Abstract
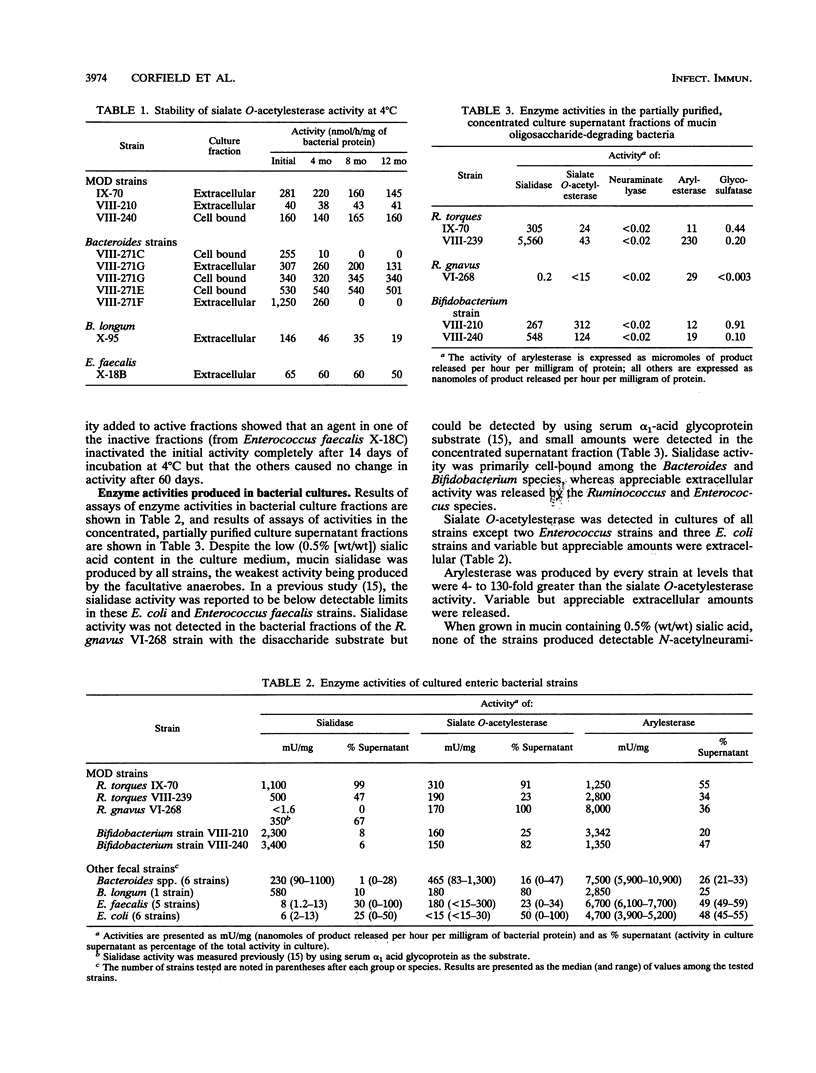
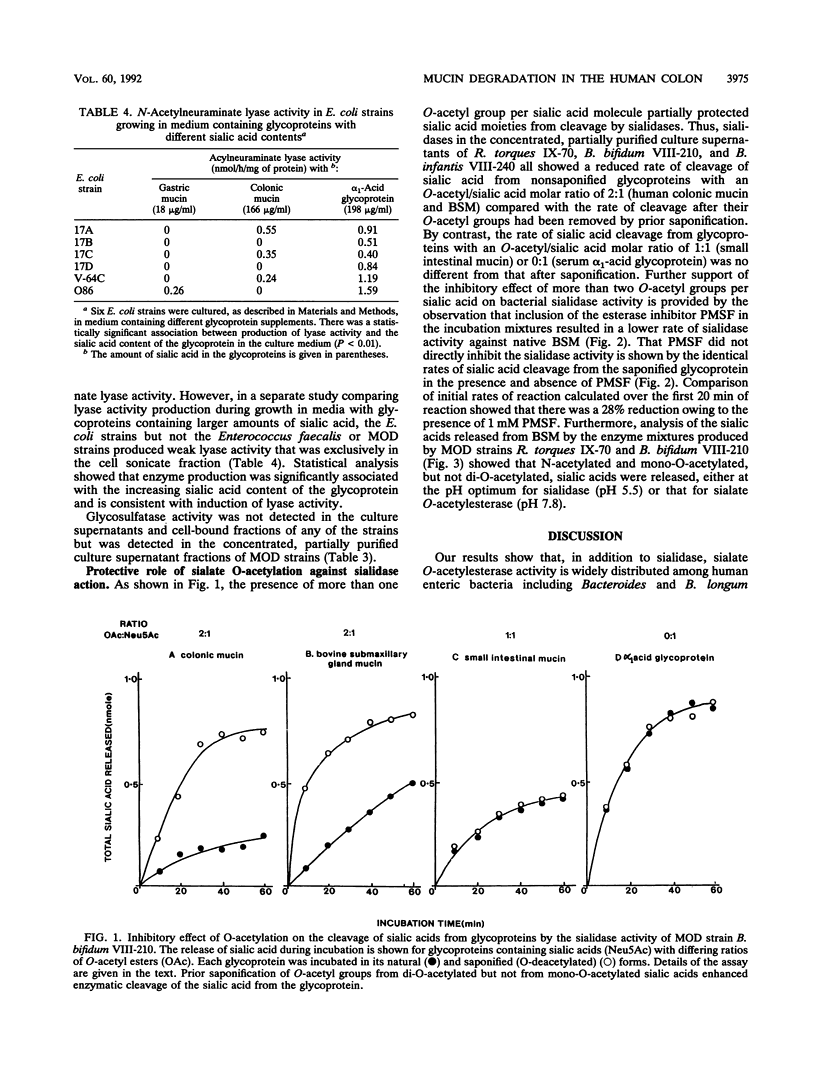
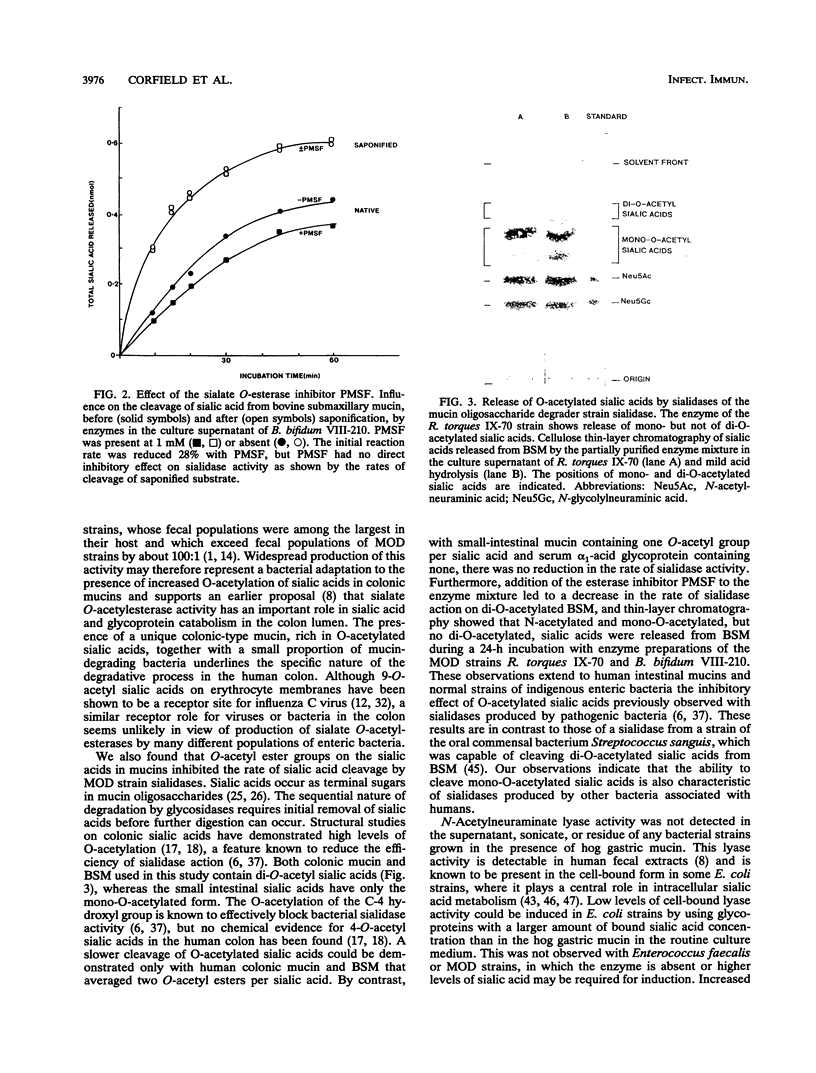
Oligosaccharide side chains of human colonic mucins contain O-acetylated sialic acids and glycosulfate esters. Although these substituents are considered to protect the chains against degradation by bacterial glycosidases, sialate O-acetylesterase, N-acetylneuraminate lyase, and glycosulfatase activities have been found in fecal extracts. To better define the source of these activities, we measured extracellular and cell-bound sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities produced by 23 isolates of human fecal bacteria grown anaerobically in a hog gastric mucin culture medium; these represented dominant populations of fecal anaerobes, facultative anaerobes, and the subset of mucin oligosaccharide-degrading bacteria. Every strain produced sialidase and high levels of arylesterase, and all but five facultative anaerobes produced sialate O-acetylesterase. Sialic acids containing 2 mol or more of O-acetyl ester per mol of sialic acid were cleaved from mucin glycoproteins more slowly by sialidases of mucin oligosaccharide-degrading stains than were sialic acids containing 1 or 0 mol, and only N-acetyl- and mono-O-acetylated sialic acids were recovered from enzyme digests of a mucin containing di-O-acetylated sialic acids. No detectable N-acetylneuraminate lyase activity was produced by any strain, but low activity was induced by increasing the glycoprotein-bound sialic acid concentration in the culture medium of six Escherichia coli strains. Using lactitol-6-sulfate as a substrate, we found weak glycosulfatase activity in the partially purified, concentrated enzyme mixture in the culture supernatants of four mucin oligosaccharide-degrading strains but in none of the unconcentrated culture fractions. We conclude that the presence of two or more O-acetyl groups on sialic acids inhibits enteric bacterial sialidases but that production of sialate O-acetylesterases by several populations of enteric bacteria lessens the likelihood that mucin oligosaccharide chains terminating in O-acetylated sialic acids are protected from degradation. Sialate O-acetylesterases have a role in bacterial degradation of mucin glycoproteins in the human colon.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clamp J. R. Chemical aspects of mucus. General considerations. Br Med Bull. 1978 Jan;34(1):25–27. doi: 10.1093/oxfordjournals.bmb.a071455. [DOI] [PubMed] [Google Scholar]
- Corfield A. P., Clamp J. R., Wagner S. A. The metabolism of sialic acids in isolated rat colonic mucosal cells. Biochem J. 1985 Feb 15;226(1):163–174. doi: 10.1042/bj2260163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield A. P., Sander-Wewer M., Veh R. W., Wember M., Schauer R. The action of sialidases on substrates containing O-acetylsialic acids. Biol Chem Hoppe Seyler. 1986 May;367(5):433–439. doi: 10.1515/bchm3.1986.367.1.433. [DOI] [PubMed] [Google Scholar]
- Corfield A. P., Wagner S. A., Paraskeva C., Clamp J. R., Durdey P., Reuter G., Schauer R. Loss of sialic acid O-acetylation in human colorectal cancer cells. Biochem Soc Trans. 1992 May;20(2):94S–94S. doi: 10.1042/bst020094s. [DOI] [PubMed] [Google Scholar]
- Corfield A. P., Williams A. J., Clamp J. R., Wagner S. A., Mountford R. A. Degradation by bacterial enzymes of colonic mucus from normal subjects and patients with inflammatory bowel disease: the role of sialic acid metabolism and the detection of a novel O-acetylsialic acid esterase. Clin Sci (Lond) 1988 Jan;74(1):71–78. doi: 10.1042/cs0740071. [DOI] [PubMed] [Google Scholar]
- Falk P., Hoskins L. C., Larson G. Bacteria of the human intestinal microbiota produce glycosidases specific for lacto-series glycosphingolipids. J Biochem. 1990 Sep;108(3):466–474. doi: 10.1093/oxfordjournals.jbchem.a123223. [DOI] [PubMed] [Google Scholar]
- Filipe M. I. Mucins in the human gastrointestinal epithelium: a review. Invest Cell Pathol. 1979 Jul-Sep;2(3):195–216. [PubMed] [Google Scholar]
- Herrler G., Rott R., Klenk H. D., Müller H. P., Shukla A. K., Schauer R. The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. EMBO J. 1985 Jun;4(6):1503–1506. doi: 10.1002/j.1460-2075.1985.tb03809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Agustines M., McKee W. B., Boulding E. T., Kriaris M., Niedermeyer G. Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J Clin Invest. 1985 Mar;75(3):944–953. doi: 10.1172/JCI111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Boulding E. T. Mucin degradation in human colon ecosystems. Evidence for the existence and role of bacterial subpopulations producing glycosidases as extracellular enzymes. J Clin Invest. 1981 Jan;67(1):163–172. doi: 10.1172/JCI110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins J. T., Reading C. L. Characterization of mono-, di-, and tri-O-acetylated sialic acids on human cells. J Cell Biochem. 1988 May;37(1):37–48. doi: 10.1002/jcb.240370105. [DOI] [PubMed] [Google Scholar]
- Hutchins J. T., Reading C. L., Giavazzi R., Hoaglund J., Jessup J. M. Distribution of mono-, di, and tri-O-acetylated sialic acids in normal and neoplastic colon. Cancer Res. 1988 Jan 15;48(2):483–489. [PubMed] [Google Scholar]
- LLOYD A. G. Fractionation of the products of the direct sulphation of monosaccharides on anion-exchange resin. Biochem J. 1962 Jun;83:455–460. doi: 10.1042/bj0830455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson G., Falk P., Hoskins L. C. Degradation of human intestinal glycosphingolipids by extracellular glycosidases from mucin-degrading bacteria of the human fecal flora. J Biol Chem. 1988 Aug 5;263(22):10790–10798. [PubMed] [Google Scholar]
- Miller R. S., Hoskins L. C. Mucin degradation in human colon ecosystems. Fecal population densities of mucin-degrading bacteria estimated by a "most probable number" method. Gastroenterology. 1981 Oct;81(4):759–765. [PubMed] [Google Scholar]
- Podolsky D. K. Oligosaccharide structures of human colonic mucin. J Biol Chem. 1985 Jul 15;260(14):8262–8271. [PubMed] [Google Scholar]
- Podolsky D. K. Oligosaccharide structures of isolated human colonic mucin species. J Biol Chem. 1985 Dec 15;260(29):15510–15515. [PubMed] [Google Scholar]
- Poon H., Reid P. E., Ramey C. W., Dunn W. L., Clay M. G. Removal of O-acetylated sialic acids from rat colonic epithelial glycoproteins by cell-free extracts of rat faeces. Can J Biochem Cell Biol. 1983 Aug;61(8):868–874. doi: 10.1139/o83-111. [DOI] [PubMed] [Google Scholar]
- Reuter G., Pfeil R., Stoll S., Schauer R., Kamerling J. P., Versluis C., Vliegenthart J. F. Identification of new sialic acids derived from glycoprotein of bovine submandibular gland. Eur J Biochem. 1983 Jul 15;134(1):139–143. doi: 10.1111/j.1432-1033.1983.tb07542.x. [DOI] [PubMed] [Google Scholar]
- Rhodes J. M., Black R. R., Gallimore R., Savage A. Histochemical demonstration of desialation and desulphation of normal and inflammatory bowel disease rectal mucus by faecal extracts. Gut. 1985 Dec;26(12):1312–1318. doi: 10.1136/gut.26.12.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. M., Gallimore R., Elias E., Allan R. N., Kennedy J. F. Faecal mucus degrading glycosidases in ulcerative colitis and Crohn's disease. Gut. 1985 Aug;26(8):761–765. doi: 10.1136/gut.26.8.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Aparicio L. B., Reglero A., Luengo J. M. Uptake of N-acetylneuraminic acid by Escherichia coli K-235. Biochemical characterization of the transport system. Biochem J. 1987 Sep 1;246(2):287–294. doi: 10.1042/bj2460287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G. N., Herrler G., Paulson J. C., Klenk H. D. Influenza C virus uses 9-O-acetyl-N-acetylneuraminic acid as a high affinity receptor determinant for attachment to cells. J Biol Chem. 1986 May 5;261(13):5947–5951. [PubMed] [Google Scholar]
- Ruseler-van Embden J. G., van Lieshout L. M. Increased faecal glycosidases in patients with Crohn's disease. Digestion. 1987;37(1):43–50. doi: 10.1159/000199486. [DOI] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., West S. E., Vercellotti J. R., Wilkins T. D. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl Environ Microbiol. 1977 Nov;34(5):529–533. doi: 10.1128/aem.34.5.529-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer R. Characterization of sialic acids. Methods Enzymol. 1978;50:64–89. doi: 10.1016/0076-6879(78)50008-6. [DOI] [PubMed] [Google Scholar]
- Schauer R., Faillard H. Zur Wirkungsspezifität der Neuraminidase. Das Verhalten isomerer N.O-Diacetylneuraminsäureglykoside im Submaxillarismucin von Pferd und Rind bei Einwirkung bakterieller Neuraminidase. Hoppe Seylers Z Physiol Chem. 1968 Aug;349(8):961–968. [PubMed] [Google Scholar]
- Sellers L. A., Allen A., Morris E. R., Ross-Murphy S. B. Mucus glycoprotein gels. Role of glycoprotein polymeric structure and carbohydrate side-chains in gel-formation. Carbohydr Res. 1988 Jul 15;178:93–110. doi: 10.1016/0008-6215(88)80104-6. [DOI] [PubMed] [Google Scholar]
- Smith A. C., Podolsky D. K. Colonic mucin glycoproteins in health and disease. Clin Gastroenterol. 1986 Oct;15(4):815–837. [PubMed] [Google Scholar]
- Stanley R. A., Ram S. P., Wilkinson R. K., Roberton A. M. Degradation of pig gastric and colonic mucins by bacteria isolated from the pig colon. Appl Environ Microbiol. 1986 May;51(5):1104–1109. doi: 10.1128/aem.51.5.1104-1109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H. H., Sunderland D., Gibson G. R., Hart C. A., Rhodes J. M. A novel mucin sulphatase from human faeces: its identification, purification and characterization. Clin Sci (Lond) 1992 Apr;82(4):447–454. doi: 10.1042/cs0820447. [DOI] [PubMed] [Google Scholar]
- Variyam E. P., Hoskins L. C. In vitro degradation of gastric mucin. Carbohydrate side chains protect polypeptide core from pancreatic proteases. Gastroenterology. 1983 Mar;84(3):533–537. [PubMed] [Google Scholar]
- Varki A., Diaz S. A neuraminidase from Streptococcus sanguis that can release O-acetylated sialic acids. J Biol Chem. 1983 Oct 25;258(20):12465–12471. [PubMed] [Google Scholar]
- Vimr E. R., Troy F. A. Identification of an inducible catabolic system for sialic acids (nan) in Escherichia coli. J Bacteriol. 1985 Nov;164(2):845–853. doi: 10.1128/jb.164.2.845-853.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Troy F. A. Regulation of sialic acid metabolism in Escherichia coli: role of N-acylneuraminate pyruvate-lyase. J Bacteriol. 1985 Nov;164(2):854–860. doi: 10.1128/jb.164.2.854-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]