Abstract
The human papillomavirus (HPV) E7 oncoprotein has been shown to associate with cyclin/CDK2 complexes. Here we present evidence that HPV E7 proteins can associate with cyclin A/CDK2 and cyclin E/CDK2 complexes in cells that lack retinoblastoma tumor suppressor family members through sequences outside of the core retinoblastoma tumor suppressor binding site. Moreover, we show that HPV16 E7 can directly associate with cyclin A/CDK2 and cyclin E/CDK2 complexes. These results suggest that cyclin/CDK2 complexes may be components of HPV E7-associated cellular complexes that do not contain retinoblastoma tumor suppressor family members.
Keywords: cyclin dependent kinase, human papillomaviruses, retinoblastoma tumor suppressor, centrosome duplication
Introduction
Viruses are obligatory intracellular parasites and, since they do not encode a full complement of enzymes that are necessary for the replication of their genomes, they critically depend upon host cellular factors for their own survival and propagation. Human papillomaviruses (HPVs) replicate their genomes and produce viral progeny in a manner that is dependent upon the terminal differentiation of infected squamous epithelial cells. This poses a challenge to the virus as differentiated epithelial cells inherently withdraw from the cell division cycle and, hence, while the differentiated state of the host cell is imperative for the early to late switch of viral gene expression and viral progeny production, such cells are intrinsically incapable of supporting viral genome replication (reviewed in Howley and Lowy, 2007). Thus, a fundamentally important aspect of the life cycle of these viruses is to be able to establish and/or maintain S-phase competence in terminally differentiated epithelial cells. This underlies the basic pathology that these viruses cause in their infected hosts: the induction of hyperplastic epithelial lesions, commonly referred to as warts.
Cyclin-dependent kinase (CDK) 2 and its regulatory subunits cyclins E and A play important roles in S-phase entry and progression, respectively (reviewed in Sherr, 1994). Hence it is not surprising that HPVs have developed multiple strategies to dysregulate CDK2 activity. Most significantly, the majority of HPV-encoded E7 proteins contain an LXCXE amino acid sequence motif, which serves as the core binding site for the retinoblastoma tumor suppressor protein, pRB, and the related p107 and p130 proteins (reviewed in Munger et al., 2004). One common activity of pRB family members is that they negatively regulate G1 to S phase progression through associations with E2F complexes and consequent modulation of E2F transcription factor activity. In addition to a variety of other cellular activities, E2F transcription factors are critical for the concerted temporal expression of regulatory proteins, including cyclins E and A, that are rate limiting for G1 to S phase entry and progression (reviewed in Dyson, 1998). Despite the fact that HPV E7 and E2F complexes associate with different pRB sequences (Wu et al., 1993), HPV E7/pRB association abrogates the transcriptional repressor activity of pRB/E2F complexes, resulting in dysregulated expression of E2F target genes including cyclins E and A. In addition, low-risk as well as high-risk mucosatropic HPV E7 proteins associate with and inactivate the cyclin-dependent kinase inhibitors (CKIs) p21CIP1 and p27KIP1, which have been implicated in regulating cell cycle withdrawal in differentiating keratinocytes (Funk et al., 1997; Jones et al., 1997; Zerfass-Thome et al., 1996).
Previous studies have reported associations between HPV16 E7 and cyclin E and A/CDK2 complexes (Arroyo et al., 1993; Davies et al., 1993; Dyson et al., 1992) but these observed associations were commonly attributed to p107 or p130 bridges (McIntyre et al., 1996). Tomassino and colleagues, however, performed Far-Western assays to show that purified recombinant HPV16 E7 can directly bind to purified recombinant cyclin A (Tommasino et al., 1993). More recently it was reported that purified recombinant high-risk HPV16 and HPV31 and low-risk HPV6b E7 proteins could directly activate cyclin E and A/CDK2 complexes (He et al., 2003). In addition, these authors used chemical crosslinking experiments to show that HPV16 E7 derived peptides corresponding to amino acid residues 9 to 48 could be crosslinked to cyclin A/CDK2 complexes and to recombinant cyclin A but not to recombinant CDK2 alone (He et al., 2003). Here we provide additional evidence that a full-length recombinant HPV16 E7 protein can associate with cyclin A/CDK2 and cyclin E/CDK2 complexes independent of pRB/p107/p130 and that purified recombinant HPV16 E7 can associate with purified recombinant cyclin A/CDK2 as well as cyclin E/CDK2 complexes. Moreover, we show that this association involves HPV16 E7 sequences outside of the known pRB binding site and that low-risk HPV6b and HPV11 E7 proteins also associate with CDK2 complexes independently of pRB, p107 and p130.
Materials and Methods
Cells
NIH 3T3 mouse embryo fibroblasts were obtained from the ATCC and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) supplemented with 10% calf serum, 50 units/ml penicillin, and 50 µg/ml streptomycin. Triple knockout pRB/p107/p130−/− mouse embryo fibroblasts (TKO MEFs) were previously described (Dannenberg et al., 2000) and were maintained in DMEM supplemented with 10% fetal bovine serum, 50 units/ml penicillin, and 50 µg/ml streptomycin.
GST-pulldown Assay
For glutathione S-transferase (GST)-pulldowns, whole cell lysates were produced using ML buffer [20 mM Tris pH8, 1 mM EDTA, 300 mM NaCl, 0.5% NP-40, and protease inhibitors (Complete EDTA-free tablets; Roche Diagnostics)] and cleared by centrifugation (4°C, 20 min, 16,000 × g). GST-control, -wild type HPV16, HPV11 and HPV6b E7, and -HPV16 E7 mutant fusion proteins were expressed in BL21(DE3)pLysS cells (Invitrogen). When the OD600 reached ~0.7, the cells were induced with 100 µM isopropyl-β-D-thiogalactopyranoside (IPTG) overnight at 30°C. For each pulldown, 500 µl of induced bacterial culture was pelleted, lysed using ML buffer (room temperature, 20 min), and cleared by centrifugation (4°C, 20 min, 16,000 × g). The supernatant was mixed with 50 µl glutathione-Sepharose 4B slurry (GE Healthcare) (previously equilibrated with ML buffer) and incubated for 1hr at 4°C. The Sepharose beads were then washed several times with ML buffer and incubated with NIH 3T3 or TKO MEF whole cell lysates. After 4 hours at 4°C, beads were washed 3× with ML buffer and, when indicated, proteins were eluted using Thrombin Protease (Amersham Biosciences) per the manufacturer’s protocol for 1 hour at room temperature; the elution step helped minimize background binding that was frequently seen in pulldowns using TKO MEF lysates. Samples were then analyzed via Western blotting. Samples were separated on sodium dodecyl sulfate-12% polyacrylamide gels and electrotransferred onto polyvinylidene difluoride membranes (Millipore). The membranes were probed with anti-cyclin A (CY-A1, Sigma), anti-CDK2 (M2, Santa Cruz Biotechnology), anti-GST (3818-1, Clontech), and anti-pRB (G3-245, BD Pharmingen) antibodies. Proteins were visualized using Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer Life Sciences, Inc.) and exposed on Kodak BioMax XAR film or electronically acquired with a Kodak Image Station 4000R equipped with Kodak Imaging software, version 4.0.
In vitro association of purified complexes
Purified CDK2/CycA Kinase and CDK2/CycE Kinase complexes produced in insect cells were obtained from Cell Signaling Technology; all components are GST-tagged. Full-length HPV16 E7 was polymerase chain reaction (PCR) cloned into the Nde1 and Xho1 sites of the pET-23a(+)-RGS6xHis bacterial expression plasmid which produces a C-terminally hexa-His tagged HPV16 E7. His6-control (empty vector) and His6-tagged HPV16 E7 were expressed in BL21(DE3)pLysS cells (Invitrogen). When the OD600 reached ~0.45–0.65, the cells were induced with 200 µM isopropyl-β-D-thiogalactopyranoside (IPTG) overnight at 30°C. 5 ml aliquots were frozen at −80°C. To make lysates, pellets were resuspended in ML buffer [20 mM Tris pH 8, 1 mM EDTA, 300 mM NaCl, 0.5% NP-40, and protease inhibitors (Complete EDTA-free tablets; Roche Diagnostics)], and sonicated 3× for 20 sec each. Bacterial lysates were then cleared via centrifugation at 4°C for 15 min at 16,000 × g. Bacterial lysates were incubated with pre-equilibrated Ni2+-charged nitrilotriacetic acid (NTA) agarose beads (Invitrogen) for 1 hour at 4°C and then washed with ML buffer. A quarter of the beads were incubated with either the CDK2/CycE complex or the CDK2/CycA complex for 4 hours at 4°C. Beads were then washed with ML buffer and analyzed via Western blotting, as described above. The membranes were probed with anti-CDK2 (M2, Santa Cruz Biotechnology) or anti-E7 (ED19, from our laboratory) antibodies.
Results
HPV16 E7 can associate with CDK2-containing complexes independent of pRB, p107, or p130
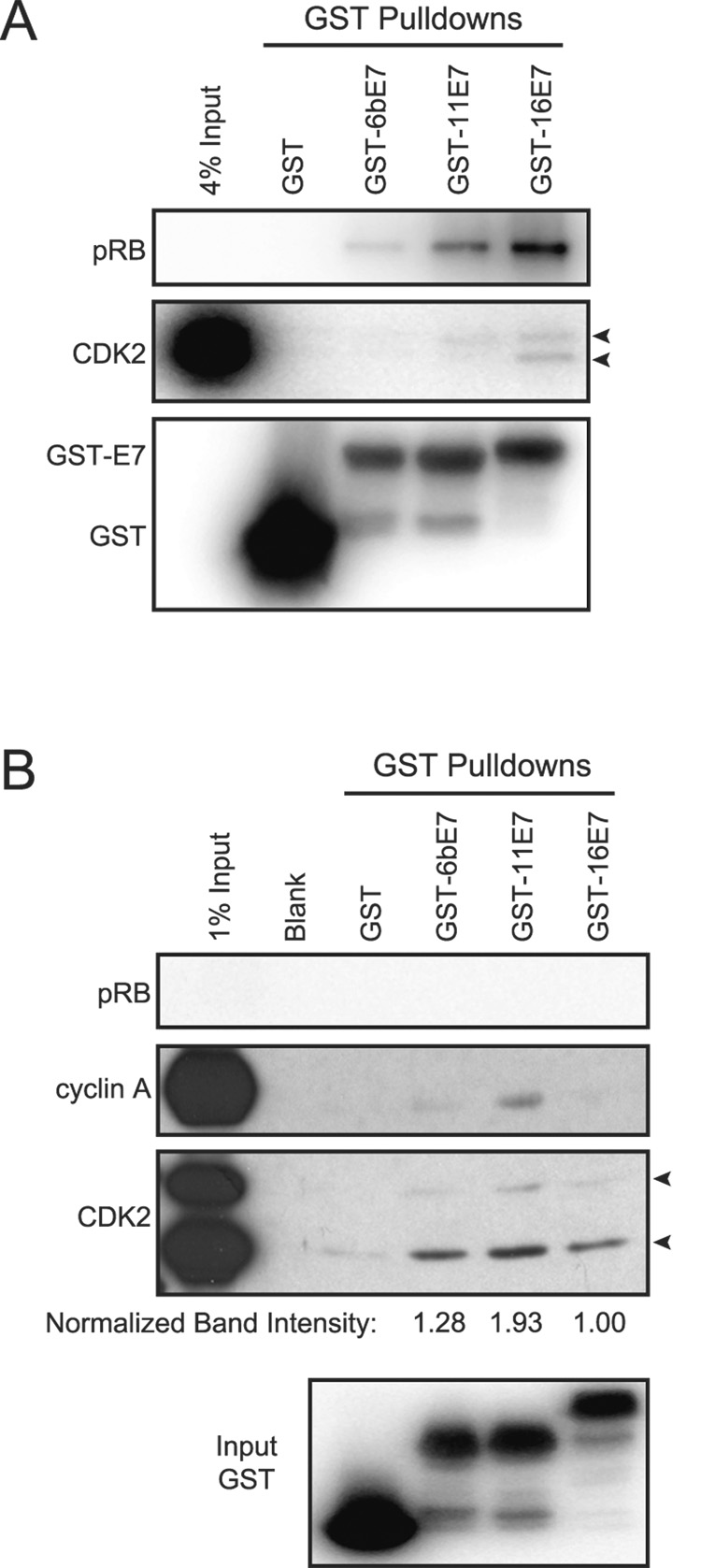
To determine whether HPV16 E7 could directly associate with cyclin/CDK2 complexes in the absence of the pRB, p107, and p130 pocket proteins, we initially attempted to generate pRB/p107/p130−/− (“triple knockout” – TKO) mouse embryo fibroblasts (MEFs) with stable expression of a C-terminally HA/FLAG epitope tagged HPV16 E7 (C-E7) (Huh et al., 2005). Despite numerous attempts we did not obtain any clones with detectable expression of this protein (data not shown), despite the fact that we could readily express this protein at detectable levels in NIH 3T3 cells (Nguyen et al., 2007). Hence we mixed recombinant, purified GST-HPV16 E7 (GST-16E7) or unfused GST immobilized on glutathione Sepharose beads with whole cell extracts from either TKO MEFs or NIH 3T3 cells as a control. After incubation, beads were washed and analyzed by SDS PAGE followed by Western blotting with CDK2 specific antibodies. The data from these experiments suggest that GST-16E7 associates with CDK2 from NIH 3T3 as well as TKO MEF derived whole cell lysates (Figure 1A and 1B). Hence, the association between HPV16 E7 and CDK2 does not strictly rely upon any of the pRB family members.
Figure 1. GST-HPV E7 associates with CDK2 in a pRB/p107/p130 independent manner.
(A) Western blot analysis of GST, GST-6bE7, GST-11E7, and GST-16E7 pulldowns in NIH 3T3 cells. pRB is shown as a positive control as it is known to associate with HPV16 E7. The blot was also probed for CDK2 (arrows indicate known isoforms). (B) Western blot analysis of eluted proteins from GST, GST-6bE7, GST-11E7, and GST-16E7 pulldowns in triple knockout pRB/p107/p130−/− mouse embryo fibroblasts (TKO MEFs), as indicated. The blot was probed for pRB, cyclin A, and CDK2. Quantified band intensities shown were determined for the lower CDK2 isoforms. Western blot analysis of input GST proteins is also provided.
HPV6b and HPV11 E7 proteins also associate with CDK2 complexes in pRB/p107/p130 deficient cells
To determine whether the observed interaction with CDK2 complexes is specific to high-risk HPV16 E7 or conserved with low-risk HPV E7 proteins, we performed similar experiments as described above with purified recombinant GST-HPV6b or -HPV11 E7 fusion proteins (GST-6bE7 and GST-11E7). As with GST-16E7, GST-6bE7 and GST-11E7 were able to bind CDK2 in TKO MEFs. Interestingly GST-6bE7 bound CDK2 at similar levels as GST-16E7, whereas GST-11E7 bound CDK2 more effectively than GST-6E7 or GST-16 E7 (Figure 1B; see normalized band intensities).
Association of HPV16 E7 with CDK2 in TKO MEFs is independent of the HPV16 E7 pRB binding site
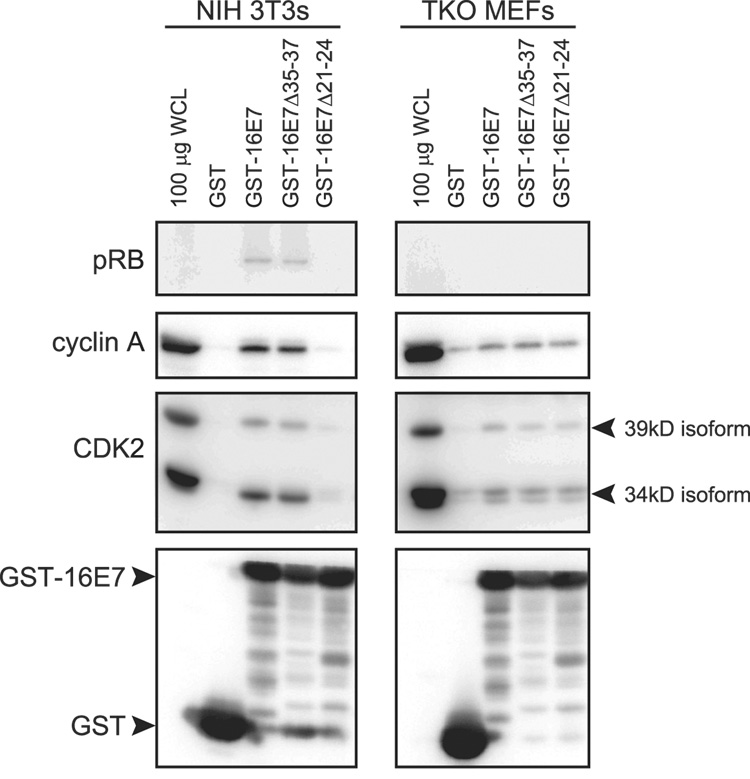
We next wanted to map the sequences of HPV16 E7 that contribute to CDK2 association in cells that do or do not contain pRB family members. To address this question, we performed similar mixing experiments using unfused GST as a control, and GST fused to full length HPV16 E7 (GST-16E7), the pRB binding deficient mutant HPV16 E7Δ21-24 (GST-16E7Δ21-24), and a deletion mutant HPV16 E7Δ35-37 (GST-16E7Δ35-37), which targets an acidic domain adjacent to the pRB binding site and affects casein kinase 2 phosphorylation (Phelps et al., 1992). Associated proteins were analyzed by SDS PAGE and Western blotting. Association with pRB was used as a control and GST-16E7 and the GST-16E7Δ35-37 efficiently associated with pRB from NIH 3T3 derived lysates. As expected GST-16E7Δ21-24 did not associate with pRB in experiments with NIH 3T3 derived lysates and no pRB signals were detected with TKO MEF extracts. Interestingly, the association of GST-16E7Δ21-24 with CDK2 and its regulatory subunit cyclin A was dramatically diminished in the mixing experiments using NIH 3T3 derived cell extracts. Nevertheless, the GST-16E7Δ21-24 mutant associated with CDK2 and cyclin A at similar levels as wild type GST-16 E7 or the GST-16E7Δ35-37 mutant in the experiments using TKO MEF derived cell extracts. This suggests that, in pRB/p107/p130 expressing cells, the majority of CDK2 complexes is co-precipitated as a consequence of associations with pRB family members, while a small pool is associated with HPV16 E7 independent of pRB family members and through HPV16E7 sequences other than the pRB binding domain or the adjacent acidic domain (Figure 2).
Figure 2. Mapping the association between CDK2 and HPV16 E7.
Western blot analysis of GST, GST-16E7, and GST-16E7 mutant pulldowns in NIH 3T3 cells and TKO MEFs. The blot was probed for pRB (positive control, as expected GST-Δ21-24 does not associate with pRB), cyclin A, CDK2, and GST. 100µg whole cell lysate (WCL) represents 5% of the input.
Purified HPV16 E7 and cyclin/CDK2 complexes interact in vitro
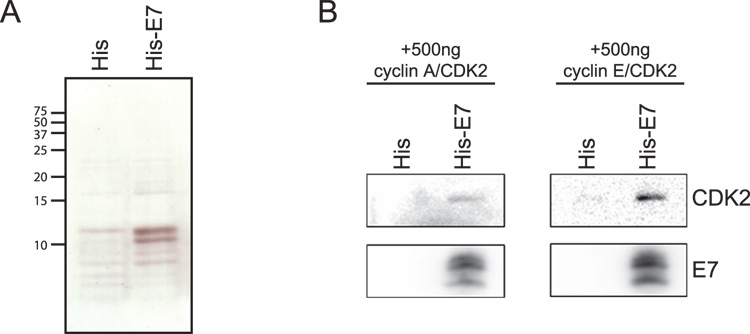
The previous experiments established that HPV16 E7 has the capacity to associate with CDK2 complexes independent of pRB family members. These experiments, however, do not rule out the possibility that the association is indirect and that other cellular proteins may be acting as bridging proteins between HPV16 E7 and CDK2. Therefore, we wanted to examine whether recombinant, purified HPV16 E7 could associate with recombinant, purified cyclin/CDK2 complexes. Hexa-histidine tagged HPV16 E7 (His6-16E7) was produced in bacteria, purified on Ni2+-charged nitrilotriacetic acid (NTA) beads (Figure 3A), and incubated with 500 ng of recombinant, purified cyclin A/CDK2 or cyclin E/CDK2 produced in insect cells. A Ni2+-NTA pulldown of His6-16E7 co-precipitated both cyclin A/CDK2 and cyclin E/CDK2 complexes (Figure 3B). Hence, HPV16 E7 can interact with cyclin/CDK2 complexes independently of other cellular bridging proteins as well.
Figure 3. His-tagged HPV16 E7 associates with purified cyclin/CDK2 complexes.
(A) A silver-stained SDS-PAGE gel containing 10% of the purified His6-control (empty vector) or His6-16E7 used in the pulldowns in part B. Molecular weight standards are indicated to the left of the gel. (B) Western blot analysis of Ni2+-NTA pulldowns using purified His6-control or His6-16E7 together with 500ng of purified cyclin E or A/CDK2. The blot was probed for CDK2 and E7.
Discussion
Due to its ability to cooperate with the ras oncogene for transformation of baby rat kidney cells (Matlashewski et al., 1987; Phelps et al., 1989) and vital association with the nuclear pRB tumor suppressor, HPV16 E7 has traditionally been regarded as a “typical nuclear” oncoprotein. HPV16 E7 is actively transported to the nucleus (Angeline et al., 2003; Fujikawa et al., 1994) but biochemical fractionation and immunofluorescence experiments have revealed that a significant amount of HPV16 E7 is localized in cytoplasmic structures (Huh et al., 2005; Nguyen et al., 2007; Ressler et al., 2007; Smotkin and Wettstein, 1987). Consistent with this notion, a number of studies have implicated HPV16 E7 in the regulation of non-nuclear processes. Some intriguing examples include the ability of HPV16 E7 to associate with the glycolytic enzyme pyruvate kinase and induce a metabolic shift reminiscent of the Warburg effect (Mazurek et al., 2001; Zwerschke et al., 1999), to bind to the cytoplasmic microtubule-associated N-end rule ubiquitin ligase p600 (Huh et al., 2005), and to interact with the centrosomal regulatory protein gamma tubulin (Nguyen et al., 2007). Hence, HPV16 E7 associates with a number of cellular regulatory complexes that do not necessarily contain pRB family members. It has been of interest to our laboratory to identify the specific complexes with which HPV16 E7 interacts because such information would help us to better understand the mechanistic details regarding how HPV16 E7 achieves its multiple functions. Because cyclin/CDK2 complexes play important roles in the cell cycle and an essential activity of HPV16 E7 is the disruption of the cell cycle to achieve S-phase competence, it was important to more clearly dissect the nature of the interplay between HPV E7 proteins and cyclin/CDK2 complexes.
Similar to the subcellular localization of HPV16 E7, while cyclin E/CDK2 and cyclin A/CDK2 are critical components of nuclear pRB family member containing E2F transcription factor complexes (Devoto et al., 1992; Lees et al., 1992; Mudryj et al., 1991; Shirodkar et al., 1992), which HPV16 E7 is known to bind (Arroyo et al., 1993), CDK2 has also been detected in the cytoplasm and on centrosomes (Jackman et al., 2002). Therefore, HPV16 E7 would be capable of associating with cyclin/CDK2 complexes both in the nucleus, where pRB is present, as well as in the cytoplasm. Here we show that HPV16 E7 can associate with cyclin E- and cyclin A-containing CDK2 complexes through both indirect, presumably pRB family member dependent, as well as direct binding to CDK2 and/or cyclin subunits. Consistent with published studies (McIntyre et al., 1996), our results suggest that the majority of cyclin/CDK2 complexes are associated with HPV16 E7 indirectly through pRB family members. Nonetheless, the pRB family member binding deficient HPV16 E7 Δ21-24 mutant retains low level, detectable association with CDK2 in NIH 3T3 cells, and HPV16 E7 associates with CDK2 through sequences other than the pRB binding site in TKO MEFs. As such, our results agree with earlier studies that demonstrated direct association of cyclin/CDK2 with HPV16 E7 (He et al., 2003; Tommasino et al., 1993). However, our studies are the first to document direct association of HPV16 E7 with cyclin E/CDK2 complexes and to show that recombinant full length HPV6b and HPV11 proteins can also associate with CDK2 complexes independent of pRB family members.
Because the pRB family member independent association between E7 and CDK2 complexes appears to be conserved between high-risk and low-risk HPVs, future studies will be directed towards determining the functional consequences of these interactions and how they contribute to the viral life cycle. Our discovery that low-risk HPV E7 proteins can associate with CDK2 complexes in TKO MEFs suggests that such an association with high-risk HPV E7 proteins likely does not contribute to high-risk HPV E7-mediated centrosome overduplication, a process that is critically dependent on CDK2 activity (Duensing et al., 2006; Duensing et al., 2004), as we first hypothesized. We had previously shown that HPV16 E7 induces supernumerary centrosomes in TKO MEFs, and moreover, that this was dependent on the integrity of the HPV16 E7 pRB-binding site (Duensing and Munger, 2003). Furthermore, subcellular fractionation experiments revealed evidence for a centrosome-associated pool of HPV16 E7 as well as CDK2 (Nguyen et al., 2007). Our finding that the HPV16 E7Δ21-24 mutant, which is defective for induction of centrosome abnormalities in normal as well as TKO MEFs (Duensing and Munger, 2003), retains the capacity to associate with CDK2, however, further suggests that the direct association between HPV16 E7 and CDK2 and/or the consequential modulation of CDK2 activity that was suggested by He and colleagues (He et al., 2003), is unlikely to be necessary for the induction of supernumerary centrosomes. In addition, our results demonstrating that HPV11 and HPV6b E7 proteins, which are inactive for induction of supernumerary centrosomes, retain pRB/p107/p130 independent cyclin A/CDK2 association at levels that are higher (HPV11 E7) or at least comparable (HPV6b E7) to that of HPV16 E7 are consistent with this notion. Indeed, we detected a pRB family member independent association of HPV16 E7 (but not HPV6b E7) with the centrosomal regulatory protein gamma tubulin that likely contributes to this important activity of HPV16 E7 (Nguyen et al., 2007).
Interestingly, as mentioned above, He and colleagues observed a dramatic stimulation in the enzymatic activity of cyclin/CDK2 complexes upon addition of recombinant HPV16 E7 protein when histone H1 was used as a substrate (He et al., 2003). Nevertheless, they observed only minimal stimulation when a potentially more relevant substrate, a carboxyl terminal pRB domain, was evaluated. While we did not address this issue in detail, our preliminary experiments with the carboxyl terminal pRB domain substrate are consistent with their results and did not yield any evidence for significant stimulation of CDK2 activity (data not shown). Therefore, it will be important to determine the effect of E7 on CDK2 activity on additional biologically relevant substrates once these are identified. In addition, high-risk HPV E6 and E7 oncoproteins have each been shown to retarget enzymatic activities to substrates that are not normally modified by these enzymes. Examples include the p53 tumor suppressor and PDZ domain containing proteins that are ubiquitinated by the HPV E6/E6AP complex (Handa et al., 2007; Jing et al., 2007; Nakagawa and Huibregtse, 2000; Scheffner et al., 1993; Storrs and Silverstein, 2007) and/or other E6/ubiquitin ligase complexes (Massimi et al., 2008) and the pRB tumor suppressor that is ubiquitinated by the HPV16 E7/cullin 2 ubiquitin ligase complex (Huh et al., 2007). Hence one might envision that binding to E7 might result in a similar reprogramming of cyclin E/CDK2 and/or cyclin A/CDK2 complexes. Therefore, in addition to determining whether E7 association alters the activity of cyclin/CDK2 complexes on natural substrates, it will be interesting to determine whether E7 redirects cyclin/CDK2 activity to novel substrates.
Ultimately, we have shown here that both low- and high-risk HPV E7 proteins associate with CDK2 complexes independent of pRB family members and it will be important to determine the biological consequences of these specific interactions. Such experiments promise to provide further insights into both CDK2 function as well as the ways in which the HPVs reprogram cellular processes.
Acknowledgments
Supported by PHS grants R01 CA066980 (KM) and T32CA009031 (CLN). We thank Hiroyuki Hayakawa for construction of the hexa-histidine tagged HPV16 E7 expression plasmid, Kevin F. Bryant for helpful discussion, and Gary Beumms for continued support and inspiration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angeline M, Merle E, Moroianu J. The E7 oncoprotein of high-risk human papillomavirus type 16 enters the nucleus via a nonclassical Ran-dependent pathway. Virology. 2003;317(1):13–23. doi: 10.1016/j.virol.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Arroyo M, Bagchi S, Raychaudhuri P. Association of the human papillomavirus type 16 E7 protein with the S-phase specific E2F-cyclin A complex. Molecular and Cellular Biology. 1993;13:6537–6546. doi: 10.1128/mcb.13.10.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg JH, van Rossum A, Schuijff L, te Riele H. Ablation of the retinoblastoma gene family deregulates G(1) control causing immortalization and increased cell turnover under growth-restricting conditions. Genes Dev. 2000;14(23):3051–3064. doi: 10.1101/gad.847700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R, Hicks R, Crook T, Morris J, Vousden K. Human papillomavirus type 16 E7 associates with a histone H1 kinase and with p107 through sequences necessary for transformation. Journal of Virology. 1993;67:2521–2528. doi: 10.1128/jvi.67.5.2521-2528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto SH, Mudryj M, Pines J, Hunter T, Nevins JR. A cyclin A-protein kinase complex possesses sequence-specific DNA binding activity: p33cdk2 is a component of the E2F-cyclin A complex. Cell. 1992;68:167–176. doi: 10.1016/0092-8674(92)90215-x. [DOI] [PubMed] [Google Scholar]
- Duensing A, Liu Y, Tseng M, Malumbres M, Barbacid M, Duensing S. Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene. 2006;25(20):2943–2949. doi: 10.1038/sj.onc.1209310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing S, Duensing A, Lee DC, Edwards KM, Piboonniyom SO, Manuel E, Skaltsounis L, Meijer L, Munger K. Cyclin-dependent kinase inhibitor indirubin-3′-oxime selectively inhibits human papillomavirus type 16 E7-induced numerical centrosome anomalies. Oncogene. 2004;23(50):8206–8215. doi: 10.1038/sj.onc.1208012. [DOI] [PubMed] [Google Scholar]
- Duensing S, Munger K. Human papillomavirus type 16 E7 oncoprotein can induce abnormal centrosome duplication through a mechanism independent of inactivation of retinoblastoma protein family members. J Virol. 2003;77(22):12331–12335. doi: 10.1128/JVI.77.22.12331-12335.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- Dyson N, Guida P, Munger K, Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol. 1992;66(12):6893–6902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa K, Furuse M, Uwabe K, Maki H, Yoshie O. Nuclear localization and transforming activity of human papillomavirus type 16 E7-beta-galactosidase fusion protein: characterization of the nuclear localization sequence. Virology. 1994;204(2):789–793. doi: 10.1006/viro.1994.1594. [DOI] [PubMed] [Google Scholar]
- Funk JO, Waga S, Harry JB, Espling E, Stillman B, Galloway DA. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes & Development. 1997;11:2090–2100. doi: 10.1101/gad.11.16.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa K, Yugawa T, Narisawa-Saito M, Ohno S, Fujita M, Kiyono T. E6AP-dependent degradation of DLG4/PSD95 by high-risk human papillomavirus type 18 E6 protein. J Virol. 2007;81(3):1379–1389. doi: 10.1128/JVI.01712-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Staples D, Smith C, Fisher C. Direct activation of cyclin-dependent kinase 2 by human papillomavirus E7. J Virol. 2003;77(19):10566–10574. doi: 10.1128/JVI.77.19.10566-10574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley PM, Lowy DR. Papillomaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 5 ed. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2299–2354. 2 vols. [Google Scholar]
- Huh K, Zhou X, Hayakawa H, Cho JY, Libermann TA, Jin J, Harper JW, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J Virol. 2007;81(18):9737–9747. doi: 10.1128/JVI.00881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh KW, DeMasi J, Ogawa H, Nakatani Y, Howley PM, Munger K. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc Natl Acad Sci U S A. 2005;102(32):11492–11497. doi: 10.1073/pnas.0505337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman M, Kubota Y, den Elzen N, Hagting A, Pines J. Cyclin A- and cyclin E-Cdk complexes shuttle between the nucleus and the cytoplasm. Mol Biol Cell. 2002;13(3):1030–1045. doi: 10.1091/mbc.01-07-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing M, Bohl J, Brimer N, Kinter M, Vande Pol SB. Degradation of tyrosine phosphatase PTPN3 (PTPH1) by association with oncogenic human papillomavirus E6 proteins. J Virol. 2007;81(5):2231–2239. doi: 10.1128/JVI.01979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Alani RM, Münger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes & Development. 1997;11:2101–2111. doi: 10.1101/gad.11.16.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees E, Faha B, Dulic V, Reed SI, Harlow E. Cyclin E/cdk2 and cyclin A/cdk2 kinases associate with p107 and E2F in a temporally distinct manner. Genes and Development. 1992;6:1874–1885. doi: 10.1101/gad.6.10.1874. [DOI] [PubMed] [Google Scholar]
- Massimi P, Shai A, Lambert P, Banks L. HPV E6 degradation of p53 and PDZ containing substrates in an E6AP null background. Oncogene. 2008;27(12):1800–1804. doi: 10.1038/sj.onc.1210810. [DOI] [PubMed] [Google Scholar]
- Matlashewski G, Schneider J, Banks L, Jones N, Murray A, Crawford L. Human papillomavirus type 16 DNA cooperates with activated ras in transforming primary cells. Embo J. 1987;6(6):1741–1746. doi: 10.1002/j.1460-2075.1987.tb02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek S, Zwerschke W, Jansen-Durr P, Eigenbrodt E. Effects of the human papilloma virus HPV-16 E7 oncoprotein on glycolysis and glutaminolysis: role of pyruvate kinase type M2 and the glycolytic-enzyme complex. Biochem J. 2001;356(Pt 1):247–256. doi: 10.1042/0264-6021:3560247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre MC, Ruesch MN, Laimins LA. Human papillomavirus E7 oncoproteins bind a single form of cyclin E in a complex with cdk2 and p107. Virology. 1996;215:73–82. doi: 10.1006/viro.1996.0008. [DOI] [PubMed] [Google Scholar]
- Mudryj M, Devoto SH, Hiebert SW, Hunter T, Pines J, Nevins JR. Cell Cycle Regulation of the E2F transcription factor involves an interaction with cyclin A. Cell. 1991;65:1243–1253. doi: 10.1016/0092-8674(91)90019-u. [DOI] [PubMed] [Google Scholar]
- Munger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, Grace M, Huh K. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78(21):11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin- protein ligase. Mol Cell Biol. 2000;20(21):8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CL, Eichwald C, Nibert ML, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the centrosomal component gamma-tubulin. J Virol. 2007;81(24):13533–13543. doi: 10.1128/JVI.01669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps WC, Münger K, Yee CL, Barnes JA, Howley PM. Structure-function analysis of the human papillomavirus E7 oncoprotein. Journal of Virology. 1992;66:2418–2427. doi: 10.1128/jvi.66.4.2418-2427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps WC, Yee CL, Münger K, Howley PM. Functional and sequence similarities between HPV16 E7 and adenovirus E1A. Current Topics in Microbiology and Immunology. 1989;144:153–166. doi: 10.1007/978-3-642-74578-2_19. [DOI] [PubMed] [Google Scholar]
- Ressler S, Scheiden R, Dreier K, Laich A, Muller-Holzner E, Pircher H, Morandell D, Stein I, Viertler HP, Santer FR, Widschwendter A, Even J, Jansen-Durr P, Capesius C, Zwerschke W. High-risk human papillomavirus E7 oncoprotein detection in cervical squamous cell carcinoma. Clin Cancer Res. 2007;13(23):7067–7072. doi: 10.1158/1078-0432.CCR-07-1222. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP Complex Functions as a Ubiquitin-Protein Ligase in the Ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Shirodkar S, Ewen M, DeCaprio JA, Morgan J, Livingston DM, Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992;68:157–168. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- Smotkin D, Wettstein FO. The major human papillomavirus protein in cervical cancers is a cytoplasmic phosphoprotein. J Virol. 1987;61:1686–1689. doi: 10.1128/jvi.61.5.1686-1689.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrs CH, Silverstein SJ. PATJ, a tight junction-associated PDZ protein, is a novel degradation target of high-risk human papillomavirus E6 and the alternatively spliced isoform 18 E6. J Virol. 2007;81(8):4080–4090. doi: 10.1128/JVI.02545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasino M, Adamczewski JP, Carlotti F, Barth CF, Manetti R, Contorni M, Cavalieri F, Hunt T, Crawford L. HPV16 E7 protein associates with the protein kinase p33CDK2 and cyclin A. Oncogene. 1993;8:195–202. [PubMed] [Google Scholar]
- Wu EW, Clemens KE, Heck DV, Münger K. The human papillomavirus E7 oncoprotein and the cellular transcription factor E2F bind to separate sites on the retinoblastoma tumor suppressor protein. Journal of Virology. 1993;67:2402–2407. doi: 10.1128/jvi.67.4.2402-2407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerfass-Thome K, Zwerschke W, Mannhardt B, Tindle R, Botz JW, Jansen-Durr P. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene. 1996;13:2323–2330. [PubMed] [Google Scholar]
- Zwerschke W, Mazurek S, Massimi P, Banks L, Eigenbrodt E, Jansen-Durr P. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1999;96:1291–1296. doi: 10.1073/pnas.96.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]