita T., Kinsky S. C. Effect of lipid A incorporation on characterization of liposomal model membranes as thymus-independent type 1 or type 2 immunogens. J Immunol. 1982 Jan;128(1):206–210. [PubMed] [Google Scholar]
- Trubetskoy V. S., Koshkina N. V., Omel'yanenko V. G., L'vov V. L., Dmitriev B. A., Petrov A. B., Torchilin V. P. FITC-labeled lipopolysaccharide: use as a probe for liposomal membrane incorporation studies. FEBS Lett. 1990 Aug 20;269(1):79–82. doi: 10.1016/0014-5793(90)81123-6. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Mocca L. F., Frasch C. E. Immunotype epitopes of Neisseria meningitidis lipooligosaccharide types 1 through 8. Infect Immun. 1987 Jul;55(7):1652–1656. doi: 10.1128/iai.55.7.1652-1656.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



