Abstract
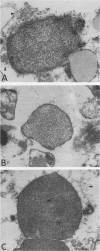
The exopolysaccharide (alginate) of mucoid strains of Pseudomonas aeruginosa is believed to be an important virulence factor. The ability of an alginate-deploymerizing enzyme (alginase) to modify the polymorphonuclear leukocyte (PMN)-directed and antibiotic-mediated phagocytosis and killing of mucoid P. aeruginosa was studied both in vitro and in vivo. In vitro, pretreatment of a mucoid P. aeruginosa strain (144MR) resulted in a significant enhancement of PMN phagocytosis and killing of the organism (P less than 0.05), to levels similar to that observed with its nonmucoid mate, strain 144NM. Moreover, alginase treatment of the mucoid strain 144MR caused a substantial removal of bacterial cell surface alginate as assessed by immunofluorescence staining with a murine monoclonal antialginate antibody. The experimental endocarditis model was used to evaluate the in vivo effect of alginase in modifying the course of a deep-seated pseudomonal infection caused by mucoid strain 144MR. In right-sided endocarditis, in which PMNs normally mediate spontaneous clearance of the organism from cardiac vegetations (A. S. Bayer, J. Yih, C. Y. Chiu, and C. C. Nast, Chemotherapy 35:278-288, 1989), the presence of the alginate exopolysaccharide on strain 144MR was associated with an inability to reduce intravegetation pseudomonal counts over a 13-day postinfection period; in contrast, right-sided vegetations infected with the nonmucoid strain 144NM underwent significant reductions in bacterial densities over this same time (P less than 0.05). Administration of alginase intravenously (i.v.) (750 enzyme units per day for 7 days) to animals with right-sided endocarditis caused by the mucoid strain 144MR was associated with a significant reduction in intravegetation pseudomonal counts (P less than 0.05), to levels similar to that seen with endocarditis caused by the nonmucoid strain. In left-sided endocarditis caused by mucoid strain 144MR, animals received either no therapy, amikacin (20 or 40 mg/kg twice a day for 7 or 14 days), or amikacin plus alginase (750 U/day [i.v.]). The coadministration of alginase for 14 days with the higher-dose amikacin regimen rendered more left-sided vegetations culture negative than those in animals receiving the antibiotic alone for 7 or 14 days (P = 0.001 and 0.056, respectively). These salutary effects of alginase in vivo were paralleled by the ability of the enzyme to remove the exopolysaccharide from the surface of mucoid pseudomonal cells within cardiac vegetations, as assessed by transmission electron microscopy.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G., Fekety F. R. Experimental endocarditis due to Pseudomonas aeruginosa. I. Description of a model. J Infect Dis. 1976 Jul;134(1):1–7. doi: 10.1093/infdis/134.1.1. [DOI] [PubMed] [Google Scholar]
- Archer G., Fekety F. R., Jr Experimental endocarditis due to Pseudomonas aeruginosa. II. Therapy with carbenicillin and gentamicin. J Infect Dis. 1977 Sep;136(3):327–335. doi: 10.1093/infdis/136.3.327. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Crowell D., Nast C. C., Norman D. C., Borrelli R. L. Intravegetation antimicrobial distribution in aortic endocarditis analyzed by computer-generated model. Implications for treatment. Chest. 1990 Mar;97(3):611–617. doi: 10.1378/chest.97.3.611. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Eftekhar F., Tu J., Nast C. C., Speert D. P. Oxygen-dependent up-regulation of mucoid exopolysaccharide (alginate) production in Pseudomonas aeruginosa. Infect Immun. 1990 May;58(5):1344–1349. doi: 10.1128/iai.58.5.1344-1349.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A. S., Selecky M., Babel K., Hirano L., Yih J., Parr T. R., Jr Bactericidal interactions of a beta-lactam and beta-lactamase inhibitors in experimental Pseudomonas aeruginosa endocarditis caused by a constitutive overproducer of type Id beta-lactamase. Antimicrob Agents Chemother. 1987 Nov;31(11):1750–1755. doi: 10.1128/aac.31.11.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A. S., Speert D. P., Park S., Tu J., Witt M., Nast C. C., Norman D. C. Functional role of mucoid exopolysaccharide (alginate) in antibiotic-induced and polymorphonuclear leukocyte-mediated killing of Pseudomonas aeruginosa. Infect Immun. 1991 Jan;59(1):302–308. doi: 10.1128/iai.59.1.302-308.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A. S., Yih J., Chiu C. Y., Nast C. C. Pathogenic effects of monocytopenia, granulocytopenia and dexamethasone on the course of experimental Pseudomonas aeruginosa endocarditis in rabbits. Chemotherapy. 1989;35(4):278–288. doi: 10.1159/000238683. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Cabral D. A., Loh B. A., Speert D. P. Mucoid Pseudomonas aeruginosa resists nonopsonic phagocytosis by human neutrophils and macrophages. Pediatr Res. 1987 Oct;22(4):429–431. doi: 10.1203/00006450-198710000-00013. [DOI] [PubMed] [Google Scholar]
- Dall L., Barnes W. G., Lane J. W., Mills J. Enzymatic modification of glycocalyx in the treatment of experimental endocarditis due to viridans streptococci. J Infect Dis. 1987 Nov;156(5):736–740. doi: 10.1093/infdis/156.5.736. [DOI] [PubMed] [Google Scholar]
- Durack D. T. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J Pathol. 1975 Feb;115(2):81–89. doi: 10.1002/path.1711150204. [DOI] [PubMed] [Google Scholar]
- Eftekhar F., Speert D. P. Alginase treatment of mucoid Pseudomonas aeruginosa enhances phagocytosis by human monocyte-derived macrophages. Infect Immun. 1988 Nov;56(11):2788–2793. doi: 10.1128/iai.56.11.2788-2793.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R. C., McElvaney N. G., Birrer P., Shak S., Robinson W. W., Jolley C., Wu M., Chernick M. S., Crystal R. G. A preliminary study of aerosolized recombinant human deoxyribonuclease I in the treatment of cystic fibrosis. N Engl J Med. 1992 Mar 19;326(12):812–815. doi: 10.1056/NEJM199203193261207. [DOI] [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980 May;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learn D. B., Brestel E. P., Seetharama S. Hypochlorite scavenging by Pseudomonas aeruginosa alginate. Infect Immun. 1987 Aug;55(8):1813–1818. doi: 10.1128/iai.55.8.1813-1818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J., Pulliam L., Dall L., Marzouk J., Wilson W., Costerton J. W. Exopolysaccharide production by viridans streptococci in experimental endocarditis. Infect Immun. 1984 Jan;43(1):359–367. doi: 10.1128/iai.43.1.359-367.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. W., Evans M. J., Slack M. P., Walmsley H. L. The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol. 1989 May;135(5):1291–1303. doi: 10.1099/00221287-135-5-1291. [DOI] [PubMed] [Google Scholar]
- Pennington J. E., Wolff S. M., Puziss M. Summary of a workshop on infections in patients with cystic fibrosis. J Infect Dis. 1979 Aug;140(2):252–256. doi: 10.1093/infdis/140.2.252. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Saunders J. M., Ames P., Edwards M. S., Auerbach H., Goldfarb J., Speert D. P., Hurwitch S. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. N Engl J Med. 1987 Sep 24;317(13):793–798. doi: 10.1056/NEJM198709243171303. [DOI] [PubMed] [Google Scholar]
- Pulliam L., Dall L., Inokuchi S., Wilson W., Hadley W. K., Mills J. Effects of exopolysaccharide production by viridans streptococci on penicillin therapy of experimental endocarditis. J Infect Dis. 1985 Jan;151(1):153–156. doi: 10.1093/infdis/151.1.153. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Pier G. B. Role of Pseudomonas aeruginosa mucoid exopolysaccharide in adherence to tracheal cells. Infect Immun. 1985 Jan;47(1):1–4. doi: 10.1128/iai.47.1.1-4.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L., Alazard M. J., Borowski R. S. Serum sensitivity of a Pseudomonas aeruginosa mucoid strain. Infect Immun. 1984 Sep;45(3):748–755. doi: 10.1128/iai.45.3.748-755.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbrock-Cox V., Russell N. J., Gacesa P. The purification and chemical characterisation of the alginate present in extracellular material produced by mucoid strains of Pseudomonas aeruginosa. Carbohydr Res. 1984 Dec 15;135(1):147–154. doi: 10.1016/0008-6215(84)85012-0. [DOI] [PubMed] [Google Scholar]
- Slack M. P., Nichols W. W. The penetration of antibiotics through sodium alginate and through the exopolysaccharide of a mucoid strain of Pseudomonas aeruginosa. Lancet. 1981 Sep 5;2(8245):502–503. doi: 10.1016/s0140-6736(81)90885-0. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Eftekhar F., Puterman M. L. Nonopsonic phagocytosis of strains of Pseudomonas aeruginosa from cystic fibrosis patients. Infect Immun. 1984 Mar;43(3):1006–1011. doi: 10.1128/iai.43.3.1006-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiver H. G., Zachidniak K., Speert D. P. Inhibition of polymorphonuclear leukocyte chemotaxis by the mucoid exopolysaccharide of Pseudomonas aeruginosa. Clin Invest Med. 1988 Aug;11(4):247–252. [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Yersin B. R., Glauser M. P., Freedman L. R. Effect of nitrogen mustard on natural history of right-sided streptococcal endocarditis in rabbits: role for cellular host defenses. Infect Immun. 1982 Jan;35(1):320–325. doi: 10.1128/iai.35.1.320-325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]