Abstract
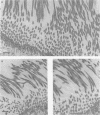
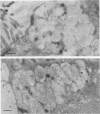
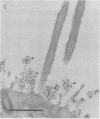
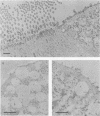
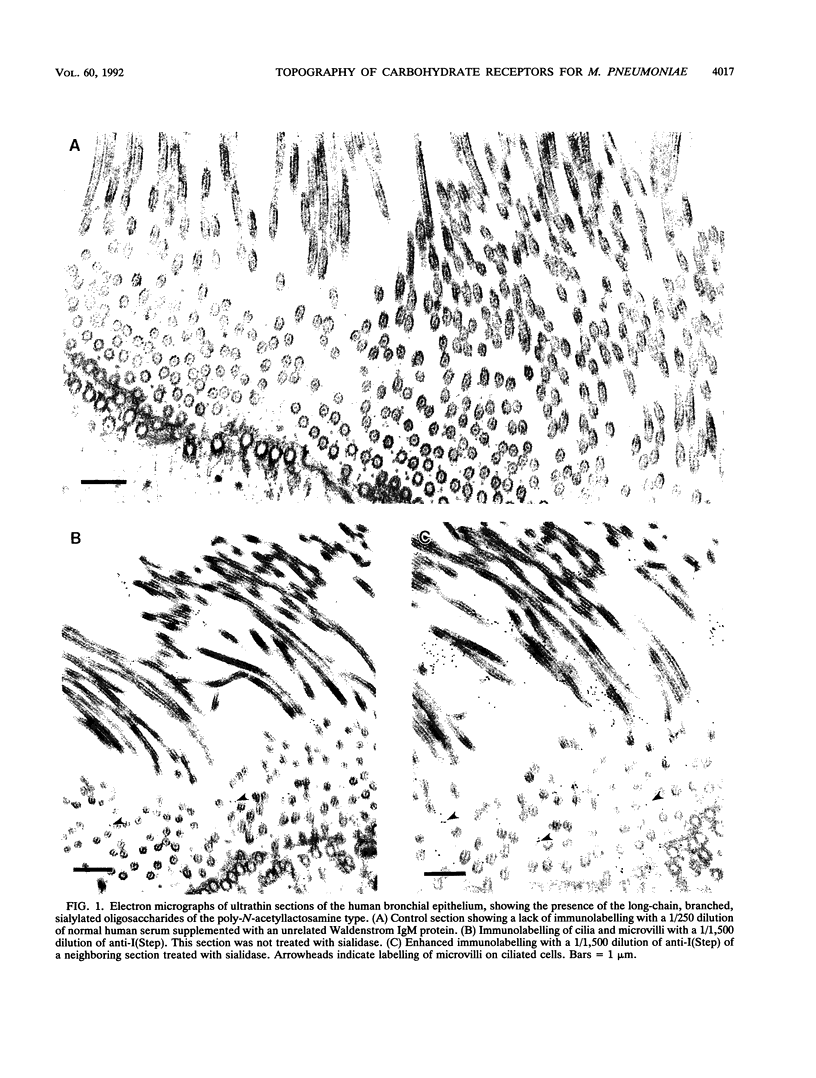
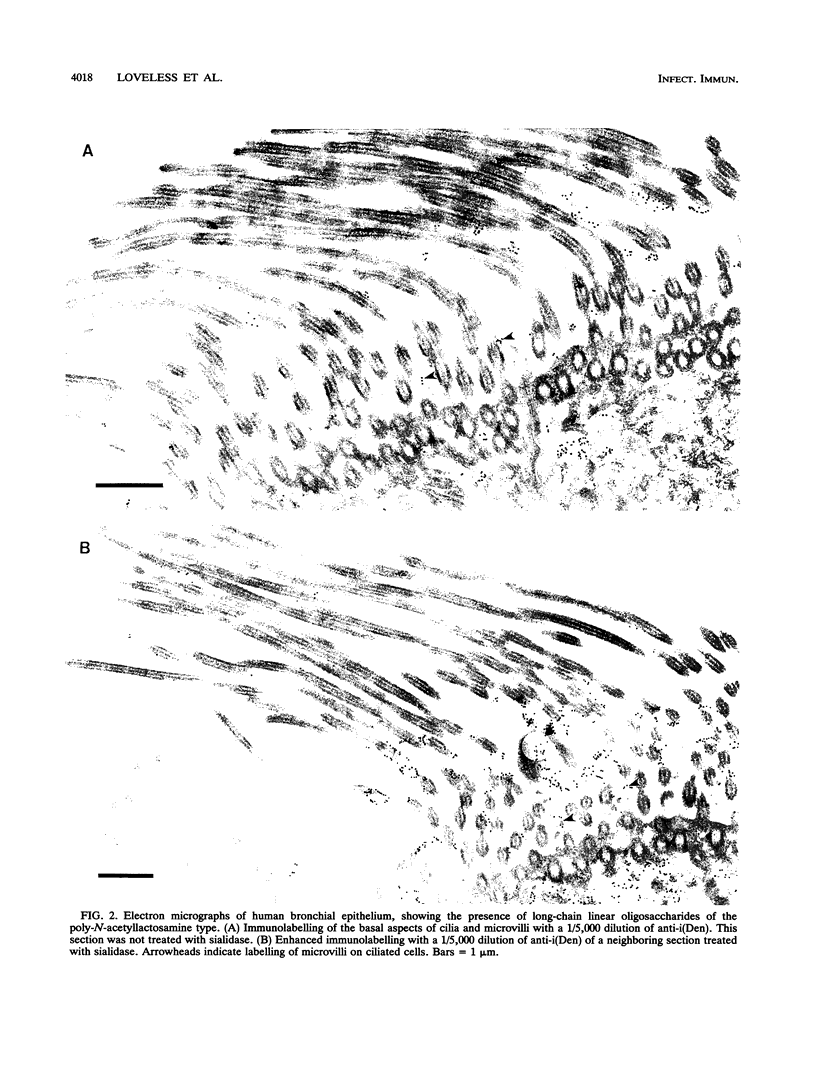
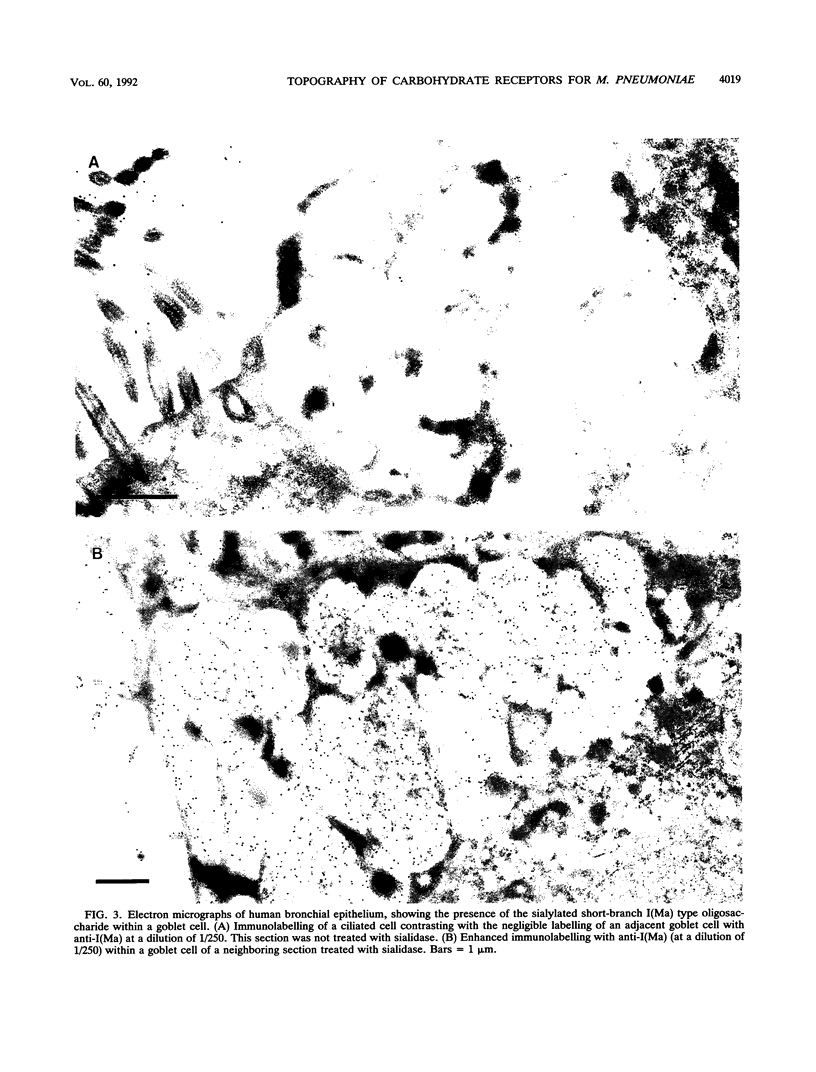
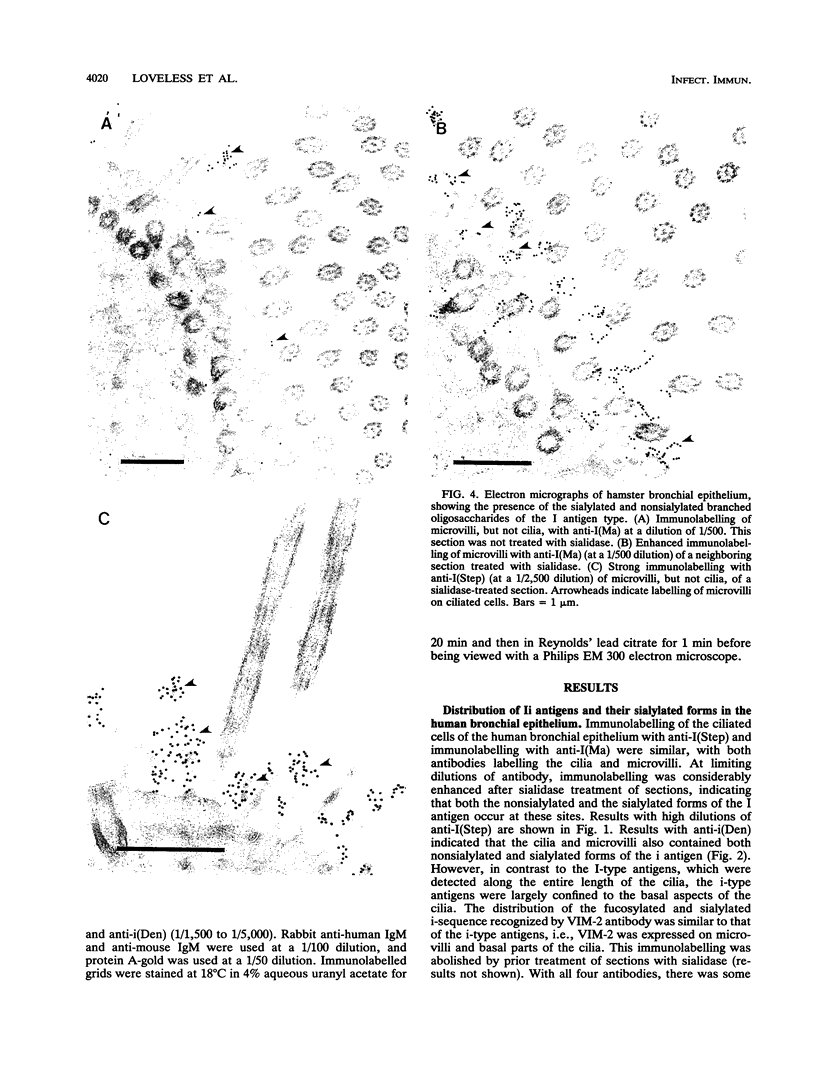
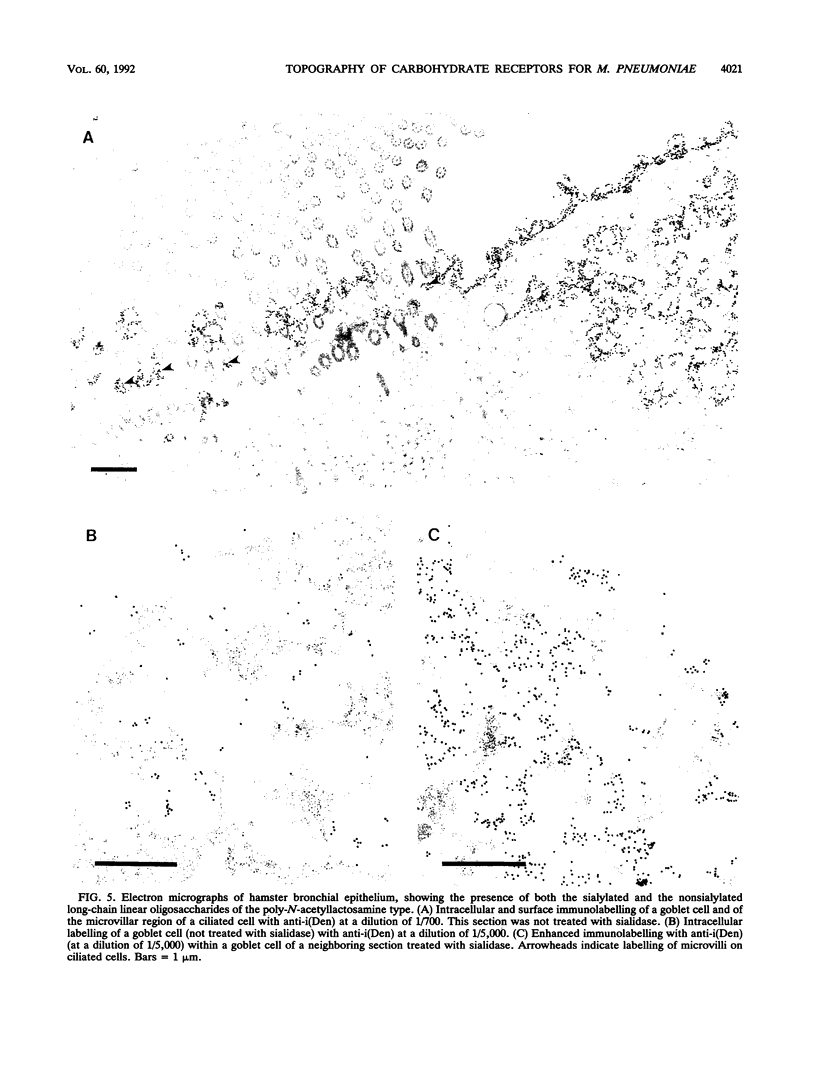
Long-chain sialo-oligosaccharides with poly-N-acetyllactosamine backbones (Ii antigen type) are major host cell receptors for the human pathogen Mycoplasma pneumoniae. Previous immunofluorescence studies of the human bronchial epithelium, using sequence-specific monoclonal antibodies to the branched I-type and linear i-type backbones, have indicated that sialylated and nonsialylated long-chain sequences of both types are richly expressed on the ciliated cells, where they are polarized at the apical aspects. These sequences are lacking in the goblet cells. In the present study, the display of these oligosaccharides has been investigated by electron microscopy (immunogold labelling) in the human bronchial epithelium and in that of the hamster, an animal model commonly used for M. pneumoniae infection. In the human bronchial epithelium, the long-chain branched sequences have been detected along the entire length of the cilia and on microvilli, whereas the linear sequences are confined to the microvilli and the basal aspects of the cilia. On the ciliated epithelial cells of the hamster, by contrast, the branched and linear sequences (sialo- and asialo-) have been detected exclusively on microvilli. A further striking difference is that in the hamster these structures are expressed in abundance on the goblet cells and in the intracellular globules. We suggest that the latter finding may partly explain the relatively large doses of M. pneumoniae required to establish experimental infection in the hamster, as the receptor-bearing secreted mucus may have a protective role in binding to the microorganisms, leading to their clearance by bronchociliary action.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armbruster B. L., Carlemalm E., Chiovetti R., Garavito R. M., Hobot J. A., Kellenberger E., Villiger W. Specimen preparation for electron microscopy using low temperature embedding resins. J Microsc. 1982 Apr;126(Pt 1):77–85. doi: 10.1111/j.1365-2818.1982.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr Appearance of Mycoplasma pneumoniae in lungs of experimentally infected hamsters and sputum from patients with natural disease. Am Rev Respir Dis. 1974 Dec;110(6):765–773. doi: 10.1164/arrd.1974.110.6P1.765. [DOI] [PubMed] [Google Scholar]
- DAJANI A. S., CLYDE W. A., Jr, DENNY F. W. EXPERIMENTAL INFECTION WITH MYCOPLASMA PNEUMONIAE (EATON'S AGENT). J Exp Med. 1965 Jun 1;121:1071–1086. doi: 10.1084/jem.121.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T., Hounsell E. F., Alais J., Veyrières A., David S. Further definition of the size of the blood group-i antigenic determinant using a chemically synthesised octasaccharide of poly-N-acetyllactosamine type. Carbohydr Res. 1992 Apr 10;228(1):289–297. doi: 10.1016/s0008-6215(00)90566-4. [DOI] [PubMed] [Google Scholar]
- Feizi T. The blood group Ii system: a carbohydrate antigen system defined by naturally monoclonal or oligoclonal autoantibodies of man. Immunol Commun. 1981;10(2):127–156. doi: 10.3109/08820138109050693. [DOI] [PubMed] [Google Scholar]
- Gooi H. C., Williams L. K., Uemura K., Hounsell E. F., McIlhinney R. A., Feizi T. A marker of human foetal endoderm defined by a monoclonal antibody involves Type 1 blood group chains. Mol Immunol. 1983 Jun;20(6):607–613. doi: 10.1016/0161-5890(83)90005-6. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat E. A., Liao J., Shyong J., Osserman E. F. A monoclonal IgM lambda macroglobulin with specificity for lacto-N-tetraose in a patient with bronchogenic carcinoma. J Immunol. 1982 Feb;128(2):540–544. [PubMed] [Google Scholar]
- Krivan H. C., Olson L. D., Barile M. F., Ginsburg V., Roberts D. D. Adhesion of Mycoplasma pneumoniae to sulfated glycolipids and inhibition by dextran sulfate. J Biol Chem. 1989 Jun 5;264(16):9283–9288. [PubMed] [Google Scholar]
- Loomes L. M., Uemura K., Childs R. A., Paulson J. C., Rogers G. N., Scudder P. R., Michalski J. C., Hounsell E. F., Taylor-Robinson D., Feizi T. Erythrocyte receptors for Mycoplasma pneumoniae are sialylated oligosaccharides of Ii antigen type. Nature. 1984 Feb 9;307(5951):560–563. doi: 10.1038/307560a0. [DOI] [PubMed] [Google Scholar]
- Loomes L. M., Uemura K., Feizi T. Interaction of Mycoplasma pneumoniae with erythrocyte glycolipids of I and i antigen types. Infect Immun. 1985 Jan;47(1):15–20. doi: 10.1128/iai.47.1.15-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless R. W., Feizi T. Sialo-oligosaccharide receptors for Mycoplasma pneumoniae and related oligosaccharides of poly-N-acetyllactosamine series are polarized at the cilia and apical-microvillar domains of the ciliated cells in human bronchial epithelium. Infect Immun. 1989 Apr;57(4):1285–1289. doi: 10.1128/iai.57.4.1285-1289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSH W. L. Anti-i: a cold antibody defining the Ii relationship in human red cells. Br J Haematol. 1961 Apr;7:200–209. doi: 10.1111/j.1365-2141.1961.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Macher B. A., Buehler J., Scudder P., Knapp W., Feizi T. A novel carbohydrate, differentiation antigen on fucogangliosides of human myeloid cells recognized by monoclonal antibody VIM-2. J Biol Chem. 1988 Jul 25;263(21):10186–10191. [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Haemadsorption and haemagglutination by mycoplasmas. J Gen Microbiol. 1968 Mar;50(3):465–478. doi: 10.1099/00221287-50-3-465. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Sadler J. E., Hill R. L. Restoration of specific myxovirus receptors to asialoerythrocytes by incorporation of sialic acid with pure sialyltransferases. J Biol Chem. 1979 Mar 25;254(6):2120–2124. [PubMed] [Google Scholar]
- Roberts D. D., Olson L. D., Barile M. F., Ginsburg V., Krivan H. C. Sialic acid-dependent adhesion of Mycoplasma pneumoniae to purified glycoproteins. J Biol Chem. 1989 Jun 5;264(16):9289–9293. [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]