Abstract
Environmental exposures, including tobacco smoke and occupational exposure to aromatic amines, have been implicated in bladder cancer etiology. However, the pathogenesis of urinary bladder transitional cell carcinoma remains incompletely defined. In epidemiologic studies, family history confers a two-fold increase in bladder cancer risk, but it is uncertain whether this represents evidence of a genetic and/or a shared environmental basis for familial aggregation. Polymorphisms in genes involved in the metabolism of environmental toxins (e.g., NAT2) clearly modify individual susceptibility to bladder cancer. A genetic predisposition has also been suggested by case reports describing multiple-case families, and the development of bladder cancer in association with several well-described Mendelian disorders (e.g., HNPCC, retinoblastoma). Here we update a previously-reported family, report a new multiple-case kindred, critically review previously-reported bladder cancer families and the epidemiologic literature related to family history of transitional cell carcinoma of the urinary tract (TCCUT) as a risk factor, as well as provide a brief summary of genetic factors that have been implicated in TCCUT risk. We conclude that familial TCCUT is either very uncommon or significantly under-reported, perhaps on the assumption that this is an environmental rather than a genetic disorder. The interaction between multiple genetic and environmental factors has made it challenging to identify genetic components responsible for many common diseases; therefore, a proposed genome-wide association study (GWAS) for urinary bladder cancer may help to clarify the etiologic role of the candidate genetic pathways reviewed here, as well as characterize gene/environment interactions that contribute to TCCUT carcinogenesis.
Keywords: Urinary bladder neoplasms, transitional cell carcinoma, hereditary neoplastic syndromes, genetic polymorphism
INTRODUCTION
Bladder cancer is the fifth most common cancer in the US, accounting for 5–10% of all malignancies; it primarily affects individuals over age 65. The incidence in white men is twice that of black men, and four times that of white women. Since 1975, bladder cancer incidence has continued to rise modestly at approximately the same rate in all sexes/races, but mortality rates are declining.[1, 2] Lifetime bladder cancer risks are 3.6% (1 in 28) and 1.1% (1 in 87) in men and women, respectively.[3] More than 90% of bladder tumors are epithelial transitional cell carcinomas (TCC); squamous cell and adenocarcinoma comprise the remainder. Approximately 55–60% of newly-diagnosed bladder cancers are low-grade, superficial, non-invasive papillary TCC. However, the recurrence rate is high (up to 80% of patients have ≥1 recurrence, 16% to 25% of which are higher-grade, muscle-invading tumors). Most urinary tract TCC originate in the bladder; a minority arise in the renal pelvis or ureter.[4] We will use the term transitional cell carcinoma of the urinary tract (TCCUT) to describe these three sites in the aggregate.
Numerous environmental exposures have been implicated in the etiology of bladder cancer. Cigarette smoking is universally regarded as the most prevalent risk factor, with an estimated 65% of male, and 20 to 30% of female bladder cancers attributed to smoking, whereas smoking cessation has been associated with a 30 to 60% reduction in risk.[2, 5] Cigarette smoke contains more than 60 carcinogens, including aromatic amines.[6] Occupational exposure to aromatic amines has been a known bladder cancer risk factor for more than a century; polycyclic aromatic hydrocarbons (PAHs) have also been implicated in bladder carcinogenesis. Estimated proportions of bladder cancer attributable to all occupational exposures combined range from 10 to 25%. Exposure to aromatic amines and PAHs occurs in various occupations, including dye, rubber, textile, chemical, leather, aluminum, iron and steel, and transport industry workers. Other environmental exposures implicated as bladder cancer risk factors include arsenic in drinking water, low fluid consumption, chronic Schistosoma haematobium urinary tract infections, acidic urine pH, urinary stasis, and cyclophosphamide chemotherapy. However, only a small fraction of individuals exposed to these risk factors actually develop bladder cancer.[2, 7]
Common variants in low-penetrance genes involved in the metabolism of environmental toxins have been shown to modify individual susceptibility to bladder carcinogens.[2] Furthermore, a genetic predisposition to bladder cancer is suggested by the occurrence of TCCUT in several Mendelian disorders[8–11], epidemiologic studies showing that a positive TCCUT family history increases bladder cancer risk[12–24], and a limited number of case reports describing multiple-case TCCUT families.[25–41] Therefore, both genetic and environmental factors play a role in the development of bladder and related urinary tract cancers. Here we update a previously-reported family, report a new multiple-case kindred, critically review the familial TCCUT literature, and provide a brief summary of genetic factors that have been implicated in TCCUT risk.
SUMMARY OF NCI FAMILIAL BLADDER CANCER FAMILIES
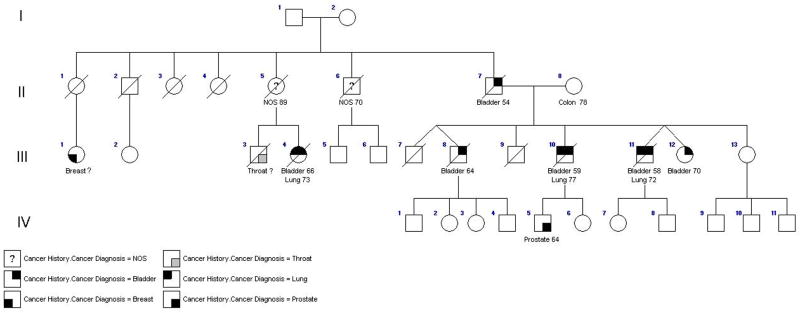
We have updated the family first reported by Fraumeni and Thomas in 1967.[28, 31] As of 1991, a father, three of seven of his offspring, and a niece had been diagnosed with TCCUT (Figure 1). Another son was diagnosed with squamous cell carcinoma of the bladder. Lung cancer had developed in two male bladder cancer cases, and the niece also developed lung cancer. All affected individuals reported substantial tobacco smoke exposure; no other proven or suspected environmental bladder cancer risk factors were documented.
Figure 1.
The rapid acetylation phenotype (based on NAT-2, N-acetyltransferase) is associated with accelerated detoxification of arylamines; it would be expected to protect against bladder cancer.[2] Recent meta-analyses indicate that the slow acetylation phenotype is associated with increased bladder cancer risk, particularly in smokers (see section on Low-Penetrance Genes).[42] However, it was previously reported that all bladder cases in this family were rapid acetylators, whereas an unaffected sibling was a slow acetylator (Table 1). The N-acetyltransferase phenotype for individuals III-10,-11,-12, and -13 was determined by administering a standard 10 gram dose of sulfamethazine orally; timed urine and blood collections were obtained and excretion products were measured according to a previously-published protocol.[43] We have since evaluated six additional unaffected offspring, among whom one male offspring developed prostate cancer at age 64. Due to the relative safety of caffeine, the N-acetyltransferase phenotype for these individuals was determined by measuring urinary caffeine metabolites in a timed urine collection following a 300mg oral dose of caffeine.[44, 45] All those newly tested with adequate samples were rapid acetylators. Debrisoquine hydroxylase is a Phase 1 enzyme; its poor metabolizer phenotype has been associated with reduced bladder cancer susceptibility in some, but not all, studies.[46, 47] We evaluated the debrisoquine hydroxylase metabolizer phenotype by measuring urinary debrisoquine metabolites after administration of 10 mg of debrisoquine in these newly-tested family members, and found no consistent relationship between metabolizer phenotype and cancer incidence.[46–49]
Table 1.
Family #1 CYP2D6 and NAT2 Polymorphism Phenotypes
| ID# | Gender | Cancer(s) | Age at Dx | Smoker | CYP2D6 (Debrisoquine metabolism)* | NAT2 (Caffeine metabolism)** | NAT2 (Sulfa-methazine metabolism) |
|---|---|---|---|---|---|---|---|
| III-10 | M | Bladder
Lung |
59
77 |
? | 85.9% (rapid) | ||
| III-11 | M | Bladder
Lung |
58
72 |
Y | 86.4% (rapid) | ||
| III-12 | F | Bladder | 70 | Y | 81.5% (rapid) | ||
| III-13 | F | N | N | 52.7% (slow) | |||
| IV-2 | F | N | Y | MR=1.32 (intermediate) | N/D | ||
| IV-3 | F | N | Y | MR=3.89 (intermediate) | N/D | ||
| IV-5 | M | Prostate | 64 | N | MR=20.96 (poor) | N/D | |
| IV-6 | F | N | N | MR=20.74 (poor) | MR=0.89 (rapid) | ||
| IV-7 | F | N | N | MR=0.91 (extensive) | MR=0.04 (rapid) | ||
| IV-8 | M | N | N | MR=0.82 (extensive) | refused |
MR = Metabolic ratio of debrisoquine to 2-hydroxy-debrisoquine. The following values are used to assign the genotype based on the MR: Extensive metabolizer = 0–1, homozygous dominant genotype; intermediate metabolizer=1–12, heterozygous genotype; and deficient (poor) metabolizer >12, homozygous recessive genotype.
MR = Molar ratio of 5-acetylamino-6-formylamino-3methyluracil (AFMU) to 1-methylxanthine (1X)
N/D = sample inadequate
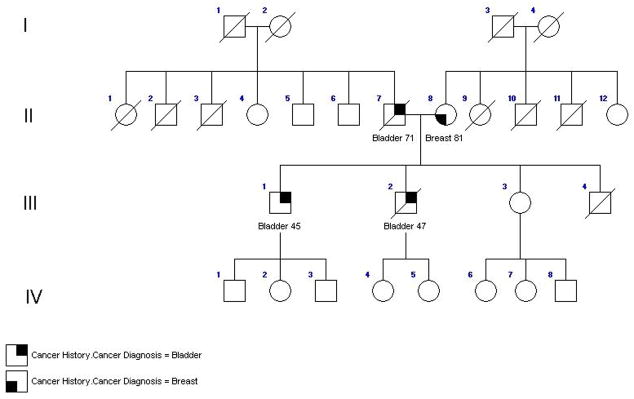
In addition, we evaluated a previously-unreported family comprised of a father and two sons with bladder cancer (Figure 2), diagnosed at ages 71, 45 and 47, respectively. Smoking history was unavailable on the father; both sons reported substantial tobacco exposure. Their mother developed breast cancer at age 81. An unaffected sibling and the offspring of the affected sons were also evaluated; all who had adequate samples were rapid NAT2 acetylators. The debrisoquine phenotype was unrelated to affection status (Table 2).
Figure 2.
Table 2.
Family #2 CYP26 and NAT2 Polymorphism Phenotypes
| ID# | Gender | Cancer | Age of dx | Smoker | CYP26 (Debrisoquine metabolism) | NAT2 (Caffeine metabolism) |
|---|---|---|---|---|---|---|
| III-1 | M | Bladder | 45 | Y | MR=1.64 (intermediate) | MR=0.63 (rapid) |
| III-2 | M | Bladder | 47 | Y | MR=1.0 (intermediate) | MR=0.40 (rapid) |
| III-3 | F | N | N | MR=1.38 (intermediate) | MR=1.45 (rapid) | |
| IV-1 | M | N | N | MR=1.11 (intermediate) | MR=0.08 (rapid) | |
| IV-2 | F | N | N | MR=1.29 (intermediate) | N/D | |
| IV-3 | M | N | N | MR=0.84 (extensive) | MR=0.31 (rapid) | |
| IV-4 | F | N | N | MR=0.48 (extensive) | N/D | |
| IV-5 | F | N | N | MR=69.6 (poor) | MR=0.56 (rapid) |
MR = Metabolic ratio of debrisoquine to 2-hydroxy-debrisoquine. The following values are used to assign the genotype based on the MR: Extensive metabolizer = 0–1, homozygous dominant genotype; intermediate metabolizer=1–12, heterozygous genotype; and deficient (poor) metabolizer >12, homozygous recessive genotype.
MR = Molar ratio of 5-acetylamino-6-formylamino-3methyluracil (AFMU) to 1-methylxanthine (1X)
N/D = sample inadequate
CASE REPORTS OF FAMILIAL TCCUT
A literature review revealed 16 multiple-case TCCUT reports, documenting 32 families with 86 affected individuals (Table 3).[25–41] The number of cases per family was two (n=15), three (n=15), six (n=1), and one family with 5 TCCUT and one squamous cell bladder cancer. When tumor site was specified, eleven families had bladder TCC only, nine included TCC of the bladder and renal pelvis/ureter, and one presented only ureteral cancer. Nine individuals had more than one urinary tract TCC. The average ages at TCCUT diagnosis were 56.6, 55.8, and 58.4 among all cases, males, and females, respectively.
Table 3.
Familial TCCUT - Literature Summary of Multiple-Case Families
| Article | Number Affected ### |
Relation to probands |
Gender | Bladder TCC |
Age at Onset |
Other TCC |
Age at Onset |
Other CA |
Age at Onset |
Family History of CA# |
Smoke | Other Exposures## |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Burkland, 1966 | 2 | Self | F | Y | 70 | Ureter | 68 | Sister-Colon | ||||
| Son | M | N | Ureter | 53 | Y | |||||||
| Fraumeni, 1967
Blattner, 1983* |
5 | Self | M | Y | 58 | Lung (Large cell) | 72 | Mother-Colon, 78
Sibling (M)-Bladder, 64 (squamous cell) Brother-NOS, 70d Sister-NOS, 89 |
Y | |||
| Sibling | M | Y | 59 | Lung (Oat cell) | 77 | Y | ||||||
| Sibling | F | Y | 70 | Y | ||||||||
| Paternal Cousin | F | Y | 66 | Y | ||||||||
| Father | M | Y | 54 | Y | ||||||||
| Benton, 1973 | 2 | Self | M | Y | 19 | Furniture shop/Machinist | ||||||
| Father | M | Y | ? | Welder | ||||||||
| McCullough, 1975 | 6 | Self | M | Y | 36 | Ureter | 40 | Paternal uncle-Melanoma, 63
Paternal cousin**-Ovary, Brain Paternal cousin***-Cervix Paternal cousin***-Leukemia Paternal cousin***-Breast |
Y | |||
| Sibling | M | Y | 35 | Ureter/Renal pelvis | 47 | Basal Cell | ? | Y | Printer | |||
| Father | M | Y | 44 | Gastric (adenocarcinoma) | 63 | Y | Painter early 20’s | |||||
| Paternal uncle** | M | Y | 65 | Lung metastases, primary unknown | 81 | N | Mechanic | |||||
| Paternal Cousin** | F | Y | 48 | Cervix | 38 | N | Lived on farm | |||||
| Paternal uncle*** | M | Y | 60 | Prostate | 60 | Y | Farmer | |||||
| Leklem, 1976 | 2 | Self | F | Y | 65 | Mother-Breast | Y | Art teacher | ||||
| Brother | M | Y | 51 | Y | Engineer diesel train/Petroleum plant operator | |||||||
| Sharma, 1976 | 2 | Self | M | Y | 83 | Y | ||||||
| Son | M | Y | 58 | |||||||||
| 2 | Self | M | Y | 53 | ||||||||
| Sibling | M | N | Renal pelvis | 56 | ||||||||
| Purtilo, 1979 | 2 | Self | M | Y | 62 | Y | Textile dye | |||||
| Sibling | M | Y | 55 | Y | Machinist | |||||||
| 2 | Self | M | Y | 59 | Brother-Colon, 62
Sister-Breast, 58 |
Y | Rubber/textile dye | |||||
| Sibling | M | Y | 56 | Y | Leather | |||||||
| 2 | Self | M | Y | 91 | Prostate | 89 | N | |||||
| Sibling | F | Y | 89 | N | ||||||||
| 2 | Self | M | Y | 39 | Renal Cell | 57 | Y | Machinist | ||||
| Sibling | M | Y | 52 | |||||||||
| 3 | Self | M | Y | 33 | Y | Bricklayer | ||||||
| Daughter | F | Y | 19 | N | Tool and dye | |||||||
| Sibling | M | Y | 28 | Y | Bricklayer | |||||||
| 2 | Self | M | Y | 73 | Daughter-Liver, 54 | Y | Electrician | |||||
| Son | M | Y | 62 | Y | Electrician | |||||||
| Lynch, 1979 | 3 | Self | M | Y | 52 | Daughter-Neuroblastoma, 4 wks
Paternal GM, GGM-Breast Paternal uncle-NOS |
N | Organic chemist | ||||
| Father | M | Y | 68 | Y | Radiologist | |||||||
| Paternal Uncle | M | N | Renal pelvis | 62 | Y | |||||||
| 3 | Self | M | Y | 50 | Basal Cell | 50 | Father-Colon, 73 | Y | Wiring for railroad | |||
| Sibling | M | Y | 24 | Renal pelvis | 48 | Y | ||||||
| Sibling | M | Y | 49 | Y | ||||||||
| Mahboubi, 1981 | 3 | Self | F | Y | 66 | Ureter | 66 | Mother, MGM, MGGM, M aunt- Skin, Bone, Leukemia | N | |||
| Sibling | M | Y | 64 | Y | Farmer | |||||||
| Son | M | Y | 50 | Y | Pilot/Construction/Farmer | |||||||
| Marchetto, 1983 | 3 | Self | F | N | Ureter | 48 | Endometrial carcinoma | 50 | Maternal aunt-Breast, 65
Maternal uncle-Liver, 67 Maternal uncle-Lung, 73 Maternal uncle-Gastric Maternal GF-Brain, 42 |
N | ||
| Mother | F | Y | 60 | Lower Ureter | 57 | Renal cell | 57 | Y | Shoemaker | |||
| Maternal Cousin | F | Y | 69 | Lower Ureter | 69 | Endometrial carcinoma | 68 | N | Leather industry | |||
| Orphali, 1986 | 3 | Self | M | N | Renal pelvis | 64 | Sister-Cervix
Father – Lymphosarcoma 4 Paternal uncles-NOS MGF, M aunt, M uncle - NOS |
N | ||||
| Sibling | M | N | Renal pelvis | 55 | Y | |||||||
| Sibling | F | N | Upper Ureter | 60 | Y | |||||||
| Eglisson, 1993 | 3 | Self | F | Y | 76 | Daughter-Renal Cell, 67; Breast, 67;Colon, 72
Paternal 2nd cousins-Skin, 72; Kidney, 58;Breast, 54;Colon, 54 Paternal 3rd cousin-Leukemia, 9 |
N | Farm | ||||
| Son | M | Y | 81 | Renal pelvis | 78 | Y | Farm | |||||
| Daughter | F | N | Renal pelvis, Ureter | 70 | Thyroid (Papillary) | 73 | N | Farm | ||||
| Kenet, 1995 | 2 | Self | F | Y | 14 | Acute lymphocytic leukemia | 8.5 | N | Treated with Cyclophosphamide | |||
| Grandfather | M | Y | ? | |||||||||
| Schoenberg, 1996 | 2 | Self | M | Y | 29 | Renal pelvis | 29 | Brother-Melanoma, 27
Father-Prostate, 68 MGF-Lung |
Multiple SAB t(5;20)(p15;q11) | |||
| Mother | F | Y | 65 | Multiple SAB | ||||||||
| Aben, 2001 Kiemeney, 2006 | 2 | Self | F | **** | 42 | |||||||
| Mother | F | **** | 42 | |||||||||
| 2 | Self | F | **** | 54 | ||||||||
| Mother | F | **** | 74 | |||||||||
| 3 | Self | M | **** | 73 | Father-Stomach
Mother-?Bone Sibling-’Neck’,61;Colon, 60 |
|||||||
| Sibling | M | **** | 65 | |||||||||
| Sibling | M | **** | 55 | |||||||||
| 3 | Self | F | **** | 43 | Endometrial | ? | Mother-?Vulvar
Siblings-Colon, 34;Ovary, 41; Stomach, 38 Father - Colon; Stomach, 34 |
|||||
| Sibling | M | **** | 43 | |||||||||
| Maternal Grandfather | M | **** | 36 | |||||||||
| 3 | Self | M | **** | 54 | ||||||||
| Mother | F | **** | 46 | |||||||||
| Sibling | F | **** | 53 | |||||||||
| 3 | Self | M | **** | ? | ureter | 38 | ||||||
| Father | M | **** | 67 | |||||||||
| Paternal Grandfather | M | **** | 60 | |||||||||
| 3 | Self | M | **** | 66 | Lung | 54 | Sibling-Esophagus, 72
Sibling-Lung |
|||||
| Sibling | M | **** | 60 | |||||||||
| Father | M | **** | 82 | Colon | 52 | |||||||
| 3 | Self | M | **** | 72 | Father-Liver, 43
Sibling-Breast, 62 Sibling-Stomach, 53 Paternal Sibling-Stomach, 72 |
|||||||
| Sibling | M | **** | 65 | Prostate | ? | |||||||
| Paternal Uncle | M | **** | 58 | |||||||||
| 3 | Self | F | **** | 53 | Sibling-Lung, 49
Sibling-Prostate, 57 |
|||||||
| Sibling | M | **** | 61 | |||||||||
| Sibling | M | **** | ? | |||||||||
| 2 | Self | F | **** | 70 | ||||||||
| Sibling | M | **** | 58 | |||||||||
| 3 | Self | M | **** | 40 | Paternal Sibling-Breast | |||||||
| Father | M | **** | 70 | |||||||||
| Paternal Grandmother | F | **** | 92 |
Relationships cited are with reference to the proband.
Shaded occupations have been suggested as potential contributors to TCCUT.
Shaded families present features suggestive of HNPCC.
Also contains previously unpublished data, See Figure 1 for updated pedigree
Nuclear family
Nuclear family
Site not specified
NOS = Not otherwise specified
? = not reported
SAB = spontaneous abortion
Blank spaces indicate that data was not reported
Environmental exposures were infrequently reported: 38% (33 of 86 familial cases) were known smokers, and 34% (29 of 86) had an occupational exposure that may have increased their TCCUT risk. Occupational exposures could potentially contribute in 13 (41%) of the 32 families. Another family had a potential environmental etiology: one affected member received cyclophosphamide (a known bladder carcinogen) to treat leukemia.[32, 50]
Schoenberg et al. reported a 29-year-old male with bladder and renal pelvis TCC with a balanced germline translocation, 46,XY,t(5;20)(p15;q11).[40] His mother died at age 65 with metastatic bladder TCC, his father died of prostate cancer (age 68) and his brother of metastatic melanoma (age 27). Only the proband was karyotyped. Subsequent analysis of the breakpoints determined that CDC91L1 at 20q11 was the only gene whose expression was affected by the translocation. It encodes CDC91L1 (also called phosphatidylinositol glycan class U or PIG-U), and its role as a potential oncogene in bladder cancer remains unclear, as a subsequent study did not confirm these findings.[51, 52]
This and several additional multiple-case families included relatives with other cancer types, raising the possibility that familial TCCUT may include a predisposition to other malignancies. But there was no clear site-specificity, mode of inheritance, or ethnic predilection among families with non-TCCUT cancers which might have suggested a distinct familial TCCUT syndrome. However, TCCUT has been implicated as part of the hereditary nonpolyposis colorectal cancer (HNPCC) cancer spectrum[53, 54], and 9 (28%) of the previously-reported families presented features suggestive of HNPCC (Table 3).
As can be seen from this literature review, there are no uniform, widely-accepted criteria as to what constitutes familial TCCUT. Although almost half the reported families had only two affected family members, all but one family had at least one affected member under the age of 65 (TCCUT primarily affects individuals over age 65). Younger-than-usual age at cancer diagnosis is a widely-recognized clue to the presence of an underlying genetic susceptibility disorder. The remaining family had one member who had multiple primary tumors (prostate cancer), also indicative of a hereditary predisposition to cancer. Ultimately, all of these families may not represent valid examples of pure site-specific TCCUT familial aggregations, clearly highlighting the importance of recognizing the strengths and limitations of the criteria used to explore the underlying genetic predisposition to bladder cancer in families.
EPIDEMIOLOGY OF FAMILIAL TCCUT
Table 4 summarizes pertinent details of 9 case-control and 4 cohort studies in which family history of TCCUT was quantitatively evaluated as a bladder cancer risk factor.[12–24] These studies varied widely in sample size, quality of design and analysis, inclusion/exclusion of upper urinary tract sites, and the extent to which reported cancers were objectively documented, but are surprisingly similar in their estimated risk ratios. These range from 1.2 to 6.1 among male and female cases combined, with most of the results clustering between 1.4 and 1.9. The confidence intervals from the adequately-powered studies generally excluded 1.0. The largest case-control study (2900 cases; 5684 controls) demonstrated a RR = 1.5 (95% CI 1.2–1.8).[17] Familial risks tended to be higher among males (a finding not consistent among studies), younger probands (< age 45) and smokers.[17, 20]
Table 4.
Familial TCCUT Literature Summary – Epidemiological Studies
| Article | Population Studied | Exposure Measured | Adjustments Made | Results | Comments |
|---|---|---|---|---|---|
| CASE-CONTROL STUDIES | |||||
| Cartwright, 1979 | 1261 bladder cancer cases number of controls not stated | Bladder cancer in1st -or 2nd-degree relatives | Age, Sex | OR 1.3(CI not given) | No statistically significant difference.
Preliminary report, final results never published Number of controls not reported |
| Kantor, 1985 | 2900 bladder cancer cases 5684 controls | Cancer of the urinary tract in 1st-degree relatives | Race, Age, Sex, Smoking | RR 1.5 (95% CI 1.2–1.8) | Higher risk in persons younger than 45: RR 2.7 (95% CI 0.8–8.9)
Higher risk in women: RR 1.8(95% CI 1.1–2.7) Higher risk among heavy smokers 60+ cigarettes /day: RR 10.7 (95% CI 1.3–236.5) No excess with high-risk occupation RR 1.1 (95% CI 0.5–2.2) |
| Piper, 1986 | 173 bladder cancer cases (females ages 20–49) 173 controls | Bladder or kidney cancer in 1st-degree relatives | Age, Sex, Residence within an area code | OR 4.0 (95% CI 0.4–195.0) | Six cases and five controls had missing values for family history |
| Kramer, 1991 | 319 bladder cancer cases (all males) 319 controls | Bladder cancer in 1st- degree relatives | Age, Sex, Socioeconomic status, Smoking | RR 1.9 (90% CI 1.1–2.7) | For relatives who smoked RR 2.1 (90% CI 0.8–3.4)
For nonsmoking relatives RR 1.8(90% CI 0.7–2.9) Proportional hazards regression considering age, sex, and smoking status of the proband agreed with these results, but values were not reported |
| Kunze, 1992 | 531 male, 144 female lower urinary tract cancer cases matched pair controls | Bladder cancer in1st-degree relatives | Age, Sex, Smoking | Male probands OR 2.4 (95% CI 1.2–4.7) Female probands OR 1.2 (95% CI 0.7–3.9) | ---- |
| Aben, 2002 | 1,193 TCCUT cases 853 non-bloodline family member controls | TCCUT in 1st-degree relatives | Age, Sex, Smoking | HR 1.8 (95% CI 1.3 – 2.7) | Higher risk in females HR 3.7 (95% CI 1.3–10.6)d
Higher risk in nonsmokers HR 4.2 (95% CI 1.4–12.7) Higher risk in relatives of probands <= 60 HR 2.5 (95% CI 2.0–4.0 |
| Lin, 2006 | 713 bladder cancer cases 658 controls | Bladder cancer in 1st- degree relatives | Age, Sex, Ethnicity | OR 1.4 (95% CI, 0.7–2.6) Proband is smoker OR 2.3 (95% CI 1–5.5) | Study is still ongoing, complete matching of cases and controls has not been achieved for age, sex, and ethnicity.
Approximately 73.2% of cases were ever smokers, 52.4% of controls were ever smokers, and cases reported significantly greater pack-years of smoking than controls, 41.5 vs. 27.4, p<.01 |
| Randi, 2007 | 727 bladder cancer cases 1,067 controls | Bladder cancer in parents and siblings | Age, Sex, Region, Education, Body Mass Index, Smoking, Alcohol consumption, Number of siblings | OR 6.1 (95% CI 2.3–16.6) | Higher risk in cases younger than 65: OR 7.6 (95% CI 2.2–26.4)
Higher risk in cases who smoke: OR 10.7 (95% CI 2.4–48.9) Higher risk when parent affected: OR 6.4 (95% CI 1.8–22.3) |
| Murta- Nascimento, 2007 | 1,158 bladder cancer cases 1,244 controls | Bladder cancer in 1st degree relatives | Age, Sex, Region, Smoking | OR 2.3 (95% CI 1.0–5.8) | Higher risk in cases with NAT2 slow acetylator genotype
OR 4.8 (95% CI 1.3–18.1) Higher risk in cases with GSTM1-present genotype OR 4.2 (95% CI 1.3–14.1) |
| COHORT STUDIES | |||||
| Lynch, 1987 | 49 cases of bladder cancer | Bladder cancer in relatives | Sex, smoking status | RR 1.6 (CI not given) | ----- |
| Goldgar, 1994 | 1,452 cases of bladder cancer | Bladder cancer in1st -degree relatives | ----- | RR 1.5 (95% CI 1.0–2.2) | Probands less than 60 RR 5.1 (95% CI 1.0–12.5) |
| Kiemeney, 1997 | 190 cases of TCCUT (145 male/45 female) | TCCUT in1st-, 2nd-, and 3rd-degree relatives | ----- | O/E 1.2 (95% CI 0.9–1.7) | Among male relatives O/E 1.4 (95% CI 0.95–1.88)
Among female relatives O/E 0.9 (95% CI 0.39–1.78) |
| Plna, 2001 | 27,000 cases of bladder cancer | Bladder cancer according to parental or sibling bladder cancer | ----- | Offspring risk SIR 1.6 (95% CI 1.2–2.0) Sibling risk SIR 3.0 (95% CI 1.4–5.1) | Offspring risk higher in daughters SIR 2.29 (95% CI 1.5–3.3) than sons SIR 1.4 (95%CI 1.0–1.8)
Highest familial risk in brothers of bladder cancer cases diagnosed before age 45 SIR 7.26 (95% CI2.61–14.24) |
? = not reported; CI=Confidence Interval; OR=Odds Ratio; RR=Relative Risk; SIR=Standard Incidence Ratio; HR=Hazards Ratio; O/E=Observed to Expected
No significant differences in risk emerged when case-control and cohort studies were compared. Among the cohort studies, an analysis of the Mormon genealogy data base yielded a familial RR = 1.5 (95% CI 1.0–2.2);[16] the RR was 5.1 (95% CI 1.0–12.5) among probands <age 60. In one study, the risks to siblings were higher (SIR = 3.0) than the risks to offspring (SIR = 1.6) (a pattern suggestive of autosomal recessive inheritance), with the highest familial risk (SIR 7.3) seen in the brothers of bladder cancer cases diagnosed before the age of 45.[24] Higher familial risks among younger-than-usual affecteds is often cited as a clinical clue to possible underlying inherited cancer susceptibility.[55] In general, this literature suggests a modest familial component to TCCUT risk, with relative risks at the lower end of the range observed for other common adult solid tumors. These observations are consistent with either hereditary and/or shared environmental exposures as the basis for familial clustering.
However, in the highest-quality data set currently available, 1193 population-based TCCUT patients and 853 controls were ascertained; family history was obtained, and verification attempted (60% successful) for all cancers reported among first- and second-degree relatives.[12] In this large Dutch cohort, at least one affected relative was reported in 95 (8.0%) bladder cancer proband families compared with 36 control families (4.0%). These authors reported a significantly increased smoking-adjusted risk associated with a positive family history (hazard ratio = 1.8; 95% CI = 1.3–2.7), suggesting that familial aggregation cannot be fully explained by shared tobacco consumption habits and genetic susceptibility factors remain to be identified.
GENETICS AND TCCUT
High-Penetrance Genes
Mutations in high-penetrance genes that confer very high cancer risk upon affected individuals, and result in familial aggregation of malignancy, are rare genetic alterations. However, they offer powerful research opportunities to clarify carcinogenesis mechanisms. TCCUT has been implicated as part of the cancer spectrum associated with several hereditary cancer syndromes. Most notably, hereditary nonpolyposis colorectal cancer (HNPCC), is a syndrome with high lifetime probabilities of colorectal, endometrial, ovarian, renal pelvis/ureteral TCC, and other cancers (OMIM numbers: 120435, 120436, 114500, 114030, 600887). HNPCC is caused by germline mutations in the mismatch repair genes MLH1, MSH2, MSH6, and PMS2.[56] The Revised Bethesda Guidelines, which were introduced to facilitate determining which families warrant genetic assessment for HNPCC, include TCC of the renal pelvis and upper ureter, but not urinary bladder, among the syndrome-defining malignancies.[57] However, a simultaneous publication by several of the same authors does include bladder cancer as an HNPCC-related tumor.[54] There have also been several reports of bladder cancer in families with Muir-Torre syndrome, a variant of HNPCC which includes sebaceous gland tumors. Unfortunately, these families were reported prior to the availability of genetic testing.[8, 58, 59] A recently-reported family with MSH2-related HNPCC included a mutation carrier with multifocal TCC, involving the bladder.[11] An analysis of the Dutch HNPCC cohort documented a renal pelvis/ureter TCC relative risk of 14.0 (95% CI 6.7–29.5; p <0.05), with a cumulative lifetime risk of 2.6%, among first-degree relatives of mutation carriers.[60] The risk of urinary bladder cancer in this cohort was not increased. The current consensus suggests that TCC of the upper urinary tract are clearly part of HNPCC, while the association with bladder cancer is unproven.
Prior to the availability of genetic testing for mutations in RB, bladder cancer was reported in hereditary retinoblastoma families (OMIM 180200), including an 11 year-old girl with bilateral retinoblastoma and multiple osteosarcomas, whose mother had unilateral retinoblastoma. Her maternal grandfather and uncle had TCC of the bladder at ages 60 and 47, respectively.[61–64] Aherne reported two siblings with retinoblastoma whose mother developed bladder cancer at age 40, and another retinoblastoma patient whose father died from bladder cancer at age 50.[65] The elevated risk of second primary bladder cancer among retinoblastoma survivors has been attributed to radiation treatment or cyclophosphamide chemotherapy[66–68]. However, Fletcher et al., found that hereditary retinoblastoma survivors who were not exposed to high-dose radiation or chemotherapy had a substantially higher mortality from bladder cancer versus the general population (standardized mortality ratio [SMR] = 26.3; 95%, CI 8.5–61.4).[9] Therefore, bladder cancer appears to be part of the hereditary retinoblastoma cancer spectrum, independent of late effects of cancer treatment.
Costello syndrome (OMIM 218040) is a rare, autosomal dominant, multiple congenital anomaly syndrome with a predisposition to rhabdomyosarcoma, neuroblastoma, and transitional cell carcinomas of the urinary bladder.[10, 69] Mutations in the HRAS proto-oncogene have been reported in the individuals with bladder cancer, thus expanding the list of major genes implicated in TCCUT etiology.[70] A recent report describes a 4 year-old girl with Apert syndrome (OMIM 101200), a germline mutation in FGFR2, and low-grade TCCUT.[71] Apert syndrome is one of eight autosomal dominant FGFR-related craniosynostosis/multiple congenital anomaly syndromes (OMIM 123500, 101600, 123510, 602849, 123790)
Interestingly, somatic mutations in HRAS and FGFR3 occur in 30% and 70% of low-grade TCCUT, respectively; over 50% of high-grade tumors display defects in RB and/or p53. It has been suggested that TCCUT arises and progresses along two distinct genetic pathways, involving either HRAS/FGFR3 or p53/RB, characterized by low-grade or high-grade histology, respectively; successive genetic abnormalities (e.g. chromosome aberrations, somatic mutations of other oncogenes/tumor suppressor genes, epigenetic alterations) ultimately pave the way for tumor progression and, ultimately, metastasis.[73, 74] Cytogenetic and molecular genetic studies have identified large-scale structural and numerical chromosome abnormalities as predictors of bladder tumor recurrence and cancer progression. Loss of 9q is the most commonly seen abnormality in low- and high-grade tumors, suggesting that it may be a primary event in the genesis of TCCUT, but the underlying genetic mechanisms (e.g. possible tumor suppressor gene in this region) remain unclear. The use of high-throughput technologies has expanded the genomic regions of interest (http://www.progenetix.de/~pgscripts/progenetix/I81203/index.html), which will help to elucidate the genetic pathways of tumor progression, which may, in turn, increase our knowledge of genetic susceptibility.
Cytogenetic abnormalities have led to the localization of several hereditary cancer syndrome genes (e.g. retinoblastoma).[75, 76] Aben et al. attempted to identify constitutional cytogenetic abnormalities in thirty of the 95 multiple-case families from the large Dutch bladder cohort described previously.[25] The thirty families selected were those in which there were 2 or 3 affected individuals who were diagnosed at a relatively young age, and did not meet the criteria for known familial cancer syndromes. Chromosome analysis was performed only on affected probands. All 30 cases (23 male, 7 female) had normal Giemsa-banded karyotypes. Spectral karyotype analysis (SKY) on 4 TCCUT cases from families more suggestive of an inherited etiology (>2 cases and/or ≥1 early-onset case) was also normal. These two techniques detect genetic alterations from 2 to 10 Mb in size; therefore, small deletions, duplications, or single base-pair mutations were below the assays’ level of resolution. This same cohort was studied with high-resolution, array-based comparative genomic hybridization (CGH).[33] Ten cases from families most consistent with an inherited etiology were analyzed; no genomic regions were identified as likely locations for bladder cancer susceptibility genes.
A complex segregation analysis was also performed on this large Dutch bladder cancer cohort (1193 affecteds), which included all 95 multiple case families, with sex and smoking status incorporated as covariates; neither environmental nor single gene models fit the data significantly.[77] The ‘no major gene’ hypothesis did not significantly characterize the data either, but it was the most parsimonious model. Overall, these findings suggest that a major gene is unlikely to account for familial TCCUT but, since none of the Mendelian single gene models could be statistically rejected, an inherited form of TCCUT cannot be excluded. The power of this analysis was constrained by the very small number of affected first-degree relatives in the subset of multiple-case families; a segregation analysis of a larger cohort, with a special effort aimed at identifying and documenting TCCUTs among more distant relatives (permitting their inclusion in the analysis), could more definitively rule in or out the possibility of a major bladder cancer gene.
Low-Penetrance Genes
The analysis of low-penetrance, common genetic variants in genes thought to be biologically plausible candidates for genetic modifiers of human cancer susceptibility comprises one of today’s most active areas of cancer genetics research. Although these genetic alterations confer only small-to-modest levels of risk, their high prevalence potentially explains a significant proportion of a given cancer’s etiology. Numerous single nucleotide polymorphisms in many genes involved in genetic pathways such as carcinogen metabolism, DNA repair, and cell cycle control have been studied as candidate bladder cancer risk modifiers, but results have been inconsistent and meta-analyses have typically not been performed [2, 73, 78]. A complete review of these studies is beyond the scope of this manuscript; however the references cited above provide additional detail on this subject.
We will focus on the most extensively-studied variants in genes involved in carcinogen metabolism/detoxification related to bladder cancer, N-acetyltransferase 2 (NAT2) and glutathione S-transferase (GSTM1). The NAT2 slow acetylator phenotype, and the GSTM1 null genotype, present in 40–60% and 50% of Caucasians respectively, are each associated with increased bladder cancer risk.[2, 79, 80] A meta-analysis of 31 case-control studies confirmed modestly-increased bladder cancer risks, with estimated odds ratios of 1.4 (95% CI 1.2–1.6) and 1.5 (95% CI 1.3–1.6) for NAT2 and GSTM1, respectively.[42] Case-only meta-analyses evaluating genotype-smoking interactions confirmed the absence of a multiplicative interaction for the GSTM1 null variant (OR = 1.0; 95% CI 0.9–1.2), and provided evidence supporting a positive interaction with the NAT2 slow acetylator phenotype (OR = 1.2; 95% CI 1.1–1.5). Furthermore, these investigators estimated that the GSTM1 null variant and NAT2 slow acetylation genotypes together might account for 31% (95% CI 20–46) of bladder cancer among Caucasians. In their bladder cancer case-control study, Murta-Nascimento et al. showed that, among family history-positive subjects, NAT2 slow acetylator genotype cases were at greater bladder cancer risk (OR=4.8) than those who were rapid/intermediate acetylators (OR=1.2) (Table 4). This study was limited by small sample size in their subgroup analyses, but did support the hypothesis that genetic factors play a role in bladder cancer etiology. This association is biologically plausible because NAT2 detoxifies aromatic amines, one family of carcinogens found in tobacco smoke; and is one of the best-established examples of a gene/environment interaction in cancer pathogenesis.
As discussed previously, debrisoquine hydroxylase (encoded by CYP2D6) is a Phase 1 enzyme involved in metabolism of xenobiotics, and as many as 25% of all medications. It is estimated that the extreme poor- and ultra-rapid-metabolizer debrisoquine phenotypes are present in 5–10% and 5% of Caucasians, respectively, [81] but the poor metabolizer phenotype has been inconsistently associated with reduced bladder cancer susceptibility.[82, 83] Interestingly, it has been suggested that the extensive metabolizer phenotype may contribute to tobacco addiction.[84]
The presence of single low-penetrance genetic variants alone are not likely to result in familial aggregations of TCCUT, although they could potentially act together, and/or interact with environmental exposures, and/or modify the penetrance of a major cancer susceptibility gene. Recently, Wu et al. used a unique pathway-based multigenic approach to examine the association between 44 DNA repair and cell cycle control genes and bladder cancer risk in a hospital-based case (n=696)-control (n=629) study.[85] They obtained ORs of 1.2 (95% CI 0.8–1.8), 1.6 (95% CI 1.0–2.4), and 1.8 (95% CI 1.2–2.6) for individuals with 13–15, 16–17, and 18 or more adverse alleles, respectively. Their findings suggested that individuals with higher cumulative numbers of adverse genetic variants in DNA repair and cell cycle control genes are at increased risk of bladder cancer. They also showed that smokers with a larger number of genetic variants had a higher risk of bladder cancer than nonsmokers (p < .01).
Several case-control studies have attempted to assess intrinsic genetic instability and bladder cancer risk by inducing DNA damage using various assays [86, 87], and by measuring telomere length [88–90]. Overall there appears to be a small increased bladder cancer risk associated with greater susceptibility to DNA damage and shorter telomere length, but the risks associated with potential genotypes and environmental exposures such as smoking is unclear. Aben et al. evaluated mutagen sensitivity by further classifying his cases into “hereditary” (2 TCC patients diagnosed age < 60, or 3 TCC patients in one nuclear family), “familial” (2 TCC patients in one nuclear family), and sporadic (no first-degree relative with TCC) TCCUT patients and healthy controls.[86] Mutagen sensitivity was measured by bleomycin-induced chromatid breaks per cell, which were significantly increased among all TCCUT cases compared with controls (p=0.001). Sporadic and “familial” TCCUT patients had the highest mutagen sensitivity, while “hereditary” TCCUT patients were similar to controls. The authors hypothesized that mutagen sensitivity increased the risk of “non-hereditary” TCCUT, and that “hereditary” TCCUT evolves as a consequence of a germline high-penetrance mutation conferring TCCUT risk regardless of carcinogen exposure. This study was limited by small sample size, possible misclassification of TCCUT risk groups, and a high prevalence of smoking, which prevented a stratified analysis by smoking status.
DISCUSSION
TCCUT is an environmentally-driven cancer, with tobacco exposure accounting for two-thirds and one-third of cases in men and women, respectively. Other environmental exposures (e.g., aromatic amines) have also been clearly implicated in its pathogenesis. Epidemiologic studies show that family history of TCCUT is associated with an approximately two-fold increase in bladder cancer risk that cannot be fully explained by smoking, but it is currently uncertain how genes and environment contribute to the origin of these familial clusters.
Genetic determinants of TCCUT risk have been less systematically investigated. It is not widely appreciated that TCCUT is a component of several rare, hereditary cancer susceptibility disorders, including HNPCC, hereditary retinoblastoma, Costello syndrome and, possibly, Apert syndrome. Furthermore, reports of multiple-case TCCUT families are infrequent in the literature compared with the other common adult solid tumors, e.g., breast, ovary, colon, and melanoma. Our review revealed only 32 unique families.
A complex segregation analysis of a large cohort provided no support for a major TCCUT susceptibility gene, but was inadequately powered to definitively exclude this possibility. Linkage analyses of multiple-case families have not been performed, likely reflecting the limited number of families available, and the fact that most families consist of only two affected family members. Cytogenetic studies of multiple-case families employing sequentially higher resolution analytic strategies have failed to identify candidate gene locations. [25, 33]
Our research program is actively considering a major new research effort aimed at recruiting a large number of multiple-case, site-specific TCCUT families to clarify the basis for familial TCCUT aggregation. However, the limited number of families reported which might be appropriate for such studies led us to speculate that this may not be an optimal approach for TCCUT, particularly if shared environmental exposures and the known genetic disorders (e.g., HNPCC, etc.) account for some of these familial clusters. We initiated a recruiting campaign, based on mailings to members of the American Urological Association, American Society of Clinical Oncology and the National Society of Genetic Counselors. In the 10 months since the mailing, we have identified only 5 new families (although we continue to accept new referrals: Clinical Genetics Branch Family Studies Referral Nurse at 1-800-518-8474). Either such kindred are exceedingly uncommon, or they are under-reported, perhaps in the mistaken belief that TCCUT is an environmental rather than a genetic disorder. Among all familial TCCUT aggregations, a major gene might still account for a meaningful (perhaps site-specific) subset, as illustrated by the genetic disorders reviewed above, each of which includes a predisposition to bladder/ureteral cancer. This important question can only be answered by additional studies targeting extended multiple-case families.
The most provocative genetic observations related to TCCUT derive from the analyses of low-penetrance, common variants in carcinogen metabolism and detoxification genes. The most striking being the NAT2 slow acetylator phenotype as both a primary bladder cancer risk factor and a mediator of the relationship between tobacco smoking and bladder cancer risk. However, our attempt to correlate the acetylation (NAT2) and debrisoquine (CYP2D6) metabolic phenotype with familial bladder cancer risk in two families yielded no useful etiologic clues. It is likely that other genetic variants will be identified that impact TCCUT risk.
Common low-penetrance genetic factors likely contribute to familial TCCUT, and may act in concert with shared environmental exposures. While a traditional linkage analysis might employ several thousand to tens of thousands of genetic markers, genome-wide association studies (GWAS) now routinely employ 500,000 to 1,000,000 genetic markers. Thus, the genome is much more densely covered, facilitating the identification of new disease susceptibility loci. The study design, computational and biostatistical challenges of the GWAS strategy are enormous, but this approach is now yielding substantial numbers of high-impact, novel genetic observations for many different diseases, both malignant and non-malignant. A complete discussion of this new analytic approach is beyond the scope of the current manuscript, but numerous reviews are available for the interested reader.[91–92] The International Bladder Cancer Consortium is considering a GWAS, which has the potential to clarify the etiologic role of the candidate genetic pathways reviewed here, as well as characterizing gene/environment interactions that contribute to TCCUT carcinogenesis.
Acknowledgments
We are deeply indebted to our patients and their family members for their participation in NCI clinical research protocols. The patients described herein were evaluated under the auspices of NCI Protocol 78-C-0039, an IRB-approved study.
FUNDING
Drs. Mueller, Caporaso and Greene are supported through funding provided by the Intramural Research Program of the National Cancer Institute.
Footnotes
COMPETING INTERESTS
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christine M. Mueller, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, 6120 Executive Boulevard, EPS 7101, Rockville, MD 20852-7231, USA.
Neil Caporaso, Genetic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute National Institutes, of Health/DHHS, Rockville, MD, USA
Mark H. Greene, Mark H. Greene, M.D., Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health/DHHS, Rockville, MD, USA
References
- 1.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer Statistics, Trends, and Multiple Primary Cancer Analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12(1):20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 2.Silverman DT, Devesa SS, Moore LE, Rothman N. Bladder Cancer. 3. New York: Oxford University Press; 2006. [Google Scholar]
- 3.American Cancer Society. Cancer Facts and Figures 2007. American Cancer Society; Atlanta: 2007. [Google Scholar]
- 4.Messing EM. Urothelial tumors of the urinary tract. 8. Philadelphia: Elsevier Science; 2002. [Google Scholar]
- 5.Zeegers MP, Tan FE, Dorant E, van Den Brandt PA. The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer. 2000;89(3):630–9. doi: 10.1002/1097-0142(20000801)89:3<630::aid-cncr19>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3(10):733–44. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 7.Olfert SM, Felknor SA, Delclos GL. An updated review of the literature: risk factors for bladder cancer with focus on occupational exposures. South Med J. 2006;99(11):1256–63. doi: 10.1097/01.smj.0000247266.10393.72. [DOI] [PubMed] [Google Scholar]
- 8.Davis DA, Cohen PR. Genitourinary tumors in men with the Muir-Torre syndrome. J Am Acad Dermatol. 1995;33(5 Pt 2):909–12. doi: 10.1016/0190-9622(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher O, Easton D, Anderson K, Gilham C, Jay M, Peto J. Lifetime risks of common cancers among retinoblastoma survivors. J Natl Cancer Inst. 2004;96(5):357–63. doi: 10.1093/jnci/djh058. [DOI] [PubMed] [Google Scholar]
- 10.Gripp KW. Tumor predisposition in Costello syndrome. Am J Med Genet C Semin Med Genet. 2005;137(1):72–7. doi: 10.1002/ajmg.c.30065. [DOI] [PubMed] [Google Scholar]
- 11.Ong E, Joseph JV, Bramwell SP, Haites NE. Multifocal transitional cell carcinoma in a patient with hereditary nonpolyposis colon cancer. BJU Int. 2003;91(3):297. doi: 10.1046/j.1464-410x.2003.04048.x. [DOI] [PubMed] [Google Scholar]
- 12.Aben KK, Witjes JA, Schoenberg MP, Hulsbergen-van de Kaa C, Verbeek AL, Kiemeney LA. Familial aggregation of urothelial cell carcinoma. Int J Cancer. 2002;98(2):274–8. doi: 10.1002/ijc.10191. [DOI] [PubMed] [Google Scholar]
- 13.Randi G, Pelucchi C, Negri E, et al. Family history of urogenital cancers in patients with bladder, renal cell and prostate cancers. Int J Cancer. 2007;121(12):2748–52. doi: 10.1002/ijc.23037. [DOI] [PubMed] [Google Scholar]
- 14.Murta-Nascimento C, Silverman DT, Kogevinas M, et al. Risk of bladder cancer associated with family history of cancer: do low-penetrance polymorphisms account for the increase in risk? Cancer Epidemiol Biomarkers Prev. 2007;16(8):1595–600. doi: 10.1158/1055-9965.EPI-06-0743. [DOI] [PubMed] [Google Scholar]
- 15.Cartwright RA. Genetic association with bladder cancer. Br Med J. 1979;2(6193):798. doi: 10.1136/bmj.2.6193.798-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst. 1994;86(21):1600–8. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- 17.Kantor AF, Hartge P, Hoover RN, Fraumeni JF., Jr Familial and environmental interactions in bladder cancer risk. Int J Cancer. 1985;35(6):703–6. doi: 10.1002/ijc.2910350602. [DOI] [PubMed] [Google Scholar]
- 18.Kiemeney LA, Moret NC, Witjes JA, Schoenberg MP, Tulinius H. Familial transitional cell carcinoma among the population of Iceland. J Urol. 1997;157(5):1649–51. [PubMed] [Google Scholar]
- 19.Kramer AA, Graham S, Burnett WS, Nasca P. Familial aggregation of bladder cancer stratified by smoking status. Epidemiology. 1991;2(2):145–8. doi: 10.1097/00001648-199103000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Kunze E, Chang-Claude J, Frentzel-Beyme R. Life style and occupational risk factors for bladder cancer in Germany. A case-control study Cancer. 1992;69(7):1776–90. doi: 10.1002/1097-0142(19920401)69:7<1776::aid-cncr2820690721>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Spitz MR, Dinney CP, Etzel CJ, Grossman HB, Wu X. Bladder cancer risk as modified by family history and smoking. Cancer. 2006;107(4):705–11. doi: 10.1002/cncr.22071. [DOI] [PubMed] [Google Scholar]
- 22.Lynch HT, Kimberling WJ, Lynch JF, Brennan K. Familial bladder cancer in an oncology clinic. Cancer Genet Cytogenet. 1987;27(1):161–5. doi: 10.1016/0165-4608(87)90270-6. [DOI] [PubMed] [Google Scholar]
- 23.Piper JM, Matanoski GM, Tonascia J. Bladder cancer in young women. Am J Epidemiol. 1986;123(6):1033–42. doi: 10.1093/oxfordjournals.aje.a114331. [DOI] [PubMed] [Google Scholar]
- 24.Plna K, Hemminki K. Familial bladder cancer in the National Swedish Family Cancer Database. J Urol. 2001;166(6):2129–33. [PubMed] [Google Scholar]
- 25.Aben KK, Macville MV, Smeets DF, Schoenberg MP, Witjes JA, Kiemeney LA. Absence of karyotype abnormalities in patients with familial urothelial cell carcinoma. Urology. 2001;57(2):266–9. doi: 10.1016/s0090-4295(00)00905-5. [DOI] [PubMed] [Google Scholar]
- 26.Lynch HT, Walzak MP, Fried R, Domina AH, Lynch JF. Familial factors in bladder carcinoma. J Urol. 1979;122(4):458–61. doi: 10.1016/s0022-5347(17)56461-7. [DOI] [PubMed] [Google Scholar]
- 27.Benton B, Henderson BE. Environmental exposure and bladder cancer in young males. J Natl Cancer Inst. 1973;51(1):269–70. doi: 10.1093/jnci/51.1.269. [DOI] [PubMed] [Google Scholar]
- 28.Blattner WA, Greene MH, Goedert JJ. Interdisciplinary studies in the evaluation of persons at high risk of cancer. New York: Academic Press; 1983. [Google Scholar]
- 29.Burkland CE, Juzek RH. Familial occurrence of carcinoma of the ureter. J Urol. 1966;96(5):697–701. doi: 10.1016/S0022-5347(17)63333-0. [DOI] [PubMed] [Google Scholar]
- 30.Egilsson V, Einarsson GV, Thorhallsson P, Ingvarsson S. Urinary system tumours in a family. Eur J Cancer. 1993;29A(16):2335–6. doi: 10.1016/0959-8049(93)90233-6. [DOI] [PubMed] [Google Scholar]
- 31.Fraumeni JF, Jr, Thomas LB. Malignant bladder tumors in a man and his three sons. JAMA. 1967;201(7):97–9. [Google Scholar]
- 32.Kenet G, Mandel M, Mor Y, et al. Genetic predisposition and cyclophosphamide treatment in a girl with bladder carcinoma? Med Pediatr Oncol. 1995;24(4):269–70. doi: 10.1002/mpo.2950240411. [DOI] [PubMed] [Google Scholar]
- 33.Kiemeney LA, Kuiper RP, Pfundt R, et al. No evidence for large-scale germline genomic aberrations in hereditary bladder cancer patients with high-resolution array-based comparative genomic hybridization. Cancer Epidemiol Biomarkers Prev. 2006;15(1):180–3. doi: 10.1158/1055-9965.EPI-05-0714. [DOI] [PubMed] [Google Scholar]
- 34.Leklem JE, Brown RR. Abnormal tryptophan metabolism in a family with a history of bladder cancer. J Natl Cancer Inst. 1976;56(6):1101–4. doi: 10.1093/jnci/56.6.1101. [DOI] [PubMed] [Google Scholar]
- 35.Mahboubi AO, Ahlvin RC, Mahboubi EO. Familial aggregation of urothelial carcinoma. J Urol. 1981;126(5):691–2. doi: 10.1016/s0022-5347(17)54693-5. [DOI] [PubMed] [Google Scholar]
- 36.Marchetto D, Li FP, Henson DE. Familial carcinoma of ureters and other genitourinary organs. J Urol. 1983;130(4):772–3. doi: 10.1016/s0022-5347(17)51452-4. [DOI] [PubMed] [Google Scholar]
- 37.McCullough DL, Lamma DL, McLaughlin AP, 3rd, Gittes RF. Familial transitional cell carcinoma of the bladder. J Urol. 1975;113(5):629–35. doi: 10.1016/s0022-5347(17)59540-3. [DOI] [PubMed] [Google Scholar]
- 38.Orphali SL, Shols GW, Hagewood J, Tesluk H, Palmer JM. Familial transitional cell carcinoma of renal pelvis and upper ureter. Urology. 1986;27(5):394–6. doi: 10.1016/0090-4295(86)90400-0. [DOI] [PubMed] [Google Scholar]
- 39.Purtilo DT, McCarthy B, Yang JP, Friedell GH. Familial urinary bladder cancer. Semin Oncol. 1979;6(2):254–6. [PubMed] [Google Scholar]
- 40.Schoenberg M, Kiemeney L, Walsh PC, Griffin CA, Sidransky D. Germline translocation t(5;20)(p15;q11) and familial transitional cell carcinoma. J Urol. 1996;155(3):1035–6. [PubMed] [Google Scholar]
- 41.Sharma SK, Bapna BC, Singh SM. Familial profile of transitional cell carcinoma. Br J Urol. 1976;48(6):442. doi: 10.1111/j.1464-410x.1976.tb06677.x. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Closas M, Malats N, Silverman D, et al. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet. 2005;366(9486):649–59. doi: 10.1016/S0140-6736(05)67137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lower GM, Jr, Nilsson T, Nelson CE, Wolf H, Gamsky TE, Bryan GT. N-acetyltransferase phenotype and risk in urinary bladder cancer: approaches in molecular epidemiology. Preliminary results in Sweden and Denmark. Environ Health Perspect. 1979;29:71–9. doi: 10.1289/ehp.792971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant DM, Tang BK, Kalow W. Variability in caffeine metabolism. Clin Pharmacol Ther. 1983;33(5):591–602. doi: 10.1038/clpt.1983.80. [DOI] [PubMed] [Google Scholar]
- 45.Grant DM, Tang BK, Kalow W. A simple test for acetylator phenotype using caffeine. Br J Clin Pharmacol. 1984;17(4):459–64. doi: 10.1111/j.1365-2125.1984.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cartwright RA, Philip PA, Rogers HJ, Glashan RW. Genetically determined debrisoquine oxidation capacity in bladder cancer. Carcinogenesis. 1984;5(9):1191–2. doi: 10.1093/carcin/5.9.1191. [DOI] [PubMed] [Google Scholar]
- 47.Kaisary A, Smith P, Jaczq E, et al. Genetic predisposition to bladder cancer: ability to hydroxylate debrisoquine and mephenytoin as risk factors. Cancer Res. 1987;47(20):5488–93. [PubMed] [Google Scholar]
- 48.Ayesh R, Idle JR, Ritchie JC, Crothers MJ, Hetzel MR. Metabolic oxidation phenotypes as markers for susceptibility to lung cancer. Nature. 1984;312(5990):169–70. doi: 10.1038/312169a0. [DOI] [PubMed] [Google Scholar]
- 49.Green-Gallo LA, Buivys DM, Fisher KL, et al. A protocol for the safe administration of debrisoquine in biochemical epidemiologic research protocols for hospitalized patients. Cancer. 1991;68(1):206–10. doi: 10.1002/1097-0142(19910701)68:1<206::aid-cncr2820680138>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 50.Knight A, Askling J, Granath F, Sparen P, Ekbom A. Urinary bladder cancer in Wegener’s granulomatosis: risks and relation to cyclophosphamide. Ann Rheum Dis. 2004;63(10):1307–11. doi: 10.1136/ard.2003.019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo Z, Linn JF, Wu G, et al. CDC91L1 (PIG-U) is a newly discovered oncogene in human bladder cancer. Nat Med. 2004;10(4):374–81. doi: 10.1038/nm1010. [DOI] [PubMed] [Google Scholar]
- 52.Schultz IJ, Kiemeney LA, Witjes JA, et al. CDC91L1 (PIG-U) mRNA expression in urothelial cell carcinomas. Int J Cancer. 2005;116(2):282–4. doi: 10.1002/ijc.21040. [DOI] [PubMed] [Google Scholar]
- 53.Jones B, Oh C, Mangold E, Egan CA. Muir-Torre syndrome: Diagnostic and screening guidelines. Australas J Dermatol. 2006;47(4):266–9. doi: 10.1111/j.1440-0960.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 54.Umar A, Risinger JI, Hawk ET, Barrett JC. Testing guidelines for hereditary non-polyposis colorectal cancer. Nat Rev Cancer. 2004;4(2):153–8. doi: 10.1038/nrc1278. [DOI] [PubMed] [Google Scholar]
- 55.Lindor NLC, Greene MH. Hereditary neoplastic syndromes. 3. New York: Oxford University Press; 2006. [Google Scholar]
- 56.Lynch HT, Boland CR, Gong G, et al. Phenotypic and genotypic heterogeneity in the Lynch syndrome: diagnostic, surveillance and management implications. Eur J Hum Genet. 2006;14(4):390–402. doi: 10.1038/sj.ejhg.5201584. [DOI] [PubMed] [Google Scholar]
- 57.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenland JE, Weston PM, Wallace DM. Familial transitional cell carcinoma and the Lynch syndrome II. Br J Urol. 1993;72(2):177–80. doi: 10.1111/j.1464-410x.1993.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 59.Lynch HT, Ens JA, Lynch JF. The Lynch syndrome II and urological malignancies. J Urol. 1990;143(1):24–8. doi: 10.1016/s0022-5347(17)39853-1. [DOI] [PubMed] [Google Scholar]
- 60.Sijmons RH, Kiemeney LA, Witjes JA, Vasen HF. Urinary tract cancer and hereditary nonpolyposis colorectal cancer: risks and screening options. J Urol. 1998;160(2):466–70. [PubMed] [Google Scholar]
- 61.Chan H, Pratt CB. A new familial cancer syndrome? A spectrum of malignant and benign tumors including retinoblastoma, carcinoma of the bladder and other genitourinary tumors, thyroid adenoma, and a probable case of multifocal osteosarcoma. J Natl Cancer Inst. 1977;58(2):205–7. doi: 10.1093/jnci/58.2.205. [DOI] [PubMed] [Google Scholar]
- 62.Tarkkanen A, Karjalainen K. Excess of cancer deaths in close relatives of patients with bilateral retinoblastoma. Ophthalmologica. 1984;189(3):143–6. doi: 10.1159/000309401. [DOI] [PubMed] [Google Scholar]
- 63.DerKinderen DJ, Koten JW, Nagelkerke NJ, Tan KE, Beemer FA, Den Otter W. Non-ocular cancer in patients with hereditary retinoblastoma and their relatives. Int J Cancer. 1988;41(4):499–504. doi: 10.1002/ijc.2910410405. [DOI] [PubMed] [Google Scholar]
- 64.Sanders BM, Jay M, Draper GJ, Roberts EM. Non-ocular cancer in relatives of retinoblastoma patients. Br J Cancer. 1989;60(3):358–65. doi: 10.1038/bjc.1989.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aherne G. Retinoblastoma associated with other primary malignant tumours. Trans Ophthalmol Soc U K. 1974;94(4):938–44. [PubMed] [Google Scholar]
- 66.Draper GJ, Sanders BM, Kingston JE. Second primary neoplasms in patients with retinoblastoma. Br J Cancer. 1986;53(5):661–71. doi: 10.1038/bjc.1986.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moll AC, Imhof SM, Bouter LM, et al. Second primary tumors in patients with hereditary retinoblastoma: a register-based follow-up study, 1945–1994. Int J Cancer. 1996;67(4):515–9. doi: 10.1002/(SICI)1097-0215(19960807)67:4<515::AID-IJC9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 68.Schlienger P, Campana F, Vilcoq JR, et al. Nonocular second primary tumors after retinoblastoma: retrospective study of 111 patients treated by electron beam radiotherapy with or without TEM. Am J Clin Oncol. 2004;27(4):411–9. doi: 10.1097/01.coc.0000128861.46357.ee. [DOI] [PubMed] [Google Scholar]
- 69.Hennekam RC. Costello syndrome: an overview. Am J Med Genet C Semin Med Genet. 2003;117(1):42–8. doi: 10.1002/ajmg.c.10019. [DOI] [PubMed] [Google Scholar]
- 70.Aoki Y, Niihori T, Kawame H, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37(10):1038–40. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 71.Andreou A, Lamy A, Layet V, et al. Early-onset low-grade papillary carcinoma of the bladder associated with Apert syndrome and a germline FGFR2 mutation (Pro253Arg) Am J Med Genet A. 2006;140(20):2245–7. doi: 10.1002/ajmg.a.31430. [DOI] [PubMed] [Google Scholar]
- 72.Coumoul X, Deng CX. Roles of FGF receptors in mammalian development and congenital diseases. Birth Defects Res C Embryo Today. 2003;69(4):286–304. doi: 10.1002/bdrc.10025. [DOI] [PubMed] [Google Scholar]
- 73.Kim WJ, Quan C. Genetic and epigenetic aspects of bladder cancer. J Cell Biochem. 2005;95(1):24–33. doi: 10.1002/jcb.20412. [DOI] [PubMed] [Google Scholar]
- 74.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5(9):713–25. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 75.Herrera L, Kakati S, Gibas L, Pietrzak E, Sandberg AA. Gardner syndrome in a man with an interstitial deletion of 5q. Am J Med Genet. 1986;25(3):473–6. doi: 10.1002/ajmg.1320250309. [DOI] [PubMed] [Google Scholar]
- 76.Francke U, Kung F. Sporadic bilateral retinoblastoma and 13q- chromosomal deletion. Med Pediatr Oncol. 1976;2(4):379–85. doi: 10.1002/mpo.2950020404. [DOI] [PubMed] [Google Scholar]
- 77.Aben KK, Baglietto L, Baffoe-Bonnie A, et al. Segregation analysis of urothelial cell carcinoma. Eur J Cancer. 2006;42(10):1428–33. doi: 10.1016/j.ejca.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 78.Caporaso N. Genetic Modifiers of Cancer Risk. 3. New York: Oxford University Press; 2006. [Google Scholar]
- 79.Garte S, Gaspari L, Alexandrie AK, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10(12):1239–48. [PubMed] [Google Scholar]
- 80.Hein DW, Doll MA, Fretland AJ, et al. Molecular Genetics and Epidemiology of the NAT1 and NAT2 Acetylation Polymorphisms. Cancer Epidemiol Biomarkers Prev. 2000;9(1):29–42. [PubMed] [Google Scholar]
- 81.de Leon J, Armstrong SC, Cozza KL. Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 2D6 and CYP450 2C19. Psychosomatics. 2006;47(1):75–85. doi: 10.1176/appi.psy.47.1.75. [DOI] [PubMed] [Google Scholar]
- 82.Wolf CR, Smith CA, Gough AC, et al. Relationship between the debrisoquine hydroxylase polymorphism and cancer susceptibility. Carcinogenesis. 1992;13(6):1035–8. doi: 10.1093/carcin/13.6.1035. [DOI] [PubMed] [Google Scholar]
- 83.Branch RA, Chern HD, Adedoyin A, et al. The procarcinogen hypothesis for bladder cancer: activities of individual drug metabolizing enzymes as risk factors. Pharmacogenetics. 1995;(5 Spec No):S97–102. doi: 10.1097/00008571-199512001-00009. [DOI] [PubMed] [Google Scholar]
- 84.Saarikoski ST, Sata F, Husgafvel-Pursiainen K, et al. CYP2D6 ultrarapid metabolizer genotype as a potential modifier of smoking behaviour. Pharmacogenetics. 2000;10(1):5–10. doi: 10.1097/00008571-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 85.Wu X, Gu J, Grossman HB, Amos CI, et al. Bladder cancer predisposition: a multigenic approach to DNA-repair and cell-cycle-control genes. Am J Hum Genet. 2006;78(3):464–79. doi: 10.1086/500848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aben KK, Cloos J, Koper NP, Braakhuis BJ, Witjes JA, Kiemeney LA. Mutagen sensitivity in patients with familial and non-familial urothelial cell carcinoma. Int J Cancer. 2000;88(3):493–6. [PubMed] [Google Scholar]
- 87.Schabath MB, Spitz MR, Grossman HB, et al. Genetic instability in bladder cancer assessed by the comet assay. J Natl Cancer Inst. 2003;95(7):540–7. doi: 10.1093/jnci/95.7.540. [DOI] [PubMed] [Google Scholar]
- 88.Broberg K, Bjork J, Paulsson K, Hoglund M, Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis. 2005;26(7):1263–71. doi: 10.1093/carcin/bgi063. [DOI] [PubMed] [Google Scholar]
- 89.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16(4):815–9. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 90.Wu X, Amos CI, Zhu Y, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003;95(16):1211–8. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 91.Hunter DJ, Thomas G, Hoover RN, Chanock SJ. Scanning the horizon: What is the future of genome-wide association studies in accelerating discoveries in cancer etiology and prevention? Cancer Causes Control. 2007;18:479–484. doi: 10.1007/s10552-007-0118-y. [DOI] [PubMed] [Google Scholar]
- 92.Pearson JV, Huentelman MJ, Halperin RF, et al. Identification of the genetic basis for complex disorders by use of pooling-based genomewide single-nucleotide–polymorphism association studies. Am J Hum Genet. 2007;80:126–139. doi: 10.1086/510686. [DOI] [PMC free article] [PubMed] [Google Scholar]