Abstract
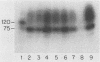
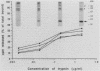
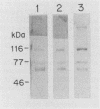
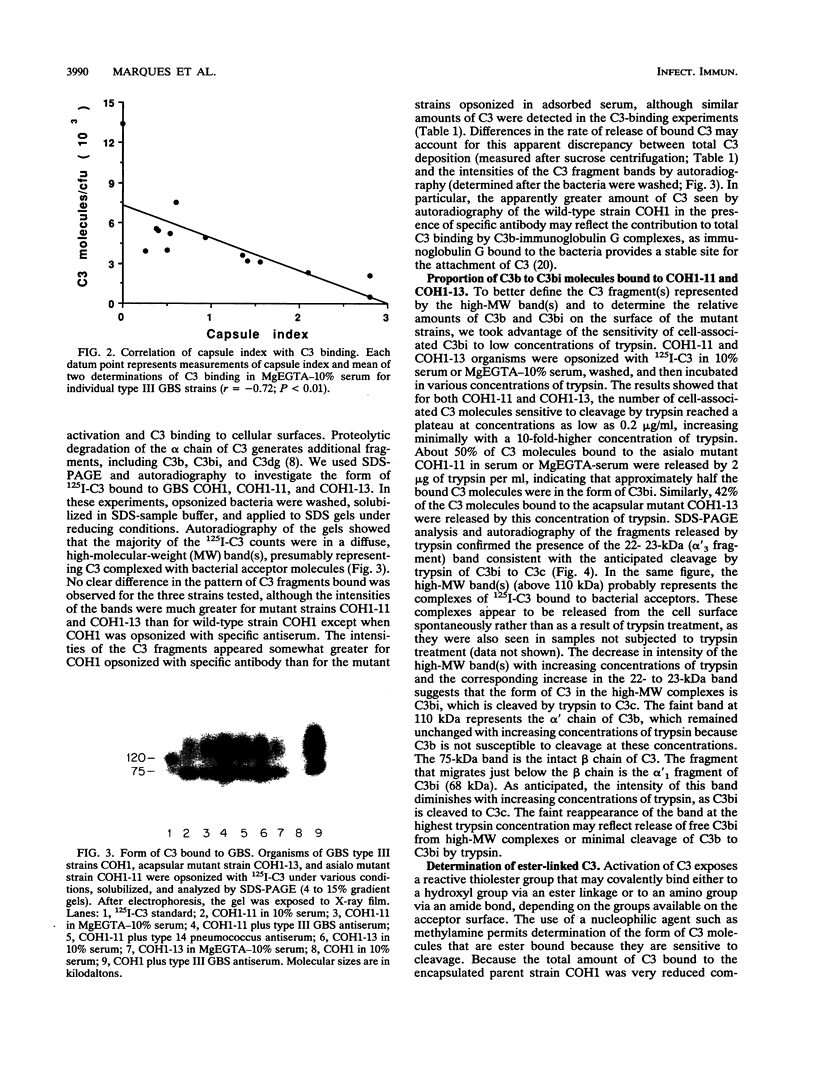
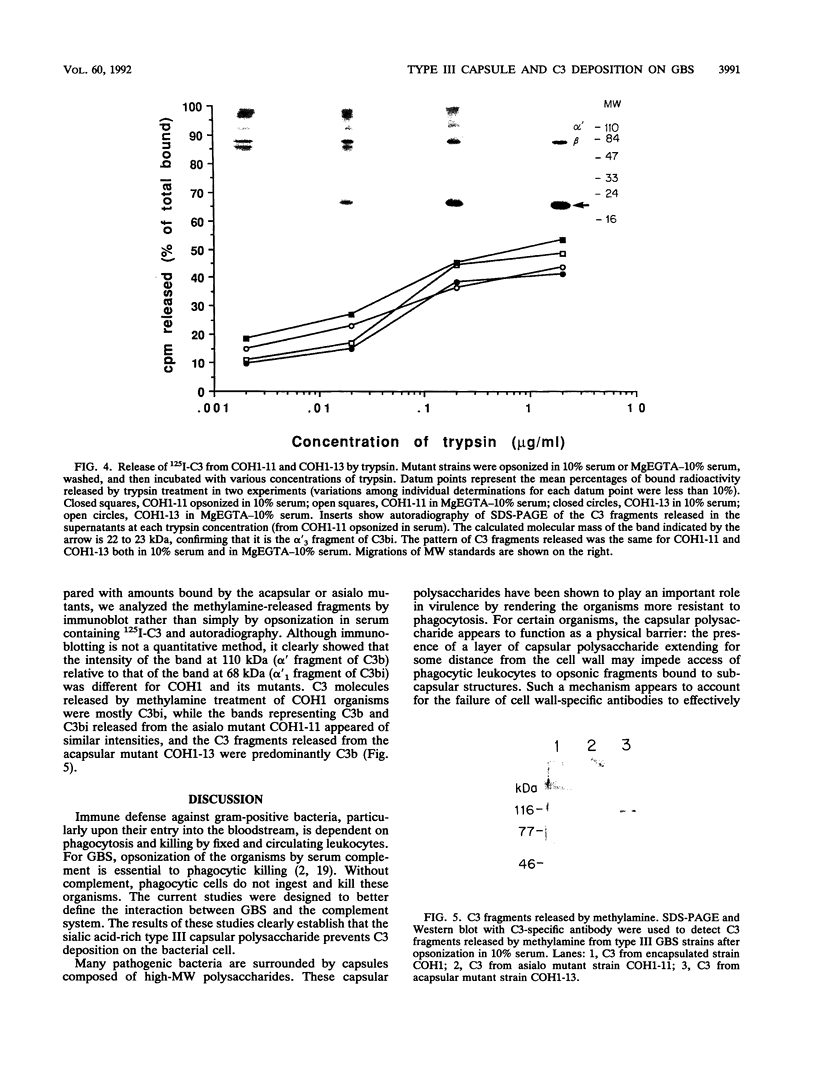
Strains of type III group B streptococci isolated from patients with neonatal sepsis are generally resistant to complement-mediated phagocytic killing in the absence of specific antibody. It has been suggested that the resistance of type III group B streptococci to phagocytosis results from inhibition of alternative-complement-pathway activation by sialic acid residues of the type III polysaccharide. To better define the relationship between structural features of the type III capsule and resistance of type III group B streptococci to complement-mediated phagocytic killing, we measured deposition of human C3 on group B streptococcal strains with altered capsule phenotypes. C3 binding was quantified by incubating bacteria with purified human 125I-C3 in 10% serum. Wild-type group B Streptococcus sp. strain COH1 bound eightfold fewer C3 molecules than did either of two isogenic mutant strains, one expressing a sialic acid-deficient capsule and the other lacking capsule completely. Similar results were obtained when the incubation with 125I-C3 was performed in serum chelated with Mg-ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'- tetraacetic acid (MgEGTA), suggesting that the majority of C3 deposition occurred via the alternative pathway. In contrast to the wild-type strain, which was relatively resistant, both mutant strains were killed by human leukocytes in 10% serum with or without MgEGTA. We also measured C3 binding to 14 wild-type strains of type III group B streptococci expressing various amounts of capsule. Comparison of degree of encapsulation with C3 binding revealed a significant inverse correlation (r = -0.72; P less than 0.01). C3 fragments released by methylamine treatment of wild-type strain COH1 were predominantly in the form of C3bi, while those released from the acapsular mutant were predominantly C3b and those from the asialo mutant represented approximately equal amounts of C3b and C3bi. We conclude from these studies that the sialylated type III capsular polysaccharide inhibits alternative-pathway activation, prevents C3 deposition on group B streptococci, and protects the organisms from phagocytic killing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore R. S., Kasper D. L., Baker C. J., Goroff D. K. Antigenic specificity of opsonophagocytic antibodies in rabbit anti-sera to group B streptococci. J Immunol. 1977 Feb;118(2):673–678. [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Hammer C. H., Burch C. G., Frank M. M. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J Clin Invest. 1982 Jan;69(1):85–98. doi: 10.1172/JCI110444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. R., Baker C. J., Edwards M. S. Deposition and degradation of C3 on type III group B streptococci. Infect Immun. 1991 Jun;59(6):1978–1983. doi: 10.1128/iai.59.6.1978-1983.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. S., Kasper D. L., Jennings H. J., Baker C. J., Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol. 1982 Mar;128(3):1278–1283. [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Wong W. W. Complement ligand-receptor interactions that mediate biological responses. Annu Rev Immunol. 1983;1:243–271. doi: 10.1146/annurev.iy.01.040183.001331. [DOI] [PubMed] [Google Scholar]
- Gaither T. A., Hammer C. H., Frank M. M. Studies of the molecular mechanisms of C3b inactivation and a simplified assay of beta 1H and the C3b inactivator (C3bINA). J Immunol. 1979 Sep;123(3):1195–1204. [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Hostetter M. K. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J Infect Dis. 1986 Apr;153(4):682–693. doi: 10.1093/infdis/153.4.682. [DOI] [PubMed] [Google Scholar]
- Jarvis G. A., Vedros N. A. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect Immun. 1987 Jan;55(1):174–180. doi: 10.1128/iai.55.1.174-180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. C3b deposition during activation of the alternative complement pathway and the effect of deposition on the activating surface. J Immunol. 1983 Oct;131(4):1930–1935. [PubMed] [Google Scholar]
- Puentes S. M., Dwyer D. M., Bates P. A., Joiner K. A. Binding and release of C3 from Leishmania donovani promastigotes during incubation in normal human serum. J Immunol. 1989 Dec 1;143(11):3743–3749. [PubMed] [Google Scholar]
- Rubens C. E., Wessels M. R., Heggen L. M., Kasper D. L. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka A. O., Jensen C. L., Pincus S. H., Hill H. R. Absolute requirement for complement in monoclonal IgM antibody-mediated protection against experimental infection with type III group B streptococci. J Infect Dis. 1984 Jul;150(1):63–70. doi: 10.1093/infdis/150.1.63. [DOI] [PubMed] [Google Scholar]
- Shohet J. M., Bergamaschini L., Davis A. E., Carroll M. C. Localization of the human complement component C3 binding site on the IgG heavy chain. J Biol Chem. 1991 Oct 5;266(28):18520–18524. [PubMed] [Google Scholar]
- Van Dijk W. C., Verbrugh H. A., van der Tol M. E., Peters R., Verhoef J. Role of Escherichia coli K capsular antigens during complement activation, C3 fixation, and opsonization. Infect Immun. 1979 Aug;25(2):603–609. doi: 10.1128/iai.25.2.603-609.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels M. R., Haft R. F., Heggen L. M., Rubens C. E. Identification of a genetic locus essential for capsule sialylation in type III group B streptococci. Infect Immun. 1992 Feb;60(2):392–400. doi: 10.1128/iai.60.2.392-400.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels M. R., Paoletti L. C., Kasper D. L., DiFabio J. L., Michon F., Holme K., Jennings H. J. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Invest. 1990 Nov;86(5):1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels M. R., Rubens C. E., Benedí V. J., Kasper D. L. Definition of a bacterial virulence factor: sialylation of the group B streptococcal capsule. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8983–8987. doi: 10.1073/pnas.86.22.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]