Abstract
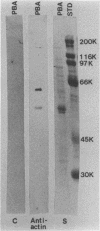
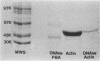
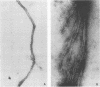
Antigens shared between Streptococcus pyogenes and heart tissue may play an important role in autoimmune cardiac injury associated with acute rheumatic fever. Antiheart/antistreptococcal antibodies found in the disease react with antigens of S. pyogenes, including M protein and a 60-kDa antigen distinct from M protein. Heart antigens recognized by these cross-reactive antistreptococcal antibodies include myosin and actin. To investigate the presence of a streptococcal actin, established protocols for the polymerization and isolation of eukaryotic actin were used to extract and concentrate actinlike proteins from M- streptococcal cells. The polymerized bacterial actin from the streptococcal extract was probed in immunoblots with an antiactin monoclonal antibody. Two proteins of about 60 kDa in the polymerized bacterial actin reacted with the antiactin antibody. Proteins in the polymerized bacterial actin extract of about 43 and 60 kDa behaved like eukaryotic actin by binding to myosin and DNase I affinity columns. Filaments were demonstrated by electron microscopy in the polymerized bacterial actinlike extract, which also enhanced the ATPase activity of eukaryotic myosin. The data suggest that proteins resembling actin are present in S. pyogenes.
Full text
PDF
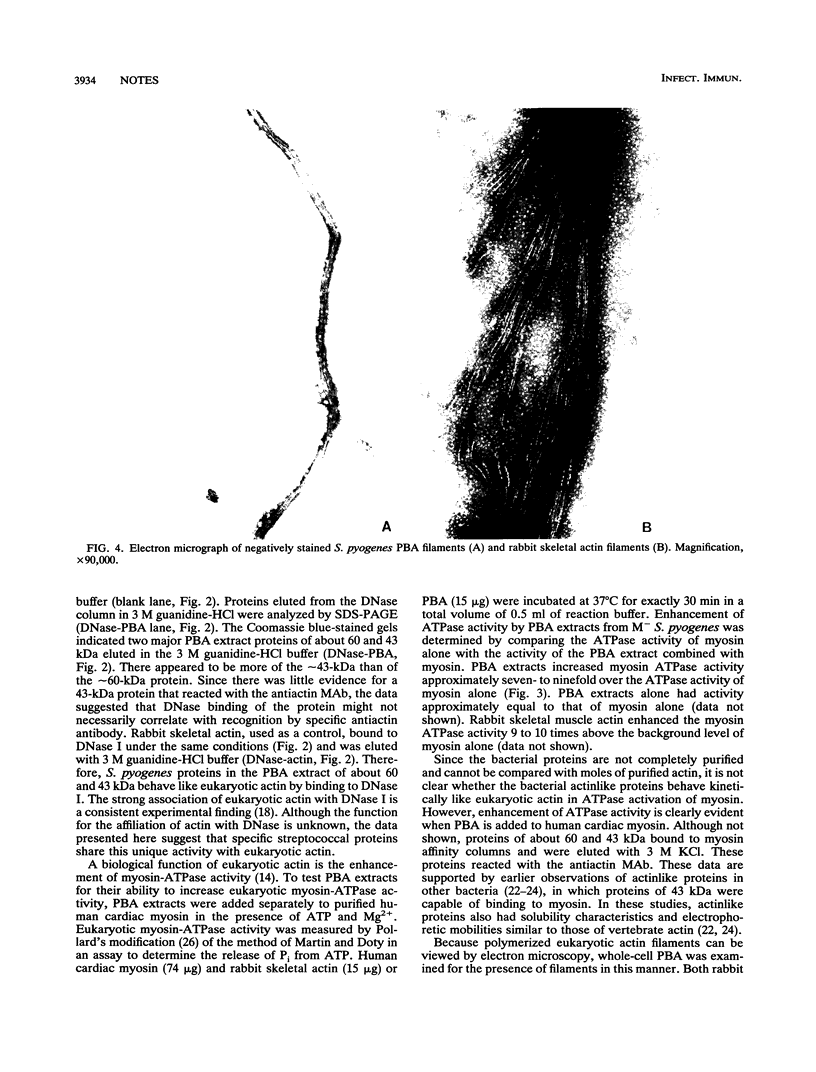


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett L. A., Cunningham M. W. A new heart-cross-reactive antigen in Streptococcus pyogenes is not M protein. J Infect Dis. 1990 Oct;162(4):875–882. doi: 10.1093/infdis/162.4.875. [DOI] [PubMed] [Google Scholar]
- Chalovich J. M., Fischetti V. A. Crosslinking of actin filaments and inhibition of actomyosin subfragment-1 ATPase activity by streptococcal M6 protein. Arch Biochem Biophys. 1986 Feb 15;245(1):37–43. doi: 10.1016/0003-9861(86)90187-6. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Biochemical and structural studies of actomyosin-like proteins from non-muscle cells. Isolation and characterization of myosin from amoebae of Dictyostelium discoideum. J Mol Biol. 1974 Jun 25;86(2):209–222. doi: 10.1016/0022-2836(74)90013-8. [DOI] [PubMed] [Google Scholar]
- Cunningham M. W., Hall N. K., Krisher K. K., Spanier A. M. A study of anti-group A streptococcal monoclonal antibodies cross-reactive with myosin. J Immunol. 1986 Jan;136(1):293–298. [PubMed] [Google Scholar]
- Cunningham M. W., Krisher K., Graves D. C. Murine monoclonal antibodies reactive with human heart and group A streptococcal membrane antigens. Infect Immun. 1984 Oct;46(1):34–41. doi: 10.1128/iai.46.1.34-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. W., McCormack J. M., Fenderson P. G., Ho M. K., Beachey E. H., Dale J. B. Human and murine antibodies cross-reactive with streptococcal M protein and myosin recognize the sequence GLN-LYS-SER-LYS-GLN in M protein. J Immunol. 1989 Oct 15;143(8):2677–2683. [PubMed] [Google Scholar]
- Cunningham M. W., McCormack J. M., Talaber L. R., Harley J. B., Ayoub E. M., Muneer R. S., Chun L. T., Reddy D. V. Human monoclonal antibodies reactive with antigens of the group A Streptococcus and human heart. J Immunol. 1988 Oct 15;141(8):2760–2766. [PubMed] [Google Scholar]
- Cunningham M. W., Russell S. M. Study of heart-reactive antibody in antisera and hybridoma culture fluids against group A streptococci. Infect Immun. 1983 Nov;42(2):531–538. doi: 10.1128/iai.42.2.531-538.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. W., Swerlick R. A. Polyspecificity of antistreptococcal murine monoclonal antibodies and their implications in autoimmunity. J Exp Med. 1986 Oct 1;164(4):998–1012. doi: 10.1084/jem.164.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. B., Beachey E. H. Sequence of myosin-crossreactive epitopes of streptococcal M protein. J Exp Med. 1986 Nov 1;164(5):1785–1790. doi: 10.1084/jem.164.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenderson P. G., Fischetti V. A., Cunningham M. W. Tropomyosin shares immunologic epitopes with group A streptococcal M proteins. J Immunol. 1989 Apr 1;142(7):2475–2481. [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Jones K. F., Manjula B. N., Johnston K. H., Hollingshead S. K., Scott J. R., Fischetti V. A. Location of variable and conserved epitopes among the multiple serotypes of streptococcal M protein. J Exp Med. 1985 Mar 1;161(3):623–628. doi: 10.1084/jem.161.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisher K., Cunningham M. W. Myosin: a link between streptococci and heart. Science. 1985 Jan 25;227(4685):413–415. doi: 10.1126/science.2578225. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Sequence homology of group A streptococcal Pep M5 protein with other coiled-coil proteins. Biochem Biophys Res Commun. 1986 Oct 30;140(2):684–690. doi: 10.1016/0006-291x(86)90786-2. [DOI] [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Tropomyosin-like seven residue periodicity in three immunologically distinct streptococal M proteins and its implications for the antiphagocytic property of the molecule. J Exp Med. 1980 Mar 1;151(3):695–708. doi: 10.1084/jem.151.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula B. N., Trus B. L., Fischetti V. A. Presence of two distinct regions in the coiled-coil structure of the streptococcal Pep M5 protein: relationship to mammalian coiled-coil proteins and implications to its biological properties. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1064–1068. doi: 10.1073/pnas.82.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Takahashi K., Watanabe S. Myosin and actin from Escherichia coli K12 C600. J Biochem. 1978 Dec;84(6):1453–1458. doi: 10.1093/oxfordjournals.jbchem.a132268. [DOI] [PubMed] [Google Scholar]
- Neimark H. C. Extraction of an actin-like protein from the prokaryote Mycoplasma pneumoniae. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4041–4045. doi: 10.1073/pnas.74.9.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Purification of muscle actin. Methods Enzymol. 1982;85(Pt B):164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. Actin-binding protein evolution. 1984 Nov 29-Dec 5Nature. 312(5993):403–403. doi: 10.1038/312403a0. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. Assays for myosin. Methods Enzymol. 1982;85(Pt B):123–130. doi: 10.1016/0076-6879(82)85015-5. [DOI] [PubMed] [Google Scholar]
- Scott J. R., Hollingshead S. K., Fischetti V. A. Homologous regions within M protein genes in group A streptococci of different serotypes. Infect Immun. 1986 May;52(2):609–612. doi: 10.1128/iai.52.2.609-612.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R., Pulliam W. M., Hollingshead S. K., Fischetti V. A. Relationship of M protein genes in group A streptococci. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1822–1826. doi: 10.1073/pnas.82.6.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M., Baldacci G., Forterre P., de Recondo A. M. Antitumor drugs inhibit the growth of halophilic archaebacteria. Eur J Biochem. 1987 Dec 1;169(2):231–236. doi: 10.1111/j.1432-1033.1987.tb13602.x. [DOI] [PubMed] [Google Scholar]
- Spudich J. A. Biochemical and structural studies of actomyosin-like proteins from non-muscle cells. II. Purification, properties, and membrane association of actin from amoebae of Dictyostelium discoideum. J Biol Chem. 1974 Sep 25;249(18):6013–6020. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B., Hsu K. C., Seegal B. C. Heart-reactive antibody associated with rheumatic fever: characterization and diagnostic significance. Clin Exp Immunol. 1970 Aug;7(2):147–159. [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]