Abstract
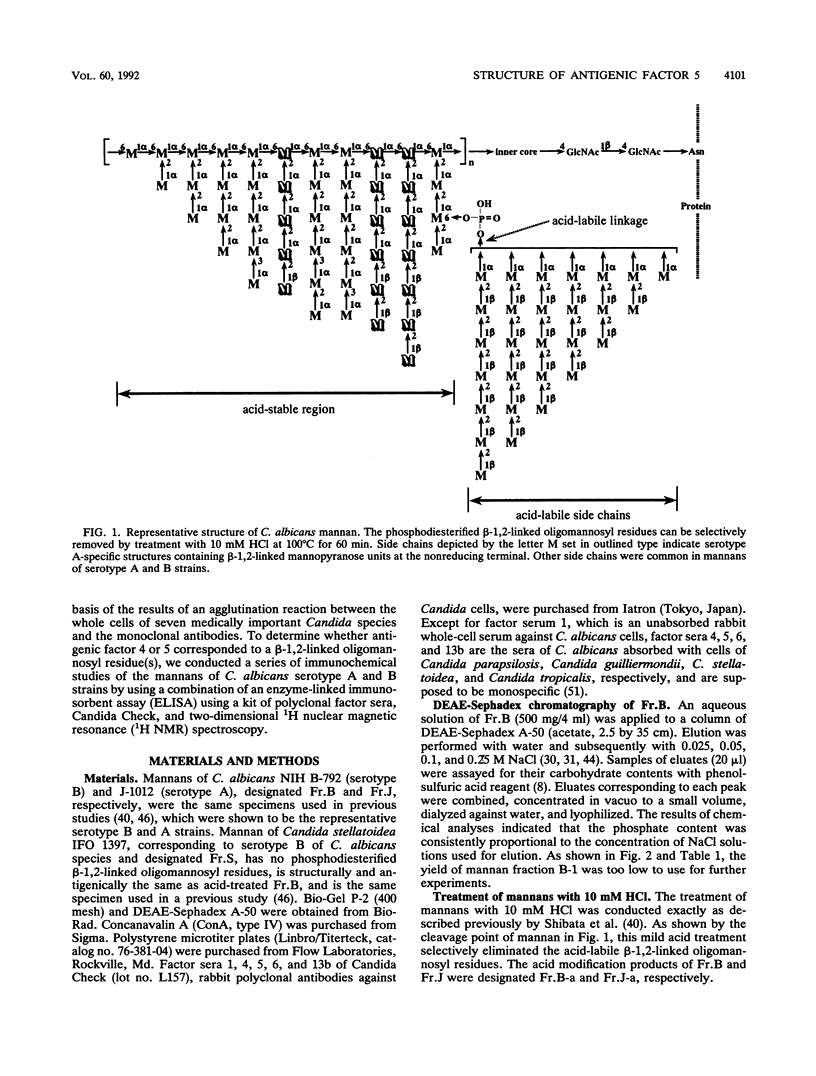
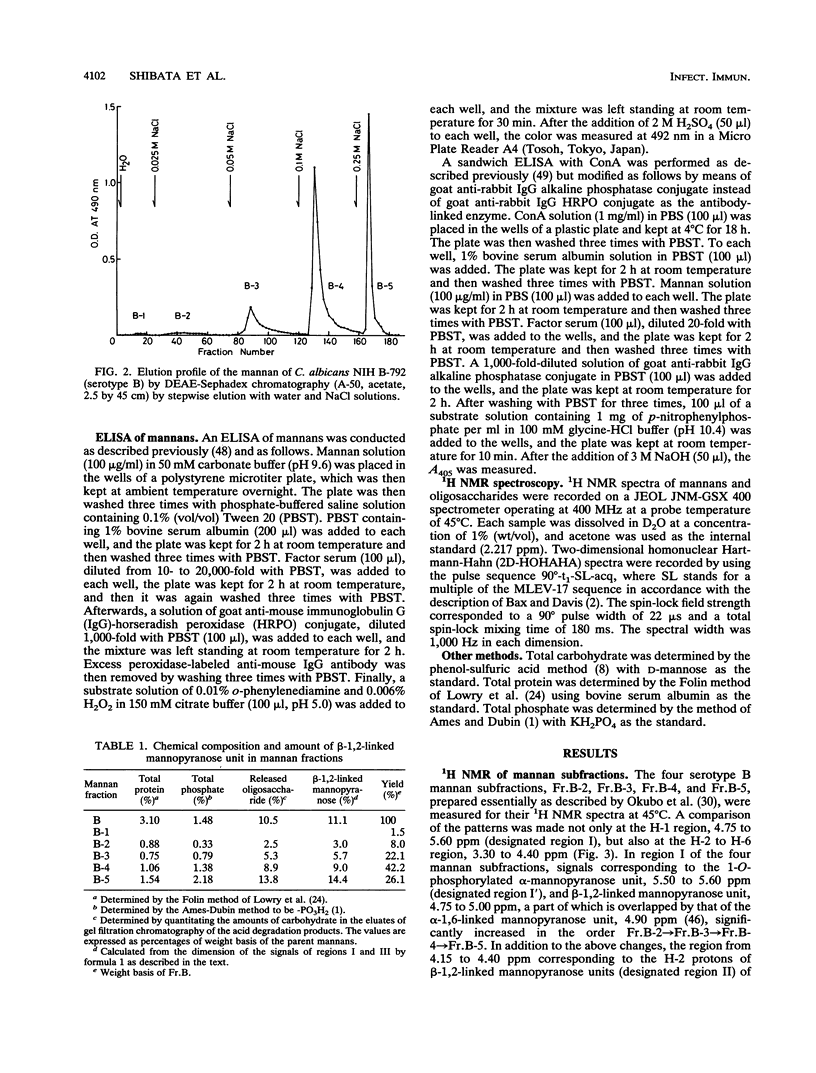
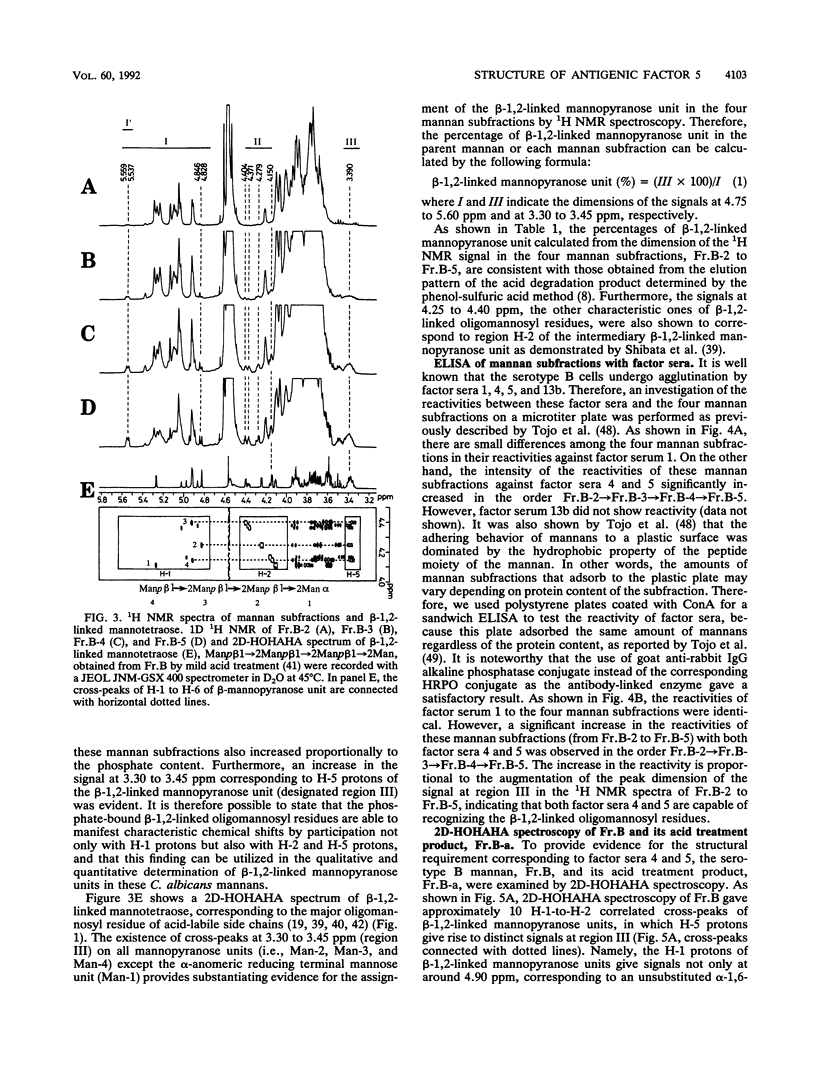
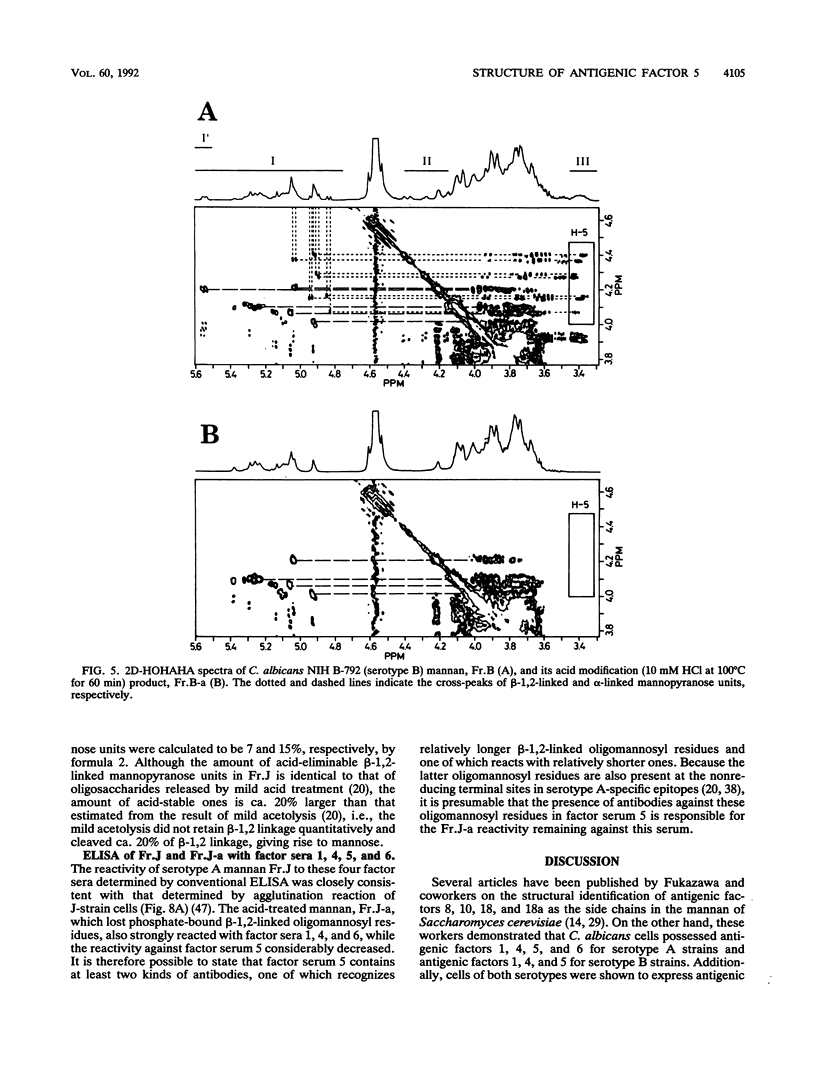
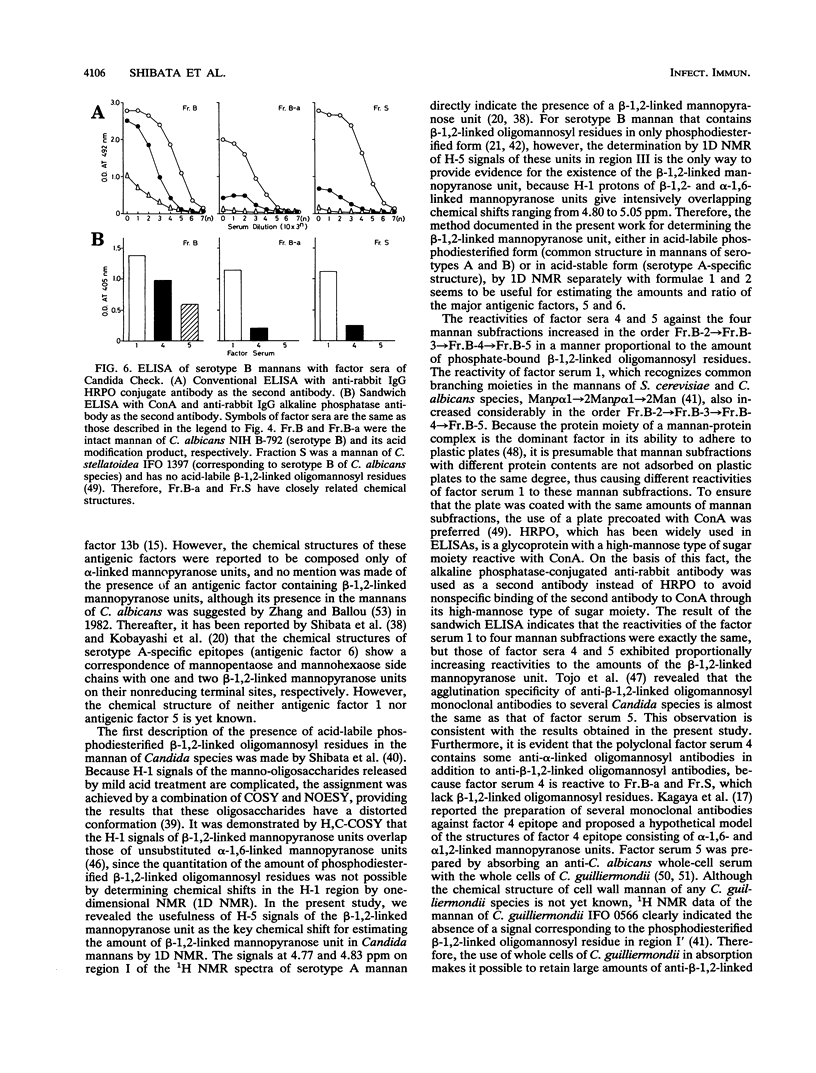
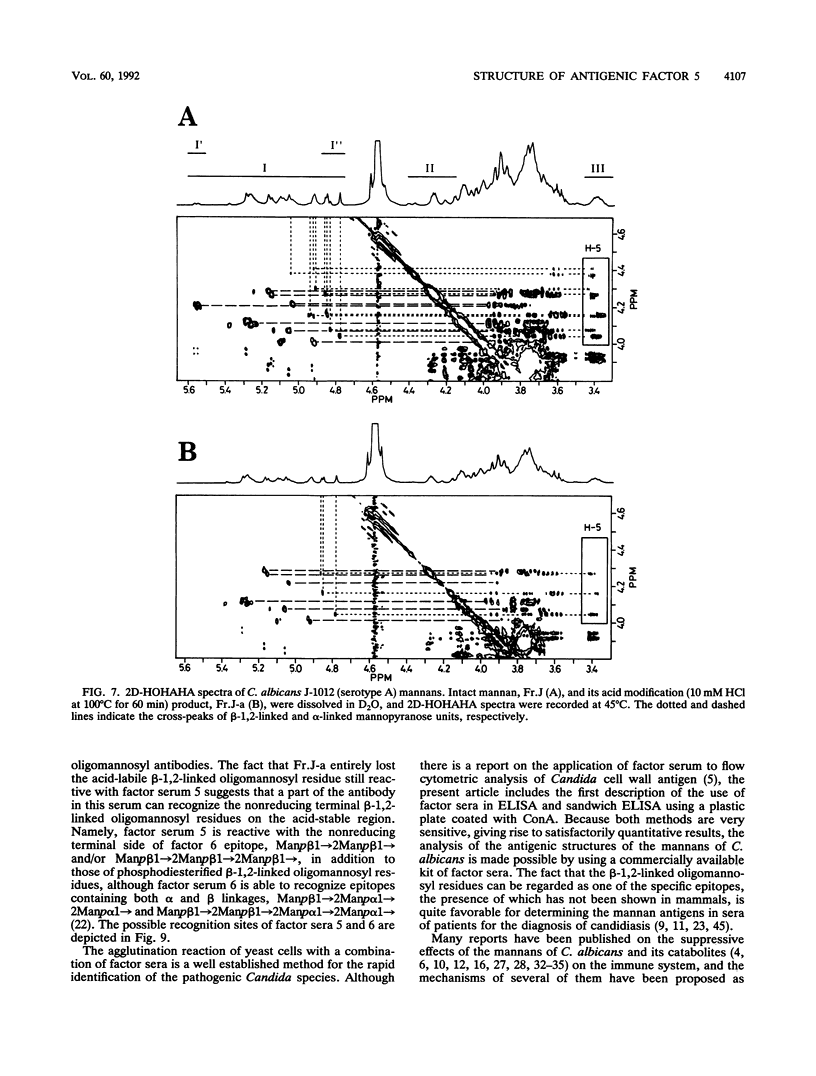
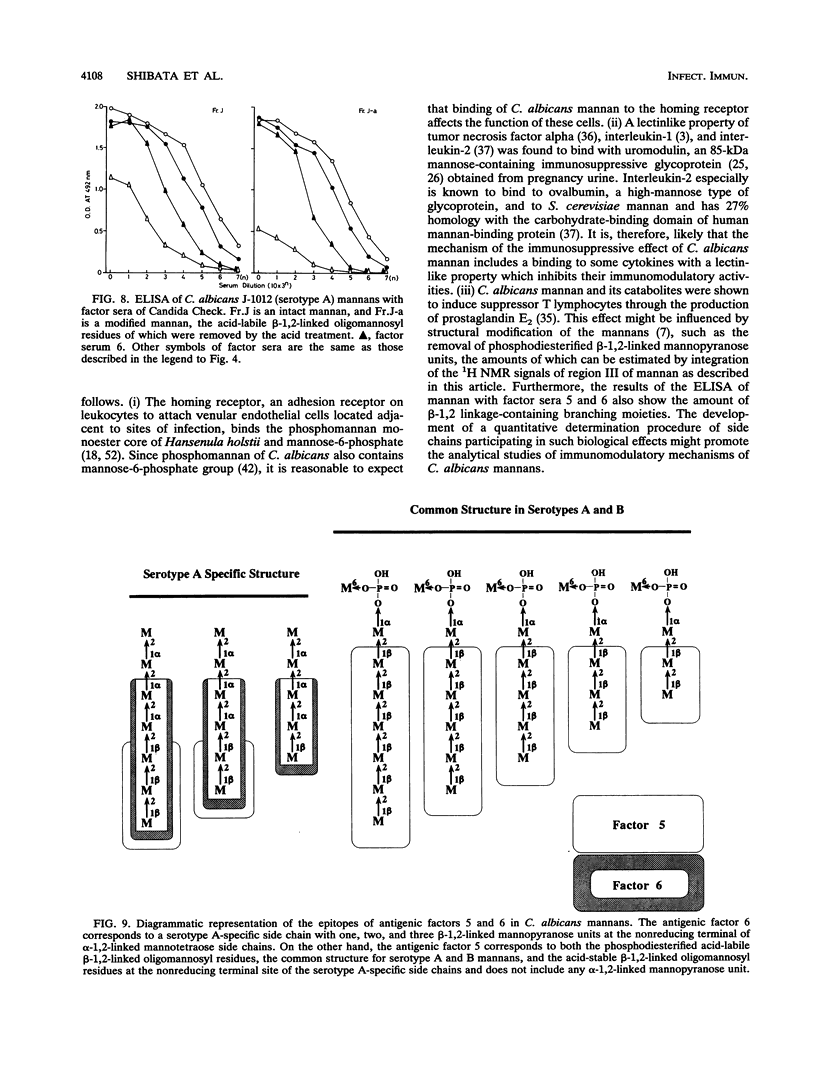
In previous articles, we reported the presence of phosphate-bound beta-1,2-linked oligomannosyl residues in the mannans of strains of Candida albicans serotypes A and B and Candida stellatoidea. To identify the antigenic factor corresponding to this type of oligomannosyl residue, a relationship between chemical structure and antigenic specificity in the mannans of C. albicans NIH B-792 (serotype B, B-strain) and C. albicans J-1012 (serotype A, J-strain) was investigated by using a combination of two-dimensional 1H nuclear magnetic resonance spectroscopy of H-1, H-2, and H-5 regions in the mannans and an enzyme-linked immunosorbent assay that employed concanavalin A-coated microtiter plates. It was shown in the present 1H nuclear magnetic resonance study that an examination of chemical shifts not only in the H-1 region but also in the H-5 region was useful for the quantitative determination of the phosphate-bound beta-1,2-linked oligomannosyl residues. In the enzyme-linked immunosorbent assay using concanavalin A-coated plates, it was revealed that, of factor sera 1, 4, and 5, only factor serum 5 showed a reactivity proportional to the densities of the beta-1,2-linked oligomannosyl residues of the mannan subfractions of different phosphate contents that had been prepared from the bulk B-strain mannan by DEAE-Sephadex chromatography. The above results indicate that the phosphate-bound beta-1,2-linked oligomannosyl residues, Manp beta 1----(2Manp beta 1----)n2Man (n = 0-5), correspond to antigenic factor 5.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Brown K. M., Muchmore A. V., Rosenstreich D. L. Uromodulin, an immunosuppressive protein derived from pregnancy urine, is an inhibitor of interleukin 1. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9119–9123. doi: 10.1073/pnas.83.23.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow E. W., Domer J. E. Immunoregulation in experimental murine candidiasis: specific suppression induced by Candida albicans cell wall glycoprotein. Infect Immun. 1985 Jul;49(1):172–181. doi: 10.1128/iai.49.1.172-181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin W. L., Ringler L., Larsen H. S. Interactions of monospecific antisera with cell surface determinants of Candida albicans. Infect Immun. 1988 Dec;56(12):3294–3296. doi: 10.1128/iai.56.12.3294-3296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff C. F., Rogers C. M., Lamb B. J., Rogers T. J. Induction of suppressor cells in vitro by Candida albicans. Cell Immunol. 1986 Jun;100(1):47–56. doi: 10.1016/0008-8749(86)90005-5. [DOI] [PubMed] [Google Scholar]
- Domer J. E., Stashak P. W., Elkins K., Prescott B., Caldes G., Baker P. J. Separation of immunomodulatory effects of mannan from Candida albicans into stimulatory and suppressive components. Cell Immunol. 1986 Sep;101(2):403–414. doi: 10.1016/0008-8749(86)90153-x. [DOI] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Le Deist F., Drouhet E., Griscelli C. Mannan-specific and mannan-induced T-cell suppressive activity in patients with chronic mucocutaneous candidiasis. J Clin Immunol. 1987 Sep;7(5):400–409. doi: 10.1007/BF00917018. [DOI] [PubMed] [Google Scholar]
- Faille C., Michalski J. C., Strecker G., Mackenzie D. W., Camus D., Poulain D. Immunoreactivity of neoglycolipids constructed from oligomannosidic residues of the Candida albicans cell wall. Infect Immun. 1990 Nov;58(11):3537–3544. doi: 10.1128/iai.58.11.3537-3544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Pichat L., Audinot M., Griscelli C. Defective handling of mannan by monocytes in patients with chronic mucocutaneous candidiasis resulting in a specific cellular unresponsiveness. Clin Exp Immunol. 1982 Mar;47(3):653–660. [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y. Antigenic structure of Candida albicans. Immunochemical basis of the serologic specificity of the mannans in yeasts. Immunol Ser. 1989;47:37–62. [PubMed] [Google Scholar]
- Funayama M., Nishikawa A., Shinoda T., Suzuki M., Fukazawa Y. Antigenic relationship between Candida parapsilosis and Candida albicans serotype B. Microbiol Immunol. 1984;28(12):1359–1371. doi: 10.1111/j.1348-0421.1984.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Garner R. E., Childress A. M., Human L. G., Domer J. E. Characterization of Candida albicans mannan-induced, mannan-specific delayed hypersensitivity suppressor cells. Infect Immun. 1990 Aug;58(8):2613–2620. doi: 10.1128/iai.58.8.2613-2620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya K., Miyakawa Y., Fujihara H., Suzuki M., Soe G., Fukazawa Y. Immunologic significance of diverse specificity of monoclonal antibodies against mannans of Candida albicans. J Immunol. 1989 Nov 15;143(10):3353–3358. [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Butcher E. C. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Giummelly P., Takahashi S., Ishida M., Sato J., Takaku M., Nishidate Y., Shibata N., Okawa Y., Suzuki S. Candida albicans serotype A strains grow in yeast extract-added Sabouraud liquid medium at pH 2.0, elaborating mannans without beta-1,2 linkage and phosphate group. Biochem Biophys Res Commun. 1991 Mar 29;175(3):1003–1009. doi: 10.1016/0006-291x(91)91664-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Shibata N., Mitobe H., Ohkubo Y., Suzuki S. Structural study of phosphomannan of yeast-form cells of Candida albicans J-1012 strain with special reference to application of mild acetolysis. Arch Biochem Biophys. 1989 Aug 1;272(2):364–375. doi: 10.1016/0003-9861(89)90230-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Shibata N., Nakada M., Chaki S., Mizugami K., Ohkubo Y., Suzuki S. Structural study of cell wall phosphomannan of Candida albicans NIH B-792 (serotype B) strain, with special reference to 1H and 13C NMR analyses of acid-labile oligomannosyl residues. Arch Biochem Biophys. 1990 Apr;278(1):195–204. doi: 10.1016/0003-9861(90)90248-w. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Shibata N., Suzuki S. Evidence for oligomannosyl residues containing both beta-1,2 and alpha-1,2 linkages as a serotype A-specific epitope(s) in mannans of Candida albicans. Infect Immun. 1992 May;60(5):2106–2109. doi: 10.1128/iai.60.5.2106-2109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lew M. A., Siber G. R., Donahue D. M., Maiorca F. Enhanced detection with an enzyme-linked immunosorbent assay of candida mannan in antibody-containing serum after heat extraction. J Infect Dis. 1982 Jan;145(1):45–56. doi: 10.1093/infdis/145.1.45. [DOI] [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M. Uromodulin. An immunosuppressive 85-kilodalton glycoprotein isolated from human pregnancy urine is a high affinity ligand for recombinant interleukin 1 alpha. J Biol Chem. 1986 Oct 15;261(29):13404–13407. [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science. 1985 Aug 2;229(4712):479–481. doi: 10.1126/science.2409603. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Herron M. J., McCormack R. T., Gehrz R. C. Two mechanisms of inhibition of human lymphocyte proliferation by soluble yeast mannan polysaccharide. Infect Immun. 1984 Mar;43(3):1041–1046. doi: 10.1128/iai.43.3.1041-1046.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Shibata N., Podzorski R. P., Herron M. J. Candida mannan: chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. Clin Microbiol Rev. 1991 Jan;4(1):1–19. doi: 10.1128/cmr.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa A., Sekine T., Ikeda R., Shinoda T., Fukazawa Y. Reassessment of antigenic determinant of Saccharomyces cerevisiae serotype Ia. Microbiol Immunol. 1990;34(10):825–840. doi: 10.1111/j.1348-0421.1990.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Okubo Y., Ichikawa T., Suzuki S. Relationship between phosphate content and immunochemical properties of subfractions of bakers' yeast mannan. J Bacteriol. 1978 Oct;136(1):63–68. doi: 10.1128/jb.136.1.63-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo Y., Shibata N., Matsumoto T., Suzuki M., Schuerch C., Suzuki S. Immunochemical studies of the mannans of Saccharomyces cerevisiae X2180-1A-5 and Saccharomyces cerevisiae 4484-24D-1 mutant strains, with special reference to their phosphate content. J Bacteriol. 1980 Oct;144(1):92–96. doi: 10.1128/jb.144.1.92-96.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Generation of suppressor cells in the response of human lymphocytes to a polysaccharide from Candida albicans. J Immunol. 1981 Jun;126(6):2151–2155. [PubMed] [Google Scholar]
- Podzorski R. P., Gray G. R., Nelson R. D. Different effects of native Candida albicans mannan and mannan-derived oligosaccharides on antigen-stimulated lymphoproliferation in vitro. J Immunol. 1990 Jan 15;144(2):707–716. [PubMed] [Google Scholar]
- Podzorski R. P., Herron M. J., Fast D. J., Nelson R. D. Pathogenesis of candidiasis. Immunosuppression by cell wall mannan catabolites. Arch Surg. 1989 Nov;124(11):1290–1294. doi: 10.1001/archsurg.1989.01410110044009. [DOI] [PubMed] [Google Scholar]
- Sherblom A. P., Decker J. M., Muchmore A. V. The lectin-like interaction between recombinant tumor necrosis factor and uromodulin. J Biol Chem. 1988 Apr 15;263(11):5418–5424. [PubMed] [Google Scholar]
- Sherblom A. P., Sathyamoorthy N., Decker J. M., Muchmore A. V. IL-2, a lectin with specificity for high mannose glycopeptides. J Immunol. 1989 Aug 1;143(3):939–944. [PubMed] [Google Scholar]
- Shibata N., Fukasawa S., Kobayashi H., Tojo M., Yonezu T., Ambo A., Ohkubo Y., Suzuki S. Structural analysis of phospho-D-mannan-protein complexes isolated from yeast and mold form cells of Candida albicans NIH A-207 serotype A strain. Carbohydr Res. 1989 Apr 15;187(2):239–253. doi: 10.1016/0008-6215(89)80006-0. [DOI] [PubMed] [Google Scholar]
- Shibata N., Hisamichi K., Kikuchi T., Kobayashi H., Okawa Y., Suzuki S. Sequential nuclear magnetic resonance assignment of beta-1,2-linked mannooligosaccharides isolated from the phosphomannan of the pathogenic yeast Candida albicans NIH B-792 strain. Biochemistry. 1992 Jun 23;31(24):5680–5686. doi: 10.1021/bi00139a036. [DOI] [PubMed] [Google Scholar]
- Shibata N., Ichikawa T., Tojo M., Takahashi M., Ito N., Okubo Y., Suzuki S. Immunochemical study on the mannans of Candida albicans NIH A-207, NIH B-792, and J-1012 strains prepared by fractional precipitation with cetyltrimethylammonium bromide. Arch Biochem Biophys. 1985 Dec;243(2):338–348. doi: 10.1016/0003-9861(85)90511-9. [DOI] [PubMed] [Google Scholar]
- Shibata N., Kobayashi H., Takahashi S., Okawa Y., Hisamichi K., Suzuki S., Suzuki S. Structural study on a phosphorylated mannotetraose obtained from the phosphomannan of Candida albicans NIH B-792 strain by acetolysis. Arch Biochem Biophys. 1991 Nov 1;290(2):535–542. doi: 10.1016/0003-9861(91)90578-7. [DOI] [PubMed] [Google Scholar]
- Shibata N., Kobayashi H., Tojo M., Suzuki S. Characterization of phosphomannan-protein complexes isolated from viable cells of yeast and mycelial forms of Candida albicans NIH B-792 strain by the action of Zymolyase-100T. Arch Biochem Biophys. 1986 Dec;251(2):697–708. doi: 10.1016/0003-9861(86)90379-6. [DOI] [PubMed] [Google Scholar]
- Shibata N., Mizugami K., Takano K., Suzuki S. Isolation of mannan-protein complexes from viable cells of Saccharomyces cerevisiae X2180-1A wild type and Saccharomyces cerevisiae X2180-1 A-5 mutant strains by the action of Zymolyase-60,000. J Bacteriol. 1983 Nov;156(2):552–558. doi: 10.1128/jb.156.2.552-558.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Taguchi H., Nishimura K., Miyaji M., Nakamura A., Nakajima H. Studies on detection of Candida antigen in the sera of mice inoculated orally with Candida albicans. Mycopathologia. 1988 Oct;104(1):7–17. doi: 10.1007/BF00437918. [DOI] [PubMed] [Google Scholar]
- TSUCHIYA T., FUKAZAWA Y., KAWAKITA S. A method for the rapid identification of the genus Candida. Mycopathol Mycol Appl. 1959 Mar 31;10(3):191–206. doi: 10.1007/BF02053014. [DOI] [PubMed] [Google Scholar]
- Tojo M., Shibata N., Ban Y., Suzuki S. Structure of the D-mannan of Candida stellatoidea IFO 1397 strain. Comparison with that of the phospho-D-mannan of Candida albicans NIH B-792 strain. Carbohydr Res. 1990 Jun 1;199(2):215–226. doi: 10.1016/0008-6215(90)84263-t. [DOI] [PubMed] [Google Scholar]
- Tojo M., Shibata N., Kobayashi M., Mikami T., Suzuki M., Suzuki S. Preparation of monoclonal antibodies reactive with beta-1,2-linked oligomannosyl residues in the phosphomannan-protein complex of Candida albicans NIH B-792 strain. Clin Chem. 1988 Mar;34(3):539–543. [PubMed] [Google Scholar]
- Tojo M., Shibata N., Osanai T., Mikami T., Suzuki M., Suzuki S. Quantitative precipitin reaction and enzyme-linked immunosorbent assay of mannans of Candida albicans NIH A-207 and NIH B-792 strains compared. Clin Chem. 1988 Dec;34(12):2423–2425. [PubMed] [Google Scholar]
- Tojo M., Shibata N., Osanai T., Mikami T., Suzuki M., Suzuki S. Sandwich enzyme-linked immunosorbent assay of D-mannans of Candida albicans NIH A-207 and NIH B-792 strains using concanavalin A and polyclonal rabbit anti-C. albicans antisera. Carbohydr Res. 1991 Jun 25;213:325–330. doi: 10.1016/s0008-6215(00)90619-0. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Fukazawa Y., Taguchi M., Nakase T., Shinoda T. Serologic aspects on yeast classification. Mycopathol Mycol Appl. 1974 Aug 30;53(1):77–91. doi: 10.1007/BF02127199. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Butcher E. C., Stoolman L. M., Rosen S. D. Receptors involved in lymphocyte homing: relationship between a carbohydrate-binding receptor and the MEL-14 antigen. J Cell Biol. 1987 Mar;104(3):725–731. doi: 10.1083/jcb.104.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. J., Ballou C. E. Saccharomyces kluyveri cell wall mannoprotein. Structures of the O- and N-linked carbohydrate components. J Biol Chem. 1981 Oct 10;256(19):10073–10079. [PubMed] [Google Scholar]


