Abstract
Objective
To determine the effect of maternal hypercholesterolemia on hepatic cholesterol metabolism in the offspring in a mouse model.
Study Design
Male and female wild type and apoE−/−KO (knockout for the apoprotein E [apoE]) gene) mice were crossbred to obtain all four possible genetic offspring types. The litters were maintained on regular chow and sacrificed at eight months of age. Liver samples were collected and the mRNA expression levels for SCAP, SREBP-1a, SREBP-2, HMGCR and LDLR determined using real-time RT-PCR.
Results
We found a significant activation of the transcriptional activity of genes involved in endogenous cholesterol synthesis, as well as LDLR, in the liver of adult mice born to hypercholesterolemic dams.
Conclusions
Reprogramming of hepatic cholesterol homeostasis may be the basis for an increased predisposition to hypercholesterolemia and atherosclerosis found in offspring of mice exposed to a high cholesterol environment during early life.
Keywords: Fetal Programming, Atherosclerosis, Hypercholesterolemia, Mouse Model, Fetal Development
Introduction
Cardiovascular disease remains the main contributor to overall morbidity and mortality in most countries. Atherosclerosis secondary to hyperlipidemia constitutes a major etiologic cause for cardiovascular disease.
Besides hereditary genetic factors, the environment in utero and during early postnatal life is evolving as a major contributor to the overall development of individuals (1–3). The basis of the importance of environmental factors during early life is presumed to be the tremendous cell turnover and tissue plasticity present during this period of differentiation and growth. The first recognition of early environmental influences on development was by David Barker and coworkers, who found an association between undernutrition during fetal development with the development of cardiovascular disease and other disorders later in life. More recently it is becoming evident that many other factors during early development - besides malnutrition - can have important influences on the long-term health of individuals (3).
Although atherosclerosis was among the first conditions for which a role of developmental programming was described, the mechanisms underlying this phenomenon have not been well studied. Among human fetuses Napoli and associates demonstrated a decreased rate of regression of fatty streak lesions in children born to hypercholesterolemic mothers as compared to controls (4–5). They also described a correlation between maternal and fetal cholesterol levels which persisted up to 26 weeks of gestation. The same group has further demonstrated an increased rate of plaque formation in litters born to hypercholesterolemic dams in a rabbit model (6). These changes were preventable when the dams received treatment with vitamin E or cholestyramine during pregnancy.
Apoprotein E (apoE) deficient mice are widely used in the study of atherosclerosis. Animals that are homozygously apoE-deficient experience spontaneous hypercholesterolemia when fed a regular diet and develop atherosclerotic lesions that are similar to those found in humans (7–8).
In a previous report we described a murine model of developmental programming of atherosclerosis using offspring of apoE deficient mice crossbred with wild-type animals (9). We showed that at eight months of age heterozygous offspring born to apoE deficient mothers had a near threefold increase in total cholesterol levels as compared to heterozygous mice born to wild-type dams. We also found a significantly increased rate of atherosclerotic plaque formation in heterozygous animals born to hypercholesterolemic mothers. We concluded that maternal hypercholesterolemia leads to elevated cholesterol levels and the development of atherosclerosis in the offspring in adulthood.
The liver is a major site of regulation of cholesterol homeostasis, controlling both cholesterol uptake and de-novo synthesis. Since our previous study showed significant dysregulation of cholesterol metabolism in response to a hypercholesterolemic environment during development, we decided to study the cholesterol metabolic pathways in the livers of affected versus control animals.
Endogenous cholesterol synthesis is primarily under the control of the SCAP-SREBP-HMGCR pathway (10,11). SCAP (SREBP-cleavage activator protein) and SREBP (sterol response element binding protein) are part of a cascade of proteins that are activated in response to decreased intracellular cholesterol levels. SREBP in turn stimulates the expression of lipogenic proteins, including HMGCR (3-hydroxy-3-methylglutaryl-coenzyme A reductase), the enzyme that catalyzes the committed step in the endogenous cholesterol synthetic pathway. SREBP exists as three distinct isoforms, SREBP-1a, SREBP-1c and SREBP-2. It is believed that activation of isoforms SREBP-1a and SREBP-2 preferentially lead to increased endogenous cholesterol synthesis. In contrast, SREBP-1c overactivation does not increase cholesterol production in transgenic animals. The LDL receptor (LDLR) is the main mediator for cholesterol uptake into the liver. LDLR expression is also stimulated through SCAP and SREBPs when intracellular cholesterol levels are decreased.
In the present study we aimed to determine the effect of maternal hypercholesterolemia on the transcriptional activation of SCAP, SREBP-1a, SREBP-2, HMGCR and LDLR, and to study the correlations between the expression of these mediators.
Materials and Methods
Mature cycling female and male mice (4–6 weeks old) homozygous for disruption of the apoE gene (apoE−/−KO, strain B6.129P2-Apoe,tm1Unc>/J, stock no. 002052) and their age-matched wild-type controls (apoE+/+WT, strain C57BL/6J, stock no. 000664) were obtained from The Jackson Laboratory (Bar Harbor, Maine). The animals were housed separately in temperature and humidity controlled quarters with constant light:dark cycles of 12h:12h and provided with food and water ad libitum. All mice (dams and litters) were maintained on regular chow. Maintenance and care was provided by certified personnel and veterinary staff according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch in Galveston.
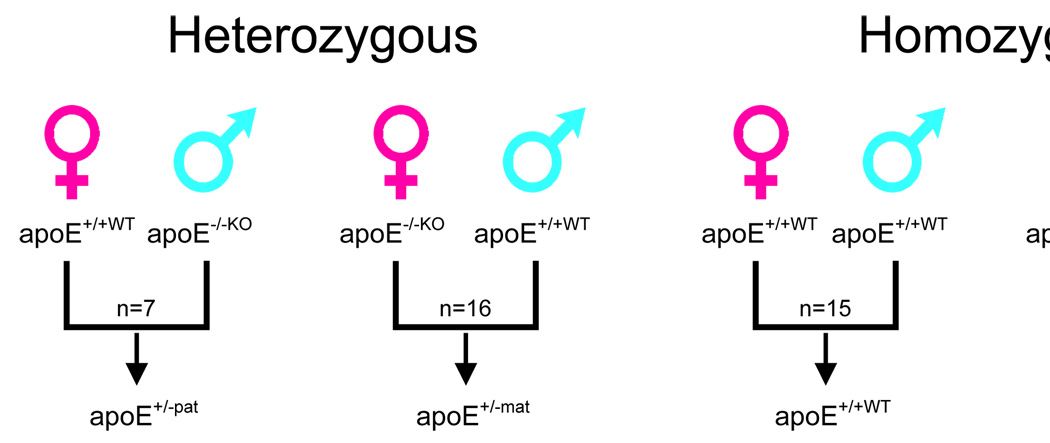
Homozygous apoE deficient-knockout mice (apoE−/−KO) and wild type (apoE+/+WT) were crossbred to obtain all four possible genetic variants of litters (figure 1). The heterozygous offspring were classified as apoE+/−mat or apoE+/−pat according to the maternal or paternal origin of the knockout apoE gene, respectively. Litters from homozygous apoE−/−KO and apoE+/+WT were used as controls.
Figure 1.
Crossbreeding pattern used to obtain the four genetic variations of offspring.
The offspring from the four litter groups were sacrificed at eight months of age using a carbon dioxide chamber. Liver samples were collected, washed in phosphate buffered saline, flash frozen in liquid nitrogen and stored at −80°C. RNA extraction was performed using the RNAqueous kit (Ambion, Austin, Texas) according to the manufacturer’s instructions. Reverse transcription into cDNA was performed using the MultiScribe RT system (Applied Biosystems, Foster City, California) according to manufacturer instructions. The cDNA was amplified on an ABI 7900 HT Fast Real Time PCR system (Applied Biosystems, Foster City, California) using mouse-specific Taqman probe sets (all purchased from Applied Biosystems, Foster City, California) for SCAP (catalog numbers Mm01250183_g1), SREBP-1a (probe sequences available upon request), SREBP-2 (catalog number Mm01306294_m1), HMGCR (catalog number Mm01282497_g1) and LDLR (catalog number Mm00440169_m1) according to the manufacturer’s protocol under the following conditions: 95 °C (20 seconds) for 1 cycle followed by 40 cycles of: 95 °C (3 seconds) and 60 °C (30 seconds). All samples were run in duplicate and were within the standard curve. Target gene expression was related to the expression levels of 18S mRNA in each specific sample (catalog number Hs99999901_s1; Applied Biosystems, Foster City, California). All PCR reactions for each sample were performed simultaneously on the same plate.
Statistical analyses were performed using one-way ANOVA, Fisher’s PLSD and linear regression analysis. All tests were two tailed and a p value of less than 0.05 was considered statistically significant. Data are expressed as mean ± standard error.
Results
Overall, 48 animals were included in this study: 15 apoE+/+WT (8 females, 7 males); 7 apoE+/−pat (3 females, 4 males); 16 apoE+/−mat (8 females, 8 males); and 10 apoE−/−KO (6 females, 4 males).
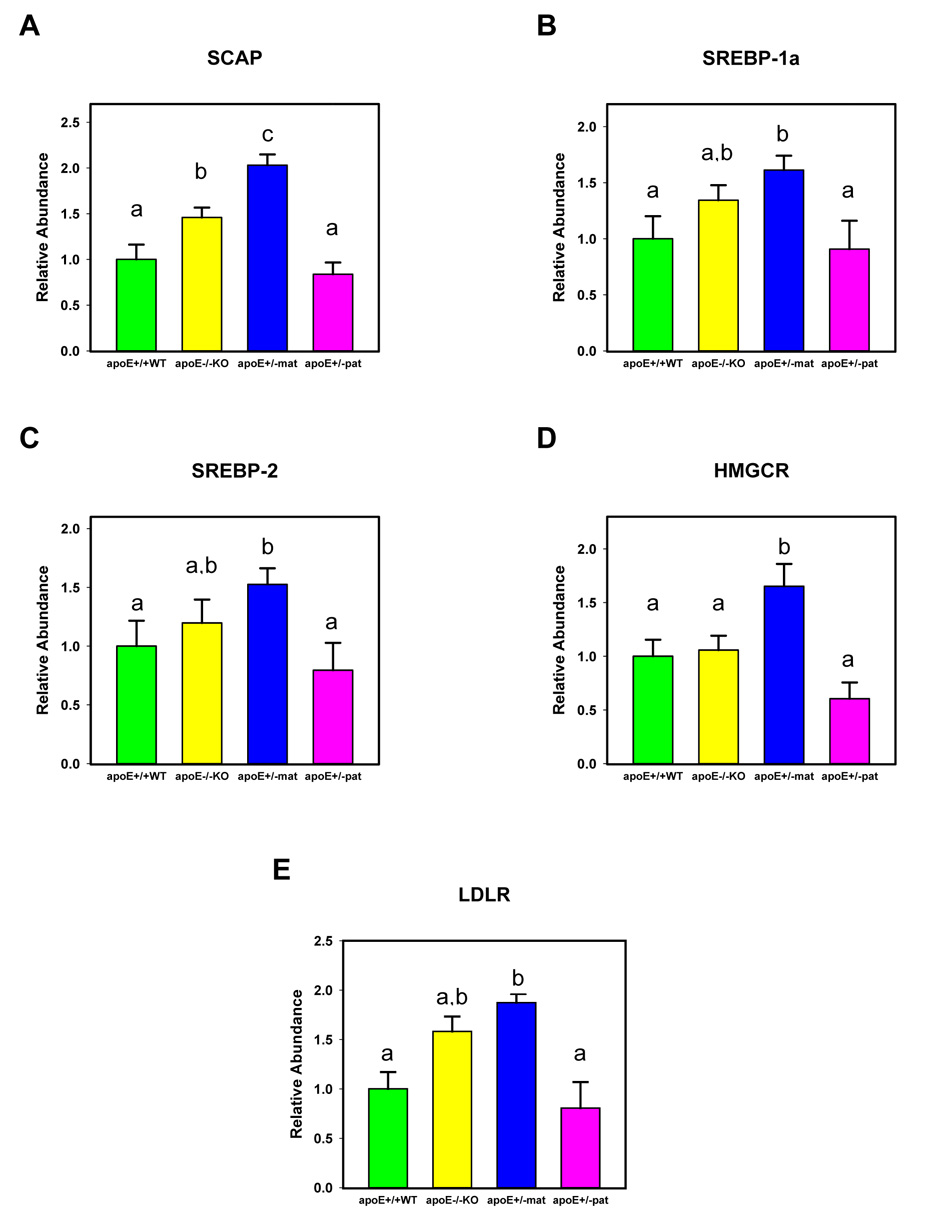
We found differential expression of the RNA message for SCAP between the four study groups (p<0.0001, ANOVA) (figure 2A). The highest level was encountered in the apoE+/−mat offspring, which was significantly increased as compared to apoE+/+WT (p<0.0001), apoE+/−pat (p<0.0001) and apoE−/−KO animals (p=0.0088).
Figure 2.
Mean relative mRNA expression levels and standard errors for SCAP (A), SREBP-1a (B), SREBP-2 (C), HMGCR (D) and LDLR (E) by study group. Labeling of columns by different letters denotes a significant difference (p<0.05) between the means in post-hoc analysis. The significance levels specifically are as follows: (A) SCAP (p<0.0001 by ANOVA): p<0.0001 for apoE+/−mat vs. apoE+/+WT, p<0.0001 for apoE+/−mat vs. apoE+/−pat, p=0.0088 for apoE+/−mat vs. apoE−/−KO; (B) SREBP-1a (p<0.0001 by ANOVA): p<0.0001 for apoE+/−mat vs. apoE+/+WT, p=0.0027 for apoE+/−mat vs. apoE+/− pat; (C) SREBP-2 (p=0.0016 by ANOVA): p=0.0004 for apoE+/−mat vs. apoE+/+WT, p=0.0046 for apoE+/−mat vs. apoE+/−pat; (D) HMGCR (p=0.0109 by ANOVA): p=0.0082 for apoE+/−mat vs. apoE+/+WT , p=0.0042 for apoE+/−mat vs. apoE+/−pat; (E) LDLR (p=0.002 by ANOVA): p=0.03 for apoE+/−mat vs. apoE+/+WT.
The pattern of SREBP-1a message expression resembled that observed for SCAP: The variation in mRNA levels was overall statistically significant (p=0.0001, ANOVA) (figure 2B). The highest levels were found in apoE+/−mat offspring. The difference in SRBEP-1a-specific message expression was significant between apoE+/−mat as compared with apoE+/+WT and apoE+/−pat groups (p<0.0001 and p=0.0027, respectively). apoE−/−KO animals showed intermediate levels of SREBP-1a mRNA, albeit the levels found were not statistically different from the apoE+/+WT and apoE+/−pat groups.
The expression of SREBP-2 mRNA was significantly different across the four study groups (p=0.0016, ANOVA). apoE+/−mat animals showed the highest SREBP-2 expression, followed by apoE−/−KO offspring (figure 2C). The differences in SREBP-2 message levels were statistically significantly different between apoE+/−mat and apoE+/+WT groups (p=0.0004), as well between apoE+/−mat and apoE+/−pat animals (p=0.0046).
HMGCR mRNA expression levels varied significantly by study group (p=0.0109, ANOVA) (figure 2D). The quantity of HMGCR message was highest in the apoE+/−mat group as compared with apoE+/+WT (p=0.0082), apoE+/−pat (p=0.0042) and apoE−/−KO offspring (p=0.0295). In contrast to the other gene products described so far, the expression of HMGCR in the apoE−/−KO animals did not show a trend towards higher levels than those in apoE+/+WT or apoE+/−pat animals.
We found evidence for differential expression of LDLR among the four study groups (p=0.002, ANOVA). LDLR mRNA levels were significantly higher in apoE+/−mat versus apoE+/+WT (p=0.0004) and apoE+/−pat (p=0.0085) offspring (figure 2E). apoE−/−KO animals displayed higher HMGCR expression levels than either apoE+/+WT or apoE+/−pat offspring, but the difference only reached statistical significance in comparison to wild-type (apoE+/+WT) mice (p=0.03).
We compared the levels of mRNA expression for SCAP, SREBP-1a, SREBP-2, HMGCR and LDLR in each group by gender, but did not find any significant difference for any of the studied gene products (data not shown).
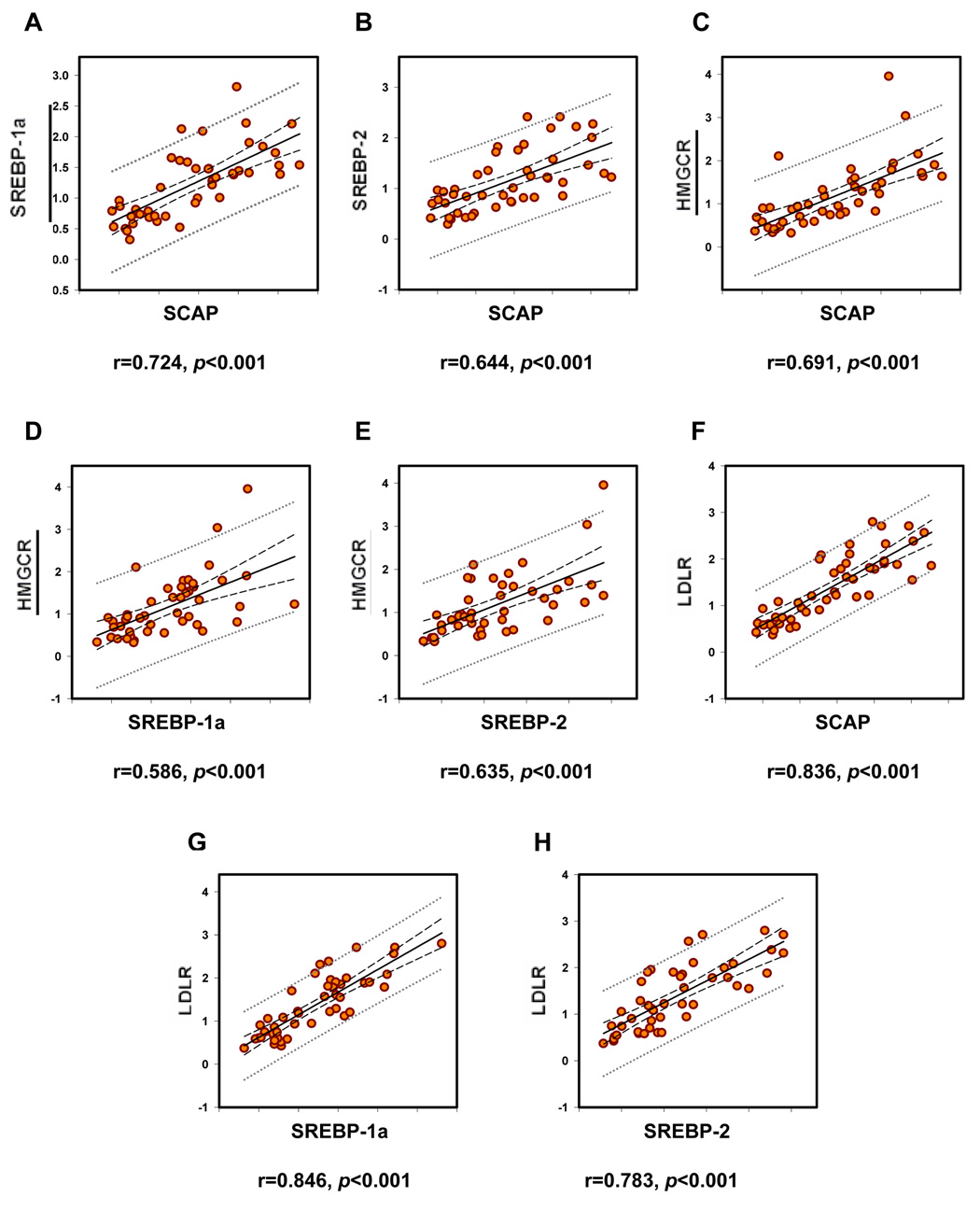
Linear regression analyses were performed to correlate the expression levels of the mRNA for SCAP, SREBP-1a, SREBP-2, HMGCR and LDLR. We found that the expression of all these gene products significantly correlated with each other. The regression plots and regression coefficients found are depicted in figure 3.
Figure 3.
Correlations between mRNA expression levels of mediators of cholesterol metabolism: SCAP and SREBP-1a (A), SCAP and SREBP-2 (B), SCAP and HMGCR (C), SREBP-1a and HMGCR (D), SREBP-2 and HMGCR (E), SCAP and LDLR (F), SREBP-1a and LDLR (G) and SREBP-2 and LDLR (H).
Comment
We have found evidence of activation of the cholesterol lipogenic pathway in the liver of animals born to hypercholesterolemic mothers. This activation is apparent across all the major components of the SCAP-SREBP-HMGCR pathway. Exposure to a high cholesterol environment during early development appears to reprogram the set point for cholesterol homeostasis in the liver of affected animals. This mechanism could explain the hypercholesterolemia observed in adult offspring born to hypercholesterolemic dams.
SCAP is the most upstream component studied in the present investigation. SCAP activity itself is affected by other regulatory proteins, most notably Insig-1 and Insig-2 (11,12). Whether Insig expression is altered in response to developmental programming events needs to be determined.
Another question that remains to be answered is the mechanism that leads to the resetting of cholesterol homeostasis and the abnormal overactivation of SCAP in the presence of high cholesterol levels. Epigenetic mechanisms, such as gene methylation events, are likely involved in this process. This needs to be further studied.
Although there is some evidence for transplacental transfer of cholesterol from the mother to the fetus in mice and in humans, we do not know for certain whether offspring of hypercholesterolemic mothers are directly exposed to a high-cholesterol environment (5,13). It is conceivable that the initial insult in the fetus may be an indirect event such as a change in the feto-placental environment as a result of endocrine and metabolic changes in the placenta in response to maternal hyperlipidemia.
In a recent follow-up to our original study we have shown a paradoxical decrease in cholesterol levels in 21-day old apoE+/−mat and apoE−/−KO offspring versus apoE+/+WT and apoE+/−pat controls (14). In contrast to eight month old offspring, preliminary studies in the 21-day old offspring fail to show evidence for an activation of hepatic cholesterol synthesis in apoE+/−mat animals. It is important to note that in our studies all offspring remain with and breastfeed from the natural dams up to three weeks of age. The lack of any evidence of a maladaptive response at this age may be hypothesized to be a reflection of a continuation of the feto-placental environment while breastfeeding from a hypercholesterolemic dam. Studies using cross-fostering of animals and/or embryo transfer experiments will be utilized in future to determine the critical time periods for the observed programming effects.
A limitation of the present study is the lack of protein and enzyme activity measurements. While this aspect is the subject of ongoing studies, we believe that the significant and consistent activation of the entire SCAP-SREBP-HMGCR pathway (and of LDLR) in the apoE+/−mat group underscores the validity of our hypothesis based on the current data.
In summary, our observations provide a plausible mechanism that may underlie the significant effects of the early developmental environment on the predisposition to develop hypercholesterolemia and atherosclerosis in the apoE mouse model. Further studies are needed to address the exact details of the mechanisms leading to the dysregulation of cholesterol synthesis in response to a hypercholesterolemic environment during early life.
Acknowledgments
This study was supported by grants R03HD056087-01 (N.G.) and K12HD001269-09 (G.D.V.H.) from the National Institutes of Child Health and Human Development.
Footnotes
This research was presented in part at the 27th Annual Meeting of the Society for Maternal–Fetal Medicine, San Francisco, CA, Feb. 5–10, 2007, and in part at the 28th Annual Meeting of the Society in Dallas, TX, Jan. 28-Feb. 2, 2008.
Condensation
Abnormal activation of hepatic endogenous cholesterol synthesis may be the basis of developmental programming of atherosclerosis in the apoE mouse model.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. In utero programming of cardiovascular disease. Theriogenology. 2000;53:555–574. doi: 10.1016/s0093-691x(99)00258-7. [DOI] [PubMed] [Google Scholar]
- 3.Byrne CD, Philips DI. Fetal origins of adult disease: epidemiology and mechanisms. J.Clin.Pathol. 2000 Nov;53:822–828. doi: 10.1136/jcp.53.11.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Napoli C, D'Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J.Clin.Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napoli C, Glass CK, Witztum JL, Deutsch R, D'Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet. 1999;354:1234–1241. doi: 10.1016/S0140-6736(99)02131-5. [DOI] [PubMed] [Google Scholar]
- 6.Palinski W, D'Armiento FP, Witztum JL, de Nigris F, Casanada F, Condorelli M, et al. Maternal hypercholesterolemia and treatment during pregnancy influence the long-term progression of atherosclerosis in offspring of rabbits. Circ.Res. 2001;89:991–996. doi: 10.1161/hh2301.099646. [DOI] [PubMed] [Google Scholar]
- 7.Reardon CA, Getz GS. Mouse models of atherosclerosis. Curr.Opin.Lipidol. 2001;12:167–173. doi: 10.1097/00041433-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Jawien J, Nastalek P, Korbut R. Mouse models of experimental atherosclerosis. J.Physiol.Pharmacol. 2004;55:503–517. [PubMed] [Google Scholar]
- 9.Goharkhay N, Sbrana E, Gamble PK, Tamayo ET, Betancourt A, Villarreal K, et al. Characterization of a murine model of fetal programming of atherosclerosis. Am.J.Obstet.Gynecol. 2007;197:416. doi: 10.1016/j.ajog.2007.08.002. e1-5. [DOI] [PubMed] [Google Scholar]
- 10.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J.Clin.Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bengoechea-Alonso MT, Ericsson J. SREBP in signal transduction: cholesterol metabolism and beyond. Curr.Opin.Cell Biol. 2007;199:215–222. doi: 10.1016/j.ceb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Sun LP, Seemann J, Goldstein JL, Brown MS. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Insig renders sorting signal in Scap inaccessible to COPII proteins. Proc.Natl.Acad.Sci. USA. 2007;104:6519–6526. doi: 10.1073/pnas.0700907104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida S, Wada Y. Transfer of maternal cholesterol to embryo and fetus in pregnant mice. J.Lipid Res. 2005:2168–2174. doi: 10.1194/jlr.M500096-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Goharkhay N, Mateus J, Egbert K, Smith O, Gamble P, Yin H, Anderson G, et al. Lack of hypercholesterolemia and of activation of cholesterol synthesis during infancy in a mouse model of developmental programming of atherosclerosis. Am.J.Obstet.Gynecol. 2007;6:S119. [Google Scholar]