Abstract
Ankle sprain is a frequent injury in humans that results in pain, swelling and difficulty in walking on the affected ankle. Currently a suitable animal model resembling human ankle sprain is lacking. Here, we describe an animal ankle sprain model induced by ankle ligament injury (ALI) in rats. Cutting combinations of the lateral ankle ligament complex produced pain, edema and difficulty of weight bearing, thereby mimicking severe (grade III) ankle sprain in humans. Analgesic compounds, morphine and indomethacin, significantly reversed the reduced weight bearing, thus indicating that reduction of weight bearing is partially due to pain. The ALI model is a new ankle sprain model that may be useful for the study of ankle sprain pain mechanisms and treatments and for the screening of new analgesic drugs.
Keywords: animal model, lateral ankle ligament injury, pain, weight bearing
Ankle sprain is one of the most common musculoskeletal injuries, accounting for about one million injuries per year for which people seek medical treatment in the United States [6, 14]. It occurs when the ankle is turned unexpectedly in any direction that is further than the ligaments are able to tolerate with the most common being due to hyper-inversion, which damages the lateral ankle ligaments [14]. Ankle sprain can be classified clinically into 3 grades: grade I involves stretching of any ligament; grade II includes incomplete tear of one or more ligaments; and grade III includes complete tear of one or more ligaments [6]. Although mild ankle sprain (grade I) responds well to conservative treatments and recovers to normal levels within 2–3 days, more than 40 percent of moderate to severe ankle sprains lead to recurrence, chronic ankle pain, complex regional pain syndrome, joint instability or joint stiffness [3, 4, 15].
In spite of its high incidence and clinical importance, proper animal models of ankle sprain are lacking for further studies of pain mechanisms and treatment paradigms. Our previous studies introduced a manually induced-ankle sprain model for the study of acupuncture analgesia [7, 8]. In this model, the ankle sprain was produced manually by overextending the foot in the direction of simultaneous inversion and plantar flexion in the rat. The procedure imitates human ankle sprain caused by hyper-inversion. These rats showed behavioral signs of ankle pain and edema similar to a human with mild ankle sprain (grade I) and then recovered to normal levels within 2–5 days. While this manually induced ankle sprain model closely mimics human ankle sprain conditions, there are two major problems with this approach. First, the manually induced ankle sprain is subject to intra- and inter-operator variations. Because this method relies on the operators’ finger force and skills that are subjective and variable depending on the operator, the outcome and the severity of ankle sprain varies considerably. Second, ligaments that are not normally involved in a typical ankle sprain, such as the calcaneocuboid, talonavicular and talocalcanean ligaments, are also affected. The ligaments that are affected by hyper-inversion induced ankle sprain in humans usually include the anterior and posterior talofibular (ATF & PTF) and calcaneofibular ligaments (CF). In an attempt to overcome the above mentioned problems of the previous model and to produce a more consistent and stereotyped ankle sprain model, this study employed standardized surgical injuries to the lateral ankle ligament complex. Surgical lesions of single or multiple ligaments with different combinations were made to simulate ankle sprain and then weight bearing and edema were measured on the affected limb. The group which produced symptoms close to those occurring in severe ankle sprain (Grade III) in humans was selected as an ankle sprain model for further study. To test the validity of the ankle ligament injury (ALI) model as a pain model, we tested the effects of two clinically relevant analgesic compounds, morphine and indomethacin.
Adult male Sprague-Dawley rats (225–275 g, Harlan Sprague–Dawley Co., Houston, TX) were used. The rats were housed in groups of 2–3 in plastic cages with soft bedding under a reversed 12-hour light/dark cycle. All procedures involving the use of animals are approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch.
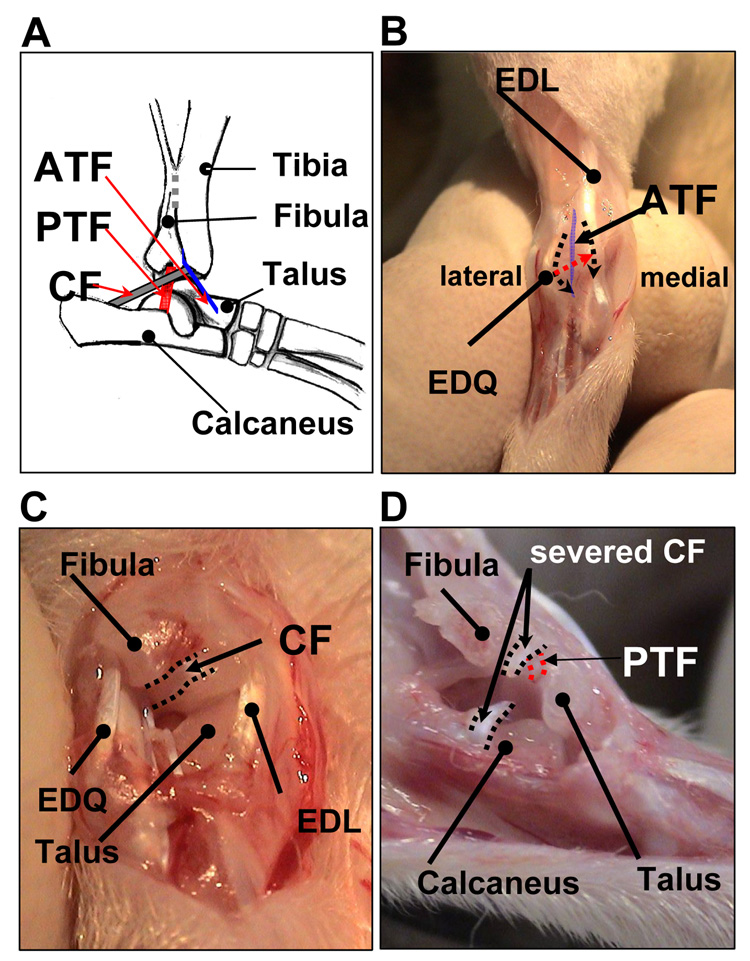
The first experiment was to explore the roles of each ligament of the lateral ankle ligament complex, the ATF, CF and PTF, in rat ankle sprain (Fig. 1A). It was necessary to cut the ATF to expose the remaining 2 ligaments, the CF and the PTF, thus rats were divided into 4 groups: 1) ATF group (n=5, only ATF was cut); 2) ATF+CF group (n=7, both ATF and CF were cut); 3) ATF+PTF group (n=8, both ATF and PTF were cut); and 4) ATF+CF+PTF group (n=8, all three ligaments were cut). To perform surgery, animals were anesthetized under isoflurane. After shaving the right ankle joint and local asepsis, the skin overlying the anterolateral aspect of the right ankle was incised longitudinally to expose the ankle joint. The three incisions of the joint capsule were made: two 3–5 mm longitudinal cuts, one along the medial margin of the extensor digiti quinti (EDQ) and the other along the lateral margin of the extensor digitorum longus (EDL) (2 black dotted arrow lines in Fig. 1B) and an oblique cut between EDQ and EDL (red dotted arrow line in Fig. 1B). During the oblique cut of the joint capsule, the ATF attached to the joint capsule was incised simultaneously (Fig. 1B). After opening the joint capsule, the CF was identified and transected in the ATF+CF and ACF+CF+PTF groups (Fig. 1C). After slight inversion of the ankle joint, the PTF was identified between the inner surface of the fibular malleolus and the lateral tubercle of talus and transected at the mid point in the ACF+PTF and ACF+CF+PTF groups (Fig. 1D). The surgical wound was irrigated with saline and closed.
Fig. 1.
(A) A schematic drawing of the lateral ankle ligaments: the anterior talofibular (ATF), the calcaneofibular (CF) and the posterior talofibular ligaments (PTF) (slightly inverted-lateral view). (B, C and D) Surgical procedures for ankle ligament injury (right foot): (B) anterior view, (C) anterolateral view, and (D) lateral view of the ankle.
To evaluate the pain levels of the affected ankle, we measured the weight bearing ratio (WBR) of the affected foot, as described in previous publications [7, 8]. Briefly, two electronic balances were placed together on one side of the walking path in a long and narrow plastic chamber (8.5 cm width, 9.5 cm high, 60 cm long) at the midway point. Each rat was allowed to walk through the chamber. The foot stepping forces from two consecutive steps were obtained for WBR. WBR was calculated as percentage of the stepping force of the affected limb to body weight.
To measure the foot volume, water displacement volumetric measurement was used as described in the previous study [8].
The group showing the most severe pain and edema was selected as the ankle ligament injury induced-ankle sprain model (ALI model). The effects of two clinically proven analgesic compounds, morphine and indomethacin, were tested on WBR and edema in the ALI model. Morphine (2 or 5 mg/kg, morphine sulfate; Sigma, St Louis, MO) and indomethacin (5 mg/kg; Sigma, St Louis, MO) were dissolved in saline and 0.5% Tween 80 in saline, respectively, and given intraperitoneally at a volume of 1 ml/kg on post-surgery day 1. The drugs were prepared immediately before each use. Control groups received the same volume of vehicle.
To examine the stiffness of the joint after ALI, the ranges of motion of the ankle (joint angle between full flexion and full extension) were measured [10] on post-surgery days 1, 2, 3 and 7 in ALI rats (n=6), and compared to that of the normal side. After the last measurements, the rats were sacrificed and the ankle joints were inspected macroscopically.
Data are presented as mean ± standard error of the mean (SEM). The data were analyzed by one- or two-way repeated-measure analysis of variance (ANOVA), followed by post-hoc testing using the Holm-Sidak method. P values ≤0.05 were considered statistically significant.
Prior to the ankle sprain surgery, the lateral ligament complex of the ankle joint was carefully examined in normal rats. The CF was a reasonably thick and round ligament, which connects from the apex of the fibular malleolus to the lateral surface of the calcaneus, and limited hyper-inversion of the calcaneus. The ATF was identified as a thin band fused with the ankle joint capsule, running from the distal anterior tip of the fibula to the lateral talar neck. The PTF was the shortest of the three and passed from the inner surface of the fibular malleolus to the lateral tubercle of talus and exerted great tension when the ankle was dorsiflexed and inverted (Fig. 1A).
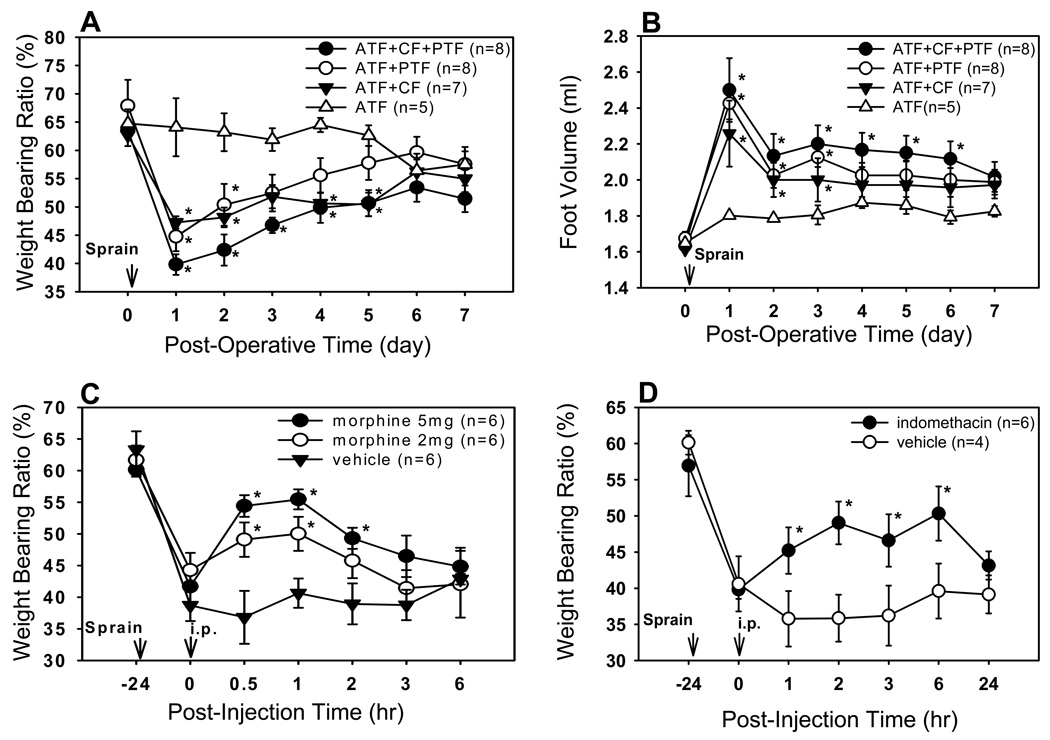
To explore the roles of the above three ligaments in ankle stability, we made surgical lesions of single or multiple ligaments with different combinations ATF, ATF+CF, ATF+PTF, and ATF+CF+PTF. Weight bearing ratios (WBR) and foot volume were measured before and after ALI. The WBR of the normal right hindlimb before surgery was on average 62–67 % of the body weight. One day after ALI, the WBRs were greatly reduced to 39.8 ± 1.8, 44.7 ± 2.6 and 47.2±1.1 % in the ATF+CF+PTF, ATF+PTF and ATF+CF groups, respectively. The animals started to recover gradually afterward, but their values stayed significantly lower than the pre-surgery values (p<0.05) up to post-surgery day 5 (Fig. 2A). The ATF group, which was subjected to ATF injury only, did not show any changes in WBR after surgery, thus it was used as a surgical control group. On the other hand, the WBR of the ATF+CF+PTF group was the lowest among the groups on post-surgery day 1. The significantly reduced WBR lasted up to post-surgery day 5 in the ATF+CF+PTF and ATF+CF groups but only to post-surgery day 2 in the ATF+PTF group, compared to the control ATF group (Fig. 2A). The changes in the foot volume were also measured before and after ALI. The average volume of the normal foot was 1.65 ± 0.01 ml. Injury to the ATF only did not show any change in foot volume compared to pre-surgery values. However, foot volumes (ml) one day after ALI were significantly increased to 2.50 ± 0.18, 2.42 ± 0.09 and 2.26 ± 0.18 ml in the ATF+CF+PTF, ATF+PTF and ATF+CF groups, respectively. Although the initial edema quickly subsided, it stayed at significantly high levels until post-surgery day 3 in the ATF+PTF and ATF+CF groups and until post-surgery day 6 in the ATF+CF+PTF group, compared to the control ATF group (p<0.05). The foot edema of the ATF+CF+PTF group was the most severe among all groups (Fig. 2B).
Fig. 2.
Changes of weight bearing ratios (% weight bearing force on injured ankle/total body weight) and foot volume following an ankle ligament injury and the effect of analgesic compounds on weight bearing ratios. (A, B) ATF+CF+PTF group showed the most severely reduced weight bearing ratios and increased foot volume among 4 groups. ATF group: only ATF was cut, ATF+CF group: ATF and CF were cut, leaving the PTF intact, ATF+PTF group: ATF and PTF were cut, leaving the CF intact, and ATF+CF+PTF group: all three ligaments were cut. *P<0.05 compared with ATF group. (C) Effect of systemic morphine on the weight bearing ratios in ATF+CF+PTF injured rats. Morphine (both 2 and 5 mg/kg) significantly improved the reduced weight bearing ratios for at least 1 hr in a dose-dependent manner. *P<0.05 compared to the vehicle group. (D) Effect of systemic indomethacin on the weight bearing ratios in ATF+CF+PTF injured rats. Indomethacin (5mg/kg, i.p.)-treated rats showed significant increases of weight bearing ratios up to 6 hr, compared to vehicle control. * P<0.05 compared to the vehicle group. The data are means ± SEM.
Since the ATF+CF+PTF group showed the most decrease in WBR and increase in edema, this group was selected as the ALI model and used to test pain in the following experiments. The effects of two clinically relevant analgesic and/or anti-inflammatory compounds, morphine and indomethacin [3, 6], on WBR and edema were tested. To test the effect of systemic morphine, rats were divided into 3 groups 1 day after ALI surgery and each received an intraperitoneal injection of one of the following drugs in a blind manner: 5 mg/kg of morphine (n=6), 2 mg/kg of morphine (n=6), or vehicle saline (n=6). Foot stepping forces were then measured at 0.5, 1, 2, 3 and 6 hr after drug injection. As shown in Fig. 2C, the WBR of the affected limb greatly decreased one day after ALI as compared to the pre-surgery value. Systemic morphine at a dose of 2 mg/kg and 5 mg/kg produced a graded increase in the WBR, which returned to the pre-treatment values by 3 hr after morphine treatment.
In another 10 one day post-surgery ALI model rats, WBR was measured before and after intraperitoneal injection of indomethacin (5 mg/kg, n=6) or vehicle (n=4). After indomethacin treatment, the WBR was significantly increased for 6 hrs as compared to vehicle treated groups (Fig. 2D). Foot edema was also measured before and after indomethacin injection in order to determine the possible anti-inflammatory effect of indomethacin. One day after ALI, the volume of the hind foot was significantly increased to 2.6 ml as compared to the pre-surgery value 1.6 ml. Although there was a tendency of reduced foot volume in the indomethacin group, this reduction was not statistically significant during the first 48 hr period after indomethacin treatment as compared to that of the control group.
The range of motion of the affected ankle was significantly decreased to 77°, 69°, 61 ° and 51 ° on day 1, day 2, day 3 and day 7 after injury to all 3 ligaments, respectively, compared to 120° in normal ankles, indicating increased joint stiffness. Post-mortem examination at 7 days after ALI revealed marked atrophy of the severed ligaments with a significant thickening of the joint capsule. Thus the results suggest that the thickening of the joint capsule might have restricted range of motion.
The aim of this study is to develop an animal model of ankle sprain that can be produced by a simple standardized method with consistent outcomes. The proposed surgical incision of individual lateral ankle ligament, though artificial, is a stereotypical procedure in which the operator's handling variations can be minimized. As a consequence, the degree of symptoms of ankle sprain primarily depends on the ligaments that are injured. Complete resection of CF, PTF, or CF+PTF in combination with ATF in the rat ankle, thus consistently produced a marked reduction in WBR and a rapid onset of swelling (edema) of the affected foot, indicating moderate to severe pain with inflammation. Ankle sprain can be categorized from grade I to grade III, depending on the severity of the injury [14]. Grade III is characterized by complete rupture of one or more ligaments, thus all the ankle ligament injured (ALI) rats in this study can be classified as grade III. All ALI rats, except the ones in the ATF group, displayed signs of pain and edema of the affected foot one day after surgery. By post-injury day 7, however, all animals recovered their WBRs up to 80–90 % of the pre-surgery values. Based on our results and post-mortem examination, the gradual improvement in the WBR after ALI is speculated to be due to thickening of the joint capsule which provided stability to the sprained ankle and subsequent improvement in gait function.
In humans, the lateral ankle ligaments consist of the ATF, the CF and the PTF [6]. From our observation, the CF and the PTF of the rat ankle have similar anatomical location and function to those in humans. The ATF, however, is a thin and weak membranous strip fused with the ankle joint capsule. Following the ATF injury only, rats did not show any change in weight bearing ratio and foot volume. The results thus indicate that the ATF ligament in the rat ankle is not a prominent structure and does not contribute significantly to ankle stability during movement. In contrast, the ATF in humans has been described as a major component of the lateral ligament complex of the ankle joint and plays an important role in limiting anterior displacement of the talus during plantar-flexion of the ankle [2, 13]. The tearing of the ATF can cause moderate pain, swelling, some loss of motion and function and mild to moderate instability of the ankle in humans [6, 12]. Our present results suggest that the PTF and CF are 2 main ligaments for stabilization of the lateral ankle in the rat and injury to these ligaments induces ankle sprain.
The partial recovery of weight bearing forces by systemic morphine in ALI rats led us to suggest that the ALI model can be used as a pain model. Indomethacin, a non-steroidal anti-inflammatory drug, has been used for alleviation of pain and inflammation in patients with ankle injury and arthritis [1, 9]. When indomethacin was injected systemically in ALI rats, the reduced WBRs were significantly recovered for several hours, thus confirming that the reduction of WBR is partially due to pain. While indomethacin is highly effective in relieving pain, its anti-inflammatory effect on edema, measured by foot volume, was not significant up to 48 hrs after the treatment when administered once at one day after the ALI surgery. A possible explanation is that since the anti-inflammatory action of indomethacin is mainly by peripheral inhibition of prostaglandin synthesis [5, 11], the compound may be less effective when injected after inflammation has fully developed. Alternatively, reduction of peripheral edema by indomethacin may require multiple treatments to be effective since it takes much longer to reach the peak concentration in joints than in the plasma [11].
In conclusion, ankle ligament injury (ALI) produces a reliable and consistent ankle sprain model which demonstrates significantly reduced weight bearing ratios, a sign of pain, and greatly increased swelling. Since this model provides reliable ankle sprain pain, it can be useful for screening new analgesic drugs.
Acknowledgements
This work was supported by an NIH/NCCAM grant R01 AT001474 and NIH/NINDS grants R01 NS 031680 and P01 NS 011255. We thank Ms. Denise Broker for her valuable assistance in editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Castro RR, Cunha FQ, Silva FS, Jr, Rocha FA. A quantitative approach to measure joint pain in experimental osteoarthritis--evidence of a role for nitric oxide. Osteoarthritis Cartilage. 2006;14:769–776. doi: 10.1016/j.joca.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Colville MR, Marder RA, Boyle JJ, Zarins B. Strain measurement in lateral ankle ligaments. Am. J. Sports Med. 1990;18:196–200. doi: 10.1177/036354659001800214. [DOI] [PubMed] [Google Scholar]
- 3.Diebschlag W, Nocker W, Lehmacher W. Treatment of acute ankle sprains. Comparison of the efficacy and tolerance of 2 indomethacin-gel preparations. Fortschr. Med. 1992;110:94–98. [PubMed] [Google Scholar]
- 4.Hernandez W, Raja A, Capuano C. Complex regional pain syndrome. J. Am. Podiatr. Med. Assoc. 1999;89:534–539. doi: 10.7547/87507315-89-10-534. [DOI] [PubMed] [Google Scholar]
- 5.Hu XH, Tang HW, Li QS, Huang XF. Central mechanism of indomethacin analgesia. Eur. J. Pharmacol. 1994;263:53–57. doi: 10.1016/0014-2999(94)90522-3. [DOI] [PubMed] [Google Scholar]
- 6.Ivins D. Acute ankle sprain: an update. Am. Fam. Physician. 2006;74:1714–1720. [PubMed] [Google Scholar]
- 7.Koo ST, Lim KS, Chung K, Ju H, Chung JM. Electroacupuncture-induced analgesia in a rat model of ankle sprain pain is mediated by spinal alpha-adrenoceptors. Pain. 2008;135:11–19. doi: 10.1016/j.pain.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koo ST, Park YI, Lim KS, Chung K, Chung JM. Acupuncture analgesia in a new rat model of ankle sprain pain. Pain. 2002;99:423–431. doi: 10.1016/S0304-3959(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 9.Oian P, Kleive I, Lereim P. Indomethacin in acute ankle torsion, Tidsskr. Nor. Laegeforen. 1981;101:91–92. [PubMed] [Google Scholar]
- 10.Okita M, Yoshimura T, Nakano J, Motomura M, Eguchi K. Effects of reduced joint mobility on sarcomere length, collagen fibril arrangement in the endomysium, and hyaluronan in rat soleus muscle. J. Muscle. Res. Cell. Motil. 2004;25:159–166. doi: 10.1023/b:jure.0000035851.12800.39. [DOI] [PubMed] [Google Scholar]
- 11.Paul AI. Analgesic-antipyrectic and antiinflammatory agents and drugs employed in the treatment of gout. Vol. 27. New York: McGraw-Hill Companies; 1996. pp. 633–635. [Google Scholar]
- 12.Samoto N, Sugimoto K, Takaoka T, Fujita T, Kitada C, Takakura Y. Comparative results of conservative treatments for isolated anterior talofibular ligament (ATFL) injury and injury to both the ATFL and calcaneofibular ligament of the ankle as assessed by subtalar arthrography. J. Orthop. Sci. 2007;12:49–54. doi: 10.1007/s00776-006-1090-1. [DOI] [PubMed] [Google Scholar]
- 13.Smith RW, Reischl S. The influence of dorsiflexion in the treatment of severe ankle sprains: an anatomical study. Foot Ankle. 1988;9:28–33. doi: 10.1177/107110078800900106. [DOI] [PubMed] [Google Scholar]
- 14.Wexler RK. The injured ankle. Am. Fam. Physician. 1998;57:474–480. [PubMed] [Google Scholar]
- 15.Wolfe MW, Uhl TL, Mattacola CG, McCluskey LC. Management of ankle sprains. Am. Fam. Physician. 2001;63:93–104. [PubMed] [Google Scholar]