Abstract
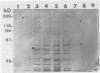
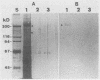
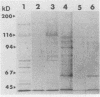
Surface proteins of Streptococcus mutans have been reported to be released into the culture filtrate at concentrations that vary with the growth conditions. The reason for this is not clear. The present study attempts to investigate the mechanism of the protein release. The results showed that whole cells and raffinose-stabilized protoplasts of S. mutans NG8, when incubated in buffers, were capable of releasing their surface proteins in a pH-dependent manner with optimal release at pH 5 to 6. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis revealed that the released proteins were very complex. Two proteins, adhesin P1, which has been previously shown to interact with a human salivary agglutinin, and glucosyltransferase have been identified among the released proteins. The release of adhesin P1 and other proteins was found to be inhibited by heat, Cu2+,Zn2+, and thiol-blocking reagents. The inhibition by heat and Cu2+ was irreversible, whereas that by the thiol-blocking reagents was reversible. EDTA, phenylmethylsulfonyl fluoride, and N-p-tosyl-L-lysyl-chloromethyl ketone had no effect on the release of P1, indicating that the release was probably not due to proteolytic activity. Adhesin P1 from Cu(2+)-inactivated S. mutans NG8 protoplasts could be released by mixing with fresh whole cells and protoplasts, but not the culture filtrate, of a P1-negative mutant of NG8, suggesting that the enzyme is located on the cell surface. This P1-releasing activity was also detected in two other strains of S. mutans and one strain each of S. gordonii, S. agalactiae, S. pneumoniae, and S. pyogenes. The biological role(s) of this enzyme activity remains to be determined. However, owing to its ability to release virulent surface proteins from the cell, it may play an important role in cell surface modulation among the pathogenic streptococci.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayakawa G. Y., Boushell L. W., Crowley P. J., Erdos G. W., McArthur W. P., Bleiweis A. S. Isolation and characterization of monoclonal antibodies specific for antigen P1, a major surface protein of mutans streptococci. Infect Immun. 1987 Nov;55(11):2759–2767. doi: 10.1128/iai.55.11.2759-2767.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. The many forms and functions of cellular proteinases. Fed Proc. 1980 Jan;39(1):9–14. [PubMed] [Google Scholar]
- Bowen W. H., Schilling K., Giertsen E., Pearson S., Lee S. F., Bleiweis A., Beeman D. Role of a cell surface-associated protein in adherence and dental caries. Infect Immun. 1991 Dec;59(12):4606–4609. doi: 10.1128/iai.59.12.4606-4609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brady L. J., Crowley P. J., Ma J. K., Kelly C., Lee S. F., Lehner T., Bleiweis A. S. Restriction fragment length polymorphisms and sequence variation within the spaP gene of Streptococcus mutans serotype c isolates. Infect Immun. 1991 May;59(5):1803–1810. doi: 10.1128/iai.59.5.1803-1810.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady L. J., Piacentini D. A., Crowley P. J., Bleiweis A. S. Identification of monoclonal antibody-binding domains within antigen P1 of Streptococcus mutans and cross-reactivity with related surface antigens of oral streptococci. Infect Immun. 1991 Dec;59(12):4425–4435. doi: 10.1128/iai.59.12.4425-4435.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Williams P. The influence of environment on envelope properties affecting survival of bacteria in infections. Annu Rev Microbiol. 1985;39:527–556. doi: 10.1146/annurev.mi.39.100185.002523. [DOI] [PubMed] [Google Scholar]
- Campbell L. K., Knox K. W., Wicken A. J. Influence of growth conditions on adherence of Streptococcus mutans ingbritt to saliva-coated hydroxyapatite. Infect Immun. 1983 Jan;39(1):445–448. doi: 10.1128/iai.39.1.445-448.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Ferretti J. J., Russell R. R., Dao M. L. Sequence analysis of the wall-associated protein precursor of Streptococcus mutans antigen A. Mol Microbiol. 1989 Apr;3(4):469–478. doi: 10.1111/j.1365-2958.1989.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester H., Hunter N., Knox K. W. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J Gen Microbiol. 1983 Sep;129(9):2779–2788. doi: 10.1099/00221287-129-9-2779. [DOI] [PubMed] [Google Scholar]
- Hardy L. N., Knox K. W., Brown R. A., Wicken A. J., Fitzgerald R. J. Comparison of extracellular protein profiles of seven serotypes of mutans streptococci grown under controlled conditions. J Gen Microbiol. 1986 May;132(5):1389–1400. doi: 10.1099/00221287-132-5-1389. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. Evidence for the presence of a phosphatidylinositol anchor on the lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J Biol Chem. 1990 Jun 5;265(16):9272–9279. [PubMed] [Google Scholar]
- Kelly C., Evans P., Bergmeier L., Lee S. F., Progulske-Fox A., Harris A. C., Aitken A., Bleiweis A. S., Lehner T. Sequence analysis of the cloned streptococcal cell surface antigen I/II. FEBS Lett. 1989 Nov 20;258(1):127–132. doi: 10.1016/0014-5793(89)81632-1. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Hardy L. N., Wicken A. J. Comparative studies on the protein profiles and hydrophobicity of strains of Streptococcus mutans serotype c. J Gen Microbiol. 1986 Sep;132(9):2541–2548. doi: 10.1099/00221287-132-9-2541. [DOI] [PubMed] [Google Scholar]
- Koga T., Asakawa H., Okahashi N., Hamada S. Sucrose-dependent cell adherence and cariogenicity of serotype c Streptococcus mutans. J Gen Microbiol. 1986 Oct;132(10):2873–2883. doi: 10.1099/00221287-132-10-2873. [DOI] [PubMed] [Google Scholar]
- Koga T., Asakawa H., Okahashi N., Takahashi I. Effect of subculturing on expression of a cell-surface protein antigen by Streptococcus mutans. J Gen Microbiol. 1989 Dec;135(12):3199–3207. doi: 10.1099/00221287-135-12-3199. [DOI] [PubMed] [Google Scholar]
- Koga T., Okahashi N., Takahashi I., Kanamoto T., Asakawa H., Iwaki M. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect Immun. 1990 Feb;58(2):289–296. doi: 10.1128/iai.58.2.289-296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. F., Forsberg C. W., Rattray J. B. Purification and Characterization of Two Endoxylanases from Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 1987 Apr;53(4):644–650. doi: 10.1128/aem.53.4.644-650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Progulske-Fox A., Bleiweis A. S. Molecular cloning and expression of a Streptococcus mutans major surface protein antigen, P1 (I/II), in Escherichia coli. Infect Immun. 1988 Aug;56(8):2114–2119. doi: 10.1128/iai.56.8.2114-2119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Progulske-Fox A., Erdos G. W., Piacentini D. A., Ayakawa G. Y., Crowley P. J., Bleiweis A. S. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II). Infect Immun. 1989 Nov;57(11):3306–3313. doi: 10.1128/iai.57.11.3306-3313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madoff L. C., Michel J. L., Kasper D. L. A monoclonal antibody identifies a protective C-protein alpha-antigen epitope in group B streptococci. Infect Immun. 1991 Jan;59(1):204–210. doi: 10.1128/iai.59.1.204-210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Song M., Krasse B., Olsson J. Biochemical and immunological differences between hydrophobic and hydrophilic strains of Streptococcus mutans. Infect Immun. 1984 Apr;44(1):68–75. doi: 10.1128/iai.44.1.68-75.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montville T. J., Cooney C. L., Sinskey A. J. Distribution of dextransucrase in Streptococcus mutans and observations on the effect of soluble dextran on dextransucrase activities. Infect Immun. 1977 Dec;18(3):629–635. doi: 10.1128/iai.18.3.629-635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Mol Microbiol. 1989 Feb;3(2):221–228. doi: 10.1111/j.1365-2958.1989.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Identification of an endogenous membrane anchor-cleaving enzyme for group A streptococcal M protein. Its implication for the attachment of surface proteins in gram-positive bacteria. J Exp Med. 1989 Dec 1;170(6):2119–2133. doi: 10.1084/jem.170.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B., Appelbaum B., Campbell L. K., Knox K. W., Wicken A. J. Chemostat studies of the effect of environmental control on Streptococcus sanguis adherence to hydroxyapatite. Infect Immun. 1982 Jan;35(1):64–70. doi: 10.1128/iai.35.1.64-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Lehner T. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23(1):7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Glycosyltransferases of Streptococcus mutans strain Ingbritt. Microbios. 1978;23(93-94):136–146. [PubMed] [Google Scholar]
- Russell R. R., Peach S. L., Colman G., Cohen B. Antibody responses to antigens of Streptococcus mutans in monkeys (Macaca fascicularis) immunized against dental caries. J Gen Microbiol. 1983 Mar;129(3):865–875. doi: 10.1099/00221287-129-3-865. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- Schilling K. M., Bowen W. H. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect Immun. 1992 Jan;60(1):284–295. doi: 10.1128/iai.60.1.284-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. L., Hurst S. F., Liberman E. S., Coleman S. E., Bleiweis A. S. Mutanolysin-induced spheroplasts of Streptococcus mutants are true protoplasts. Infect Immun. 1981 Feb;31(2):808–815. doi: 10.1128/iai.31.2.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]