Abstract
The objective was to study the competition of chloride released from a Ag/AgCl cathode on the iontophoretic delivery of dexamethasone phosphate (Dex-Phos). Iontophoresis of Dex-Phos was performed in side-by-side diffusion cells (0.78 cm2) using pig skin. A 0.3 mA constant current was applied via Ag/AgCl electrodes. The amounts of Dex-Phos and dexamethasone (Dex) were also quantified in the stratum corneum (SC), using tape stripping, after passive and iontophoretic delivery. The profiles of Dex-Phos and Dex, as a function of position in the SC, were deduced. The iontophoretic delivery of Dex-Phos from pure water was unaffected by the accumulation of Cl− released by the donor cathode when the drug’s concentration was 4.25 mM to 17 mM. At 0.85 mM, however, Cl− competition was significant and the drug flux was significantly reduced. Formulation of the drug in the presence of Cl− resulted in a non-linear dependence of flux on the molar fraction of the drug. Tape stripping experiments confirmed the enhanced delivery of Dex-Phos by iontophoresis relative to passive diffusion, with Dex-Phos concentration greater inside the barrier post-iontophoresis than that in the donor. The latter observation could explain the robustness of Dex-Phos delivery to the presence of Cl− in the donor solution.
Keywords: iontophoresis, skin, transport number, dexamethasone phosphate
1. Introduction
Iontophoresis is used to enhance the transdermal passage of charged and polar molecules, for both drug delivery and clinical monitoring applications [1–3], by the application of a small electrical current (<0.5 mA/cm2). The two principal mechanisms of transdermal iontophoretic transport, electromigration and electroosmosis have been extensively described in previous work [1, 2, 4]. According to Faraday’s law, the iontophoretic flux (Jd) of a drug “d” transported by electromigration, the main mechanism of transport for charged drugs, is directly proportional to the intensity of the current (I) applied:
| (eq. 1) |
where td is the transport number of the drug, zd its valence and F is Faraday’s constant [5].
The transport number of the drug is the fraction of the electrical current applied which it transports across the skin. The drug competes with all other ions present in the system to carry the charge and it follows that, to optimize electromigration, the presence of all other ions should be minimized. Ideally, co-ions are absent from the formulation, such that only endogenous counter-ions can compete with the drug, the transport number of which is then maximized [6, 7]. When competing co-ions are present, the transport number of the drug usually depends upon its molar fraction rather than its absolute concentration [5, 8, 9].
Electrodes are critical components of an iontophoretic system and transfer the electrical current supplied by the external circuit into ion migration across the skin [10, 11]. Ag/AgCl electrodes are commonly used for iontophoretic delivery and their electrochemistry is driven at a sufficiently low potential insuring that unwanted secondary reactions are unlikely. The electrode reactions are:
| (eq. 2a) |
| (eq. 2b) |
Importantly, compared to electrodes made of inert materials (platinum, stainless steel and glassy carbon for example), water electrolysis does not occur and pH shifts are avoided [11]. Ag/AgCl electrodes are well-adapted for the delivery of cationic drugs available as chloride salts provided there is a sufficient quantity of chloride in the donor to satisfy the electrochemistry at the anode. However, when negatively charged drugs are iontophoresed from the cathode, chloride ions released by the electrode will compete to transport the current and reduce the efficiency of delivery [12–15]. Strategies to circumvent this phenomenon have been described in the patent literature [16–18].
Previously, the iontophoretic delivery of dexamethasone phosphate (Dex-Phos) from the cathode was shown to be minimally affected by the release of Cl− when the drug was present in the donor solution at concentrations between 0.2 and 0.8% w/v in water [19]. The objective of the present study was therefore to better understand the effects of chloride anions released from the Ag/AgCl cathode on the delivery of Dex-Phos. To this end, the iontophoresis of Dex-Phos was investigated systematically as a function of the amount of Cl− present. In addition, tape stripping experiments were performed post-iontophoresis to assess the accumulation of the drug in the membrane.
2. Materials and methods
2.1. Materials
Dexamethasone (Dex) (>98%) and dexamethasone sodium phosphate (Dex-Na2-Phos) (>98%) were purchased from Sigma-Aldrich Co. (Gillingham, UK). Sodium chloride, Na2HPO4, KH2PO4 and phosphoric acid (85%) were from Acros (Geel, Belgium). Potassium citrate, methanol (HPLC grade), acetonitrile (far UV HPLC grade) were from Fisher Scientific (Loughborough, UK). NaOH 50% (ion chromatography eluent grade) was from Fluka (Buchs, Switzerland). Ag wire (> 99.99% purity) and AgCl (99.999%) were purchased from Sigma-Aldrich Co. (Gillingham, UK). All regents were at least analytical grade unless stated otherwise and all aqueous solutions were prepared using high purity deionized water (18.2 MΩ·cm, Barnstead Nanopure Diamond™, Dubuque, IA). The percentage concentration of Dex-Na2-Phos solutions is expressed in term of the Dex-Phos concentration. For example, a 0.4% Dex-Phos solution contains 4.4 mg/ml of Dex-Na2-Phos.
2.2. Skin preparation
Porcine ears were obtained locally. Ears were cleaned under cold, running water and the skin was dermatomed to a nominal thickness of 750 µm (Zimmer™ Electric Dermatome, Dover, OH). The pieces of tissue obtained (~9 cm2) were wrapped individually in Parafilm™ and stored for no more than three months at −20°C until use.
2.3. Iontophoresis
Iontophoresis experiments were performed in vitro in side-by-side diffusion cells (transport area = 0.78 cm2) with the stratum corneum side facing the donor, cathodal chamber. In all experiments, the subdermal compartment was filled with phosphate-buffered saline (PBS - 170 mM sodium, 1.4 mM potassium, 137 mM chloride and 18 mM phosphate) at pH 7.4. Both chambers held 3.5 ml of solution and were magnetically stirred. In all experiments, a 0.3 mA constant current (0.38 mA/cm2) was applied for 6 hours via Ag/AgCl electrodes connected to a power source (Yokogawa 7651 Programmable DC source, Woodburn Green, UK). Experiments were performed at room temperature with a minimum of five replicates, using skin from at least two different pigs. Every hour for the first two hours and every half-hour for the remaining 4 hours of the experiment, 1 ml of the receptor compartment was collected and replaced by the same volume of PBS.
Two series of solutions were tested. In the first set, the donor solution consisted of Dex-Phos solutions with a concentration of 0.85 mM (0.04% w/v), 4.25 mM (0.2%), 8.5 mM (0.4%) or 17 mM (0.8%) in pure water. When the donor solution was 8.5 mM Dex-Phos in water, in addition to sampling of the receptor chamber, 0.3 ml of the donor solution was also collected every hour and assayed for chloride. In one specific experiment, the donor solution (Dex-Phos 8.5 mM in water) was continuously perfused (15 ml/h) through a 1.5 ml donor compartment, using a peristaltic pump, during iontophoresis. This ensured that the concentration of Cl− never attained 1/10th of the drug’s concentration as confirmed by ion chromatography (see sample analysis). In the second set of experiments, the donor solutions consisted of 8.5 mM Dex-Phos containing 10.3 mM (0.06%), 51 mM (0.3%), 103 mM (0.6%) or 154 mM (0.9%) NaCl as background electrolyte.
2.4. Tape-stripping
In three experiments, for which the donor solution was Dex-Phos 8.5 mM in water, the amount of drug in the stratum corneum (SC) was assessed by tape stripping following (a) passive diffusion for 3h (n = 4), (b) iontophoresis for 30 minutes (n = 5), and (c) iontophoresis for 3 hours (n = 7).
At the end of the experiment, any solution remaining on the skin surface was removed using absorbent paper. The skin was then pinned to a dissecting board and a polypropylene foil template with a circular aperture (8 mm diameter) was positioned over the treated area. The SC was then removed by repeated adhesive tape-stripping (Scotch Book Tape, 3M, St. Paul, MN). Between 15 and 28 tapes were required to completely ablate the barrier. Each tape was weighed before and after stripping on a 0.1-µg precision balance (Satorius SE2-F, Epsom, UK) to determine the mass and thickness of the SC layer removed [20, 21]. Dex and Dex-Phos were completely extracted from the tapes by overnight shaking with 1 ml of 30:70 acetonitrile: pH 2 phosphate buffer. Validation of the extraction procedure involved spiking tapestripped samples of untreated skin with a known amount of Dex and/or Dex-Phos; recovery was (98 ± 2)% (n = 6).
2.5. Sample Analysis
The concentrations of Dex-Phos and Dex in the receptor chamber and tape extracts were assayed by high-performance liquid chromatography (ASI-100 automated sample injector, P680 pump, TCC-100 thermostated column compartment, PDA-100 diode array detector, Dionex, Sunnyvale, CA) under isocratic conditions. A mobile phase consisting of 30:70 (v:v) acetonitrile:phosphate buffer (0.15 M, pH 2) was pumped (0.75 ml/min) through a Lichrospher® 100 RP-18 (4×125 mm) reverse-phase column (HiChrom, Reading, UK) fitted with its guard column and thermostated at 25°C. Dex-Phos and Dex concentrations were quantified via their UV absorbance at 240 nm using their respective linear calibration curves (correlation coefficient > 0.999, relative standard deviation < 5%) obtained from a minimum of five standard solutions (made from 500 ppm stock solutions in methanol appropriately diluted in PBS pH 7.4) covering the entire range of experimental concentrations. The retention times for Dex-Phos and Dex were ~3.5 and ~10.5 minutes respectively; the detection limit for both was 0.02 µg/ml for a 25 µl sample injection (used for analysis in receptor compartment) and 0.01 µg/ml for a 50 µl sample injection (used for the analysis of tape extracts).
Ion chromatography (AS50 autosampler and thermal compartment, GP50 gradient pump, ED50 electrochemical detector, Chromeleon software, Dionex, Sunnyvale, CA) was used to measure the concentrations of chloride. The 35 mM NaOH mobile phase was pumped under isocratic conditions (1 ml/min) through an IonPac™ AS16 column (Dionex, 250×4 mm) thermostated at 30°C and the ASRS ULTRA II suppressor (Dionex, 4 mm) set at a current of 90 mA. Quantification was performed against the linear calibration curve (correlation coefficient > 0.999, relative standard deviation < 2%) obtained from at least five sodium chloride solutions.
2.6. Data analysis and statistics
Linear regressions, Lowess curves and statistics were performed using Graph Pad Prism V.4.00 (Graph Pad Software Inc., San Diego, CA, USA). Statistical differences within multiple data sets were assessed by one-way ANOVA, followed by a Tukey’s multiple comparison test. The level of statistical significance was fixed at p < 0.05. The fluxes were obtained from the slope of the cumulative amount delivered as a function of time for each replicate and are expressed as mean ± SD. The reported Dex-Phos flux is the sum of the Dex-Phos and Dex fluxes into the receptor phase to account for the partial dephosphorylation of the prodrug that occurred during transdermal passage or in the receptor phase. Transport numbers were calculated using these fluxes and equation 1. The pH of the donor solution did not significantly shift during the experiments, and was measured in the range 7.2–7.6 in all cases. As a consequence, the valence used for Dex-Phos in all calculations was 2, as at least 85% of the Dex-Phos in solution was in the di-anionic form.
3. Results and discussion
3.1. Effect of Cl−
Cl− ions are released from the Ag/AgCl electrode into the cathode compartment at a constant rate determined by the electrical current flowing in the circuit (eq. 2b). Hence, the concentration of the competing co-ion gradually increases in the donor solution even when it initially contained only Dex-Phos in water. Figure 1 compares the theoretical evolution of the Cl− concentration in the 3.5 ml donor solution, as a function of time, for a current of 0.3 mA, assuming that all the chloride formed remains in the electrode compartment, with the experimentally measured values when the Dex-Phos concentration was 0.4%. The Figure also shows the Cl− concentrations predicted (dashed line) when 20% of the charge is carried by these ions liberated at the cathode. The measured values suggest that when Dex-Phos is present at a reasonably high concentration in water, the contribution of Cl− to the transport of current is relatively modest and is less than 20% as had been previously deduced from the measurement of the transport of the counter-ions [19].
Figure 1.
Theoretical evolution of Cl− concentration in the cathode compartment over a 6 hour period of iontophoresis at 0.3 mA when either all the ions remain in the donor (solid line) or when 20% are subsequently ‘delivered’ across the skin (dashed line). Experimentally measured values when the donor solution was initially 8.5 mM Dex-Phos in water (mean ± SD, n = 11) are shown for comparison. Also shown, are the different Dex-Phos donor concentrations used (dotted lines).
Earlier results showed that the Dex-Phos fluxes from 4.25 mM, 8.5 mM and 17 mM solutions in water were statistically indistinguishable, despite the fact that the times and current doses at which the Cl− concentration reached a level equivalent to that of Dex-Phos were quite different. Respectively, these can be read from Figure 1: for 4.25 mM Dex-Phos, Cl− reached the same concentration at ~80 minutes (current dose of about 24 mA·min); for 8.5 mM at 160 minutes (48 mA·min); for 17 mM at 320 minutes (96 mA·min). The same was true when the 8.5 mM Dex-Phos in water was perfused continuously to maintain the level of Cl− at 1/10th of this concentration (Figure 2). Only for 0.85 mM is the Dex-Phos flux markedly lower (see Table 1 and Figure 2); in this case Cl− matches the Dex-Phos level after 16 minutes (4.8 mA·min).
Figure 2.
Dex-Phos fluxes from a donor solution containing drug at 8.5 mM in various concentrations of saline, compared to those obtained when the drug was dissolved in water alone. The intensity of current applied was 0.3 mA in all cases.
Table 1.
Dex-Phos fluxes and transport numbers under various conditions. The initial molar fractions of the drug in the donor solutions ([Dex-Phos]/([Dex-Phos] + [Cl−])) and their evolution with the duration of iontophoresis (t) are also shown.
| Experimental condition | Dex-Phos flux [nmol/h] | 100*Transport numbera | Dex-Phos molar fraction | |||
|---|---|---|---|---|---|---|
| t = 0 | t = 1h | t = 3h | t = 6h | |||
| Dex-Phos 8.5 mM in 154 mM NaCl | 14 ± 4 | 0.25 ± 0.07 | 0.05 | 0.05 | 0.05 | 0.05 |
| Dex-Phos 8.5 mM in 103 mM NaCl | 19 ± 9 | 0.34 ± 0.16 | 0.08 | 0.07 | 0.07 | 0.07 |
| Dex-Phos 8.5 mM in 51 mM NaCl | 37 ± 10 | 0.66 ± 0.18 | 0.14 | 0.13 | 0.12 | 0.11 |
| Dex-Phos 8.5 mM in 10.3 mM NaCl | 49 ± 22 | 0.88 ± 0.39 | 0.45 | 0.39 | 0.30 | 0.22 |
| Dex-Phos 8.5 mM in H2O. Perfused | 73 ± 25 | 1.3 ± 0.4 | 1 | > 0.9 | > 0.9 | > 0.9 |
| Dex-Phos 17 mM in H2O | 71 ± 26 | 1.3 ± 0.4 | 1 | 0.84 | 0.64 | 0.47 |
| Dex-Phos 8.5 mM in H2O | 69 ± 24 | 1.2 ± 0.4 | 1 | 0.73 | 0.47 | 0.31 |
| Dex-Phos 4.25 mM in H2O | 65 ± 24 | 1.2 ± 0.4 | 1 | 0.57 | 0.31 | 0.18 |
| Dex-Phos 0.85 mM in H2O | 27 ± 10 | 0.48 ± 0.18 | 1 | 0.21 | 0.08 | 0.04 |
Multiplication of the transport number by 100 yields a value equal to the percentage of the charge being transported across the skin.
The impact of Cl− in the drug formulation was systematically studied and the combined results of the new experiments reported here together with those from before are summarized in Figure 2 and Table 1. Clearly, the transport of the drug is progressively hampered by an increase in the presence of the competing halide.
Table 1 also includes the theoretically calculated molar fractions of Dex-Phos as a function of the time of iontophoresis assuming that all Cl− released from the cathode remained in the donor solution. It is apparent that, whenever the drug’s molar fraction was less than 50% after 1 hour of current passage, the Dex-Phos flux was compromised. In some cases, it is worth noting, the molar fraction of drug dropped precipitately during the 6 hours of iontophoresis; for example, from 1 to 0.18 and to 0.04, respectively, for 4.25 mM and 0.85 mM Dex-Phos in water. Nevertheless, it is intriguing to note that, despite the decreasing Dex-Phos molar fraction, the drug’s iontophoretic flux over the 6-hours duration of the experiment first increased over the initial period of current passage before remaining relatively constant. This phenomenon is illustrated in Figure 3 for the two donor solutions mentioned above. In other words, the anticipated falling-off of the flux with increasing time (as has been observed for other anions, such as amino acids and peptides [12, 13], piroxicam [14] and diclofenac [15] under similar conditions) was not observed.
Figure 3.
Measured Dex-Phos fluxes as a function of time of iontophoresis (square symbols, mean ± SD) compared to the calculated change in the molar fraction of the drug in the donor solution (dotted line) (upper panel: 4.25 mM Dex-Phos in water; lower panel 0.85 mM Dex-Phos in water). The intensity of current applied was 0.3 mA in all cases.
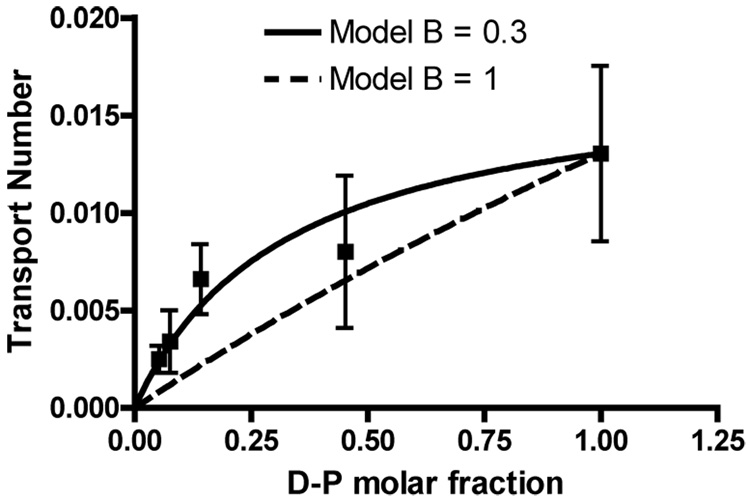
The transport numbers reported for Dex-Phos in Table 1 do not vary linearly with the initial molar fraction of Dex-Phos in the donor solution (Figure 4). In this respect, the results diverge from those reported for small cations (Na+, K+, Li+ and NH4+), and for drugs like lidocaine [8], but parallel those seen for quinine and propanolol, two relatively lipophilic cations believed to interact significantly with the fixed charge on the skin and to change its permselective properties [7]. The results reported here raise the question, therefore, as to whether Dex-Phos is perhaps altering the membrane in some way and/or accumulating in the tissue.
Figure 4.
Transport numbers of Dex-Phos as a function of its initial molar fraction in the donor solution compared to predictions of the Phipps and Gyory model [5].
In attempting to predict the effect of co-ion competition, Phipps and Gyory derived an expression for the transport number of a drug across a homogeneous uncharged membrane as a function of its molar fraction in the donor solution [5]:
| (eq. 3) |
where t0d and t0a are the transport numbers of the drug and the co-anion (Cl−) when they are the only species present (the single anion situation), B is a proportionality constant that relates the ratio of the anion concentrations in the membrane to that in the donor solution, Z is the valence ratio of the anions and X their molar fraction ratio in the donor solution. In practice, this approach has found little practical use because there is no validated method to predict the value of the parameter B [8]. In theory, when B = 1, the concentration ratio of the co-anion and the drug is conserved inside the membrane; a value of B < 1 suggests a higher concentration of the drug in the membrane relative to that of the co-anion when compared to the donor solution. The data from the experiments described here were fitted to the model assuming t0a = 0.4, the reported transport number of Cl− in the single ion situation [6]. The B value derived from the fit was 0.3 (Figure 4) suggesting that the apparent concentration of the drug relative to Cl− inside the skin is more important relative to that in the donor solution.
3.2. Tape-stripping
Tape-stripping experiments were performed to test directly whether Dex-Phos accumulated in the stratum corneum (SC) during iontophoretic delivery from a donor solution containing Dex-Phos 8.5 mM in water. The results are in Table 2. When no current was applied (passive control), the amount of drug present in the SC was an order of magnitude less than that achieved post-iontophoresis. The duration of current passage influenced the amount of drug (Dex-Phos + Dex) uptake into the SC, with about 70% more being found in the SC after 3 hours compared to 30 minutes. The higher level of Dex recovered accounted for nearly all of this difference. However, the absolute drug level in the SC, even with iontophoresis, was small compared to the measured flux (~69 nmol/h) implying that “accumulation” was not a significant factor.
Table 2.
Dex-Phos, Dex and {Dex-Phos + Dex} uptake into the SC after 3 hours of passive diffusion or following 30 minutes or 3 hours of iontophoresis.
| Iontophoresis 3 hours | Iontophoresis 30 minutes | Passive diffusion 3 hours | |
|---|---|---|---|
| Dex-Phos in SC [nmol] | 4.9 ± 2.0 | 4.6 ± 0.9 | 0.41 ± 0.17 |
| Dex in SC [nmol] | 5.0 ± 1.6 | 1.4 ± 0.2 | 0.28 ± 0.06 |
| Dex-Phos + Dex [nmol] | 9.9 ± 3.3 | 6.0 ± 1.1 | 0.69 ± 0.24 |
As the tape-stripping experiment involved individual analysis of drug on all the strips, and quantification of the amount of SC removed (achieved gravimetrically as previously described [20]), it was possible to derive concentration profiles of Dex-Phos and Dex across the SC after passive diffusion and following either 30 minutes or 3 hours of iontophoresis (Figure 5). When no current was applied, the profiles decayed rapidly from the skin surface, intercepting the concentration axis at a level similar to that of Dex-Phos in the donor solution (8.5 mM). With iontophoresis, on the other hand, while the surface level of the drug was similar, its concentration (and that of Dex as well) then increased over the first one-third or so of the SC, before falling off towards the inner boundary of the barrier (although not always returning to zero). Dex-Phos levels were obviously higher than Dex at 30 minutes but had become quite similar (as well as of greater absolute magnitude) by 3 hours of current passage.
Figure 5.
Dex-Phos (left panel) and Dex (right panel) concentration profiles as a function of relative position in the stratum corneum for (a) 3h of passive diffusion (n = 4), (b) 30 min of iontophoresis (n = 5), and (c) 3h iontophoresis (n = 7) of a solution of 8.5 mM Dex-Phos in H2O. Each piece of skin is represented by a different symbol and the full line tracks the trend of the data without following a specific model (Lowess curve).
The pattern observed in Figure 5 has been predicted theoretically from the Nernst-Plank equation (with the electroneutrality assumption) [22, 23], and shows that iontophoresis can enhance a drug’s concentration in the membrane relative to that in the delivery formulation. Whether the increased level of Dex-Phos can account for the negligible impact of Cl− released from the cathode in some of the experiments reported here cannot be unambiguously deduced. Certainly, it could be argued that an elevated presence of anions inside the already negatively-charged membrane would be more likely to raise the transport number of Na+ (moving from the receiver solution into the cathode chamber) and disadvantage the migration of Cl−, at least over the relatively short duration of these experiments.
4. Conclusions
In summary, the results presented here demonstrate that the delivery of Dex-Phos from a Ag/AgCl cathode is relatively robust to the presence of Cl− in the donor solution. From a practical standpoint, the results suggest that optimal delivery of the drug should be obtained if the cathode compartment contains sufficient Dex-Phos to ensure that its molar fraction remains > 50% for the first ~20 mA·min of the dose. The exact reasons for this phenomenon are still unclear, but quantification of the drug in the SC by tape-stripping revealed that it was not due to a significant absolute accumulation of the drug in this barrier. The concentration profile of the Dex-Phos inside the SC could, on the other hand, bring some new insight to this problem.
Acknowledgements
This research was supported by the US National Institutes of Health (EB–001420). J.-P. S. thanks BIJAB, NSERC, FQRNT and Universities UK for funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Delgado-Charro MB, Guy RH. Transdermal iontophoresis for controlled drug delivery and non-invasive monitoring S.T.P. Pharma. Sci. 2001;11(6):403–414. [Google Scholar]
- 2.Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv. Drug Deliv. Rev. 2004;56(5):619–658. doi: 10.1016/j.addr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Leboulanger B, Guy RH, Delgado-Charro MB. Reverse iontophoresis for noninvasive transdermal monitoring. Physiol. Meas. 2004;25(3):R35–R50. doi: 10.1088/0967-3334/25/3/r01. [DOI] [PubMed] [Google Scholar]
- 4.Pikal MJ. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 2001;46(1–3):281–305. doi: 10.1016/s0169-409x(00)00138-1. [DOI] [PubMed] [Google Scholar]
- 5.Phipps JB, Gyory JR. Transdermal ion migration. Adv. Drug Deliv. Rev. 1992;9:137–176. [Google Scholar]
- 6.Mudry B, Guy RH, Delgado-Charro MB. Electromigration of ions across the skin: determination and prediction of transport numbers. J. Pharm. Sci. 2006;95(3):561–569. doi: 10.1002/jps.20561. [DOI] [PubMed] [Google Scholar]
- 7.Marro D, Kalia YN, Delgado-Charro MB, Guy RH. Contributions of electromigration and electroosmosis to iontophoretic drug delivery. Pharm. Res. 2001;18(12):1701–1708. doi: 10.1023/a:1013318412527. [DOI] [PubMed] [Google Scholar]
- 8.Mudry B, Guy RH, Delgado-Charro MB. Prediction of iontophoretic transport across the skin. J. Control. Release. 2006;111(3):362–367. doi: 10.1016/j.jconrel.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Mudry B, Guy RH, Delgado-Charro MB. Transport numbers in transdermal iontophoresis. Biophys. J. 2006;90(8):2822–2830. doi: 10.1529/biophysj.105.074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott ER, Phipps B, Gyory R, Padmanabhan RV. Electrotransport System for Transdermal Delivery: A Practical Implementation of Iontophoresis. In: Wise DL, editor. Handbook of Pharmaceutical Controlled Release Technology. New York: Marcel Dekker; 2000. pp. 617–659. [Google Scholar]
- 11.Cullander C, Rao G, Guy RH. Why silver/silver chloride? Criteria for iontophoresis electrodes. In: Brain KR, James VJ, Walters KA, editors. Prediction of Percutaneous Penetration. Vol. 3b. Cardiff: STS Publishing; 1993. pp. 381–390. [Google Scholar]
- 12.Green PG, Hinz RS, Cullander C, Yamane G, Guy RH. Iontophoretic delivery of amino acids and amino acid derivatives across the skin in vitro. Pharm. Res. 1991;8(9):1113–1120. doi: 10.1023/a:1015894016235. [DOI] [PubMed] [Google Scholar]
- 13.Green PG, Hinz RS, Kim A, Szoka FC, Jr, Guy RH. Iontophoretic delivery of a series of tripeptides across the skin in vitro. Pharm. Res. 1991;8(9):1121–1127. doi: 10.1023/a:1015846100305. [DOI] [PubMed] [Google Scholar]
- 14.Gay CL, Green PG, Guy RH, Francoeur ML. Iontophoretic delivery of piroxicam across the skin in vitro. J. Control. Release. 1992;22(1):57–67. [Google Scholar]
- 15.Hui X, Anigbogu A, Singh P, Xiong G, Poblete N, Liu P, Maibach HI. Pharmacokinetic and local tissue disposition of [14C]sodium diclofenac following iontophoresis and systemic administration in rabbits. J. Pharm. Sci. 2001;90(9):1269–1276. doi: 10.1002/jps.1079. [DOI] [PubMed] [Google Scholar]
- 16.Untereker DF, Phipps JB, Cahalan PT, Brennan KR. Iontophoresis electrode. 5,395,310. U.S. Patent. 1995 March 7;
- 17.Phipps JB, Moodie LC, Gyory JR, Theeuwes F. Electrotransport system with ion exchange material competitive ion capture. 6,289,249 B1. U.S. Patent. 1999
- 18.Haak RP. Electrotransport device having improved cathodic electrode assembly. 5,503,632. U.S. Patent. 1994 April 2;
- 19.Sylvestre J-P, Delgado-Charro MB, Guy RH. In vitro optimization of dexamethasone phosphate delivery by iontophoresis. Phys. Ther. 2008 doi: 10.2522/ptj.20080043. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalia YN, Pirot F, Guy RH. Homogeneous transport in a heterogeneous membrane: water diffusion across human stratum corneum in vivo. Biophys. J. 1996;71(5):2692–2700. doi: 10.1016/S0006-3495(96)79460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herkenne C, Naik A, Kalia YN, Hadgraft J, Guy RH. Pig ear skin ex vivo as a model for in vivo dermatopharmacokinetic studies in man. Pharm. Res. 2006;23(8):1850–1856. doi: 10.1007/s11095-006-9011-8. [DOI] [PubMed] [Google Scholar]
- 22.Li SK, Zhang YH, Zhu HG, Higuchi WI, White HS. Influence of asymmetric donor-receiver ion concentration upon transscleral iontophoretic transport. J. Pharm. Sci. 2005;94(4):847–860. doi: 10.1002/jps.20293. [DOI] [PubMed] [Google Scholar]
- 23.Kasting GB. Theoretical models for iontophoretic delivery. Adv. Drug Deliv. Rev. 1992;9:177–199. [Google Scholar]