Abstract
Some actions of insulin are mediated by inositolphosphoglycan mediators. Deficient release of a putative D-chiro-inositol-containing inositolphosphoglycan (DCI-IPG) mediator may contribute to insulin resistance in women with polycystic ovary syndrome (PCOS). Previously we demonstrated that oral DCI supplementation improved ovulation and metabolic parameters in women with PCOS. However, whether oral DCI mediates an increase in the release of the DCI-IPG mediator and an improvement in insulin sensitivity in women with PCOS is unknown. We conducted a randomized controlled trial of DCI supplementation vs. placebo in 11 women with PCOS who were assessed at two-time points, 6 weeks apart. Plasma DCI, DCI-IPG release during OGTT (AUCDCI-IPG) and insulin sensitivity (Si) by FSIVGTT were assessed at baseline and end-of-study. The study was terminated early due to a sudden unavailability of the study drug. However, in all subjects without regard to treatment assignment, there was a positive correlation between the change in AUCDCI-IPG / AUCInsulin ratio and the change in Si during the 6-week period (r=0.69, p=0.02), which remained significant after adjustment for BMI (p=0.022), and after further adjustment for BMI and treatment allocation (p=0.0261). This suggests that in women with PCOS, increased glucose-stimulated DCI-IPG release is significantly correlated with improved insulin sensitivity. The significant relationship between DCI-IPG release and insulin sensitivity suggests that the DCI-IPG mediator may be a target for therapeutic interventions in PCOS.
Keywords: Polycystic ovary syndrome, Insulin sensitivity, D-chiro-inositol
Introduction
The polycystic ovary syndrome (PCOS) is a prevalent disorder that affects 6–10% of women of childbearing age, and is a major cause of female infertility in the United States. The syndrome is defined by the presence of chronic oligo- or anovulation and hyperandrogenism, with the exclusion of secondary causes of anovulation or androgen excess [1]. Recently, the presence of polycystic ovaries was included as a diagnostic criterion as well, but only in the concurrent presence of either androgen excess or ovulatory dysfunction [2].
Evidence suggests that insulin resistance and its compensatory hyperinsulinemia play an important pathogenic role in PCOS [3]. Insulin resistance is present in both obese and lean women with PCOS [4,5], and administration of insulin sensitizers, such as metformin and the thiazolidinediones, ameliorates hyperandrogenemia and increases ovulation frequency [6]. Women with PCOS are at increased risk of developing dyslipidemia, hypertension, impaired glucose tolerance, type 2 diabetes, and cardiovascular disease [7,8], and insulin resistance likely contributes to these risks.
Some actions of insulin may involve low molecular weight inositolphosphoglycan (IPG) mediators (also known as putative insulin mediators or second messengers) [9–11]. Several lines of evidence suggest that a deficiency in the putative IPG mediator containing D-chiro-inositol (DCI-IPG) [12–17], as well as myo-inositol (MYO) [18,19], may contribute to insulin resistance. DCI-IPG release, measured by a bioactivity assay, increased during an oral glucose challenge in normal individuals, but failed to change in type 2 diabetic subjects [12]. Significantly decreased muscle DCI-IPG bioactivity and decreased total DCI content have been noted in needle biopsies [13] and autopsy specimens [14] from type 2 diabetic subjects compared with controls.
Further supportive evidence of the association between insulin sensitivity and DCI-IPG release can be gathered from clinical trials comparing oral DCI or MYO supplementation (which can be converted to DCI intracellularly) vs. placebo in women with PCOS [16–19]. We have previously reported that DCI administration led to a reduction in serum testosterone levels, and an improvement in ovulation and metabolic parameters such as blood pressure and triglycerides in women with PCOS [16]. These findings have since been supported independently by Gerli et al. [17] who conducted a randomized, double-blind placebo-controlled trial of 283 women with PCOS. Frequency of ovulation was increased by almost 2-fold in women who received DCI, and serum HDL cholesterol increased, effects consistent with improved insulin sensitivity. Similar findings were found after oral administration of MYO, a precursor of DCI in vivo [18,19]. However, neither insulin sensitivity nor release of bioactive DCI-IPG was determined in any of these studies of women with PCOS. While the above data suggest that decreased DCI concentrations, and/or bioactive DCI-IPG release, may contribute to insulin resistance, the association between an increase in DCI-IPG release and improvement in insulin sensitivity has not been directly assessed.
To directly assess the effect of administration of DCI on insulin sensitivity in PCOS, we conducted a randomized controlled trial in which women with PCOS took oral DCI or placebo for 6 weeks. Insulin sensitivity was determined by the frequently sampled intravenous glucose tolerance test (FSIVGTT) and minimal model of Bergman at baseline and end-of-study. Unfortunately, the study was terminated prematurely because of a sudden unavailability of the study medication. Nonetheless, as reported in the present paper, inspection of the results yielded several novel observations. First, no improvement in insulin sensitivity was noted in the women who had received DCI. Second, DCI-IPG release did not improve in several women in the DCI group, suggesting that these women suffered from a functional defect in DCI-IPG release rather than from a simple nutritional deficiency of DCI. Thirdly, regardless of group assignment, women in whom DCI-IPG release improved also seemed to demonstrate an improvement in insulin sensitivity.
Based on these findings, the data from both the DCI and placebo groups were combined to test the new hypothesis that an increase in the release of bioactive DCI-IPG is associated with an improvement in insulin sensitivity in women with PCOS.
Patients and Methods
Subjects
We assessed the relationship between DCI-IPG release during an OGTT and insulin sensitivity in 11 women with PCOS before and after 6 weeks of administration of DCI 1200 mg (n=6) or placebo (n=5) twice daily. The women were 18–40 years old and had PCOS, as defined by chronic oligomenorrhea (eight or fewer menstrual periods annually) and hyperandrogenemia (elevated serum free testosterone concentration). Hyperprolactinemia, thyroid dysfunction, and late-onset adrenal hyperplasia were excluded by the appropriate tests [1]. None of the women had diabetes, or took oral contraceptives or any medication known to affect insulin sensitivity within 3 months prior to study enrollment. Women were studied at the General Clinical Research Center at Virginia Commonwealth University. The study was approved by the Virginia Commonwealth University institutional review board, and written informed consent was obtained from all subjects prior to study. This study was registered with clinicaltrials.gov (NCT00497653).
Study Protocol
All women were instructed to not change their usual diet for at least 3 days before the study and throughout the duration of the study, since DCI may be ingested as part of a diet rich in fruits or legumes. At baseline, all women had a serum progesterone of ≤ 2 ng/mL, documenting that they were in the equivalent of the follicular phase of the menstrual cycle.
On the first day of study, after a 12-h fast overnight, fasting blood samples were collected. A 2-h OGTT with 75g dextrose was performed; blood samples were collected every 15 minutes for determination of serum insulin and glucose concentrations, and every 30 minutes for determination of DCI-IPG activity.
On the second day, again after a 12-h fast, insulin sensitivity was determined by FSIVGTT as described by Bergman and colleagues [20,21]. At zero time, 300 mg/kg dextrose was administered as an i.v. bolus dose over 1 min, and an i.v. bolus insulin at a dose of 0.03 units/kg was administered 20 min later. Blood samples were obtained for serum determinations of glucose and insulin at 0, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 25, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 140, 160, and 180 min, as specified for the modified minimal model method by Bergman and colleagues [20,21]. Analysis of these data was performed using the Minimal Model Identification Software (MINIMOD, version 6.02)[22], which yields quantitative measurements of tissue insulin sensitivity (Si), glucose effectiveness, acute insulin response to glucose (AIRg), and disposition index (DI).
After the baseline studies, women were randomized to DCI capsules 1200 mg (Insmed Pharmaceuticals, Richmond, VA) or matched placebo capsules twice daily for 6 weeks. The women were instructed to not change their eating habits, activity levels or lifestyle during the study, but neither dietary nor exercise logs were maintained. At the end of 6 weeks, all measurements and testing performed at baseline were repeated.
The study was terminated early due to a discontinuation of the study drug supply by the drug manufacturer.
Laboratory assays
Blood samples were centrifuged immediately, and sera were stored at −70°C until assayed. All hormones were assayed as previously described [23–25]. Serum free testosterone was calculated by the method of Sodergard et al [26] using a serum albumin concentration of 4.3 g/dl. Hormone samples were analyzed in duplicate in a single assay to decrease inter-assay variability. The intra-assay coefficient of variation (CV) for the insulin assay was 5.5% and was <10% for all steroid hormone assays.
DCI concentrations
Plasma DCI concentrations were determined by gas chromatography and mass spectrometry as previously described [27]. Briefly, [2H6] racemic chiro-inositol was added to plasma as internal standard. The samples were then purified, derivatized with pentafluoropropionic anhydride, separated on a 25 m 0.25 mm i.d. Chirasil-Val capillary column (Alltech, State College, PA), and analyzed in negative ion chemical ionization mode on an Agilent 5973 mass spectrometer (Agilent Technologies, Palo Alto, CA) with methane as the reagent gas [27].
DCI-IPG insulin mediator bioactivity assay
Blood samples were centrifuged immediately after collection, and sera were stored at −70°C until assayed. The DCI-IPG mediator was extracted from serum as described previously [15]. Currently, it is not possible to measure the content of extracted DCI-IPG because its structure and exact mass are unknown, and no specific antibody suitable for an immunoassay has been developed. However, it has been well established that the DCI-IPG mediator fraction has pyruvate dehydrogenase phosphatase stimulating activity. Therefore, DCI-IPG mediator bioactivity was determined using the specific activation of pyruvate dehydrogenase phosphatase. This method has been validated in women with PCOS and was previously described in detail [15]. The interassay CV of this bioassay was 17.4% and the intra-assay CV was 6.7%. For the entire assay method, including variability during the extraction procedure, the intraassay CVs were 10.7% and 8.5%, respectively, for the absolute values of basal and peak DCI-IPG bioactivity.
To adjust for variation in basal pyruvate dehydrogenase activity from one assay to the other, and therefore from subject to subject, the water-blank activity was subtracted from the bioactivity of DCI-IPG released into serum during OGTT, which was then expressed as the percentage of its bioactivity compared to baseline (0 min).
Statistical Analysis
We analyzed the response of serum insulin concentrations and the relative bioactivity of DCI-IPG to the oral administration of glucose by calculating the areas under the respective response curves (AUCs) by the trapezoidal rule, yielding values for AUCinsulin and AUCDCI-IPG, respectively. Since insulin is thought to mediate the release of DCI-IPG after a glucose load [12] and AUCinsulin differs from one subject to the other, the ratio of AUCDCI-IPG / AUCinsulin more accurately reflects insulin-mediated release of DCI-IPG than AUCDCI-IPG alone. Hence, we used this ratio in our analyses.
Results that were not normally distributed were log-transformed for statistical analyses, and, after back-transformation, were reported in their original units as geometric means, with 95% CIs. Baseline characteristics are presented for all women in the study as a whole. Our primary objective was to determine the association between changes in the release of the bioactive DCI-IPG (ratio of AUCDCI-IPG / AUCinsulin) and changes in insulin sensitivity (Si) during the 6-week study period in all women. Before evaluating this relationship, we assessed differences in these changes between treatment groups to ascertain the appropriateness of our analyses in all women (without stratification by treatment groups). To assess the treatment effects between groups, the changes in each variable (after-treatment minus baseline) were compared using a Student two-tailed t test.
To determine the association between changes in the release of the bioactive DCI-IPG (ratio of AUCDCI-IPG / AUCinsulin) and changes in insulin sensitivity (Si) during the 6-week study period, the changes in each variable during the 6 weeks were calculated as after-treatment minus baseline. We used simple linear regression to test the relationship between changes in the ratio of AUCDCI-IPG / AUCinsulin and changes in Si, after linearity and normality of residuals were assessed. We further performed multiple linear regressions to control for potential confounders. These potential confounders were baseline BMI, changes in BMI during the study and DCI treatment assignment. Interactions among the ratio of AUCDCI-IPG / AUCinsulin and these confounders were assessed. Results on other FSIVGTT indices were also reported for completeness. Although AUCinsulin is also important parameter to assess in addition to Si, we did not evaluate the relationship between AUCinsulin and the ratio of AUCDCI-IPG / AUCinsulin because they were not independent variables. All analyses were performed using JMP 7.0 software (SAS Institute, Cary, NC).
Results
Effects of DCI administration on DCI-IPG release and insulin sensitivity
Six women with PCOS were randomized to oral DCI and five to placebo. After treatment, plasma DCI concentrations increased by 23.5 (95% CI 9.4-37.2) µmol/L in the DCI group, and remained essentially unchanged in the placebo group (p=0.04). However, despite the rise in plasma DCI concentration, DCI administration compared to placebo did not change AUCDCI-IPG (+4325.4 vs. −760.6 % • min, p=0.34), AUCDCI-IPG / AUCinsulin ratio (−0.14 vs. +0.62, p=0.42), AUCglucose (+909.3 vs. −642.6 mg•min•dL−1, p=0.31), Si (−0.48 vs. +5.64, p=0.82), AIRg (−574.4 vs. −274.0 mu • L−1 • min, p=0.41) and the disposition index (+326.7 vs. +2655.8, p=0.61). BMI (+0.7 vs. +0.4 kg/m2, p=0.62), fasting insulin concentrations (+8.8 vs. +1.3 µIU/mL, p=0.18), glucose concentrations (+6.5 vs. +1.9 mg/dL, p=0.29), total testosterone (−5.8 vs. +9.2 mg/dL, p=0.23) and free testosterone (−0.23 vs. −0.07 ng/dL, p=0.17) concentrations also did not differ with treatment in the DCI compared to the placebo group.
Relationship between change in DCI-IPG release and change in insulin sensitivity
Baseline characteristics are presented for all women in the study as a whole (Table 1) because our objective was to determine the association between changes in the release of the bioactive DCI-IPG (ratio of AUCDCI-IPG / AUCinsulin) and changes in insulin sensitivity (Si) during the 6-week study period in all women. In addition, we did not observe any difference in changes of all parameters between DCI and placebo administration, except for the expected increase in plasma DCI levels in the DCI group. We report the baseline and after-treatment characteristics for AUCDCI-IPG, ratio of AUCDCI-IPG / AUCinsulin, BMI, and indices from FSIVGTT (Si, AIRg, DI) for all women in Table 1. In all subjects, without regard to treatment assignment, the mean change in AUCDCI-IPG during the treatment period was +2013.6 %•min (95% CI −3868.5 to +7895.7 %•min). Expressed per unit of insulin released, the change in the AUCDCI-IPG / AUCinsulin ratio during the 6 weeks was +0.21 (95% CI −1.68 to +2.09). The change in Si during the same period was +2.30 (95% CI −0.40 to 8.64).
Table 1.
Baseline clinical and biochemical characteristics of women with PCOS (N=11)
| Baseline | 6 months | |
|---|---|---|
| Age (yrs) | 30.6 [26.7 to 34.6] | -- |
| BMI (kg/m2) | 36.1 [29.4 to 42.7] | 36.6 [29.8 to 43.4] |
| Waist circumference (cm) | 100.1 [86.9 to 113.3] | 100.1 [88.3 to 111.9] |
| W/H Ratio | 0.81 [0.76 to 0.85] | 0.81 [0.76 to 0.85] |
| Fasting insulin (µIU/ml) | 8.9 [2.6 to 15.2] | 7.6 [3.7 to 15.8]‡ |
| Fasting glucose (mg/dL) | 81.0 [75.6 to 86.5] | 85.5 [77.4 to 93.6] |
| Total testosterone (mg/dL) | 70.2 [45.8 to 107.7]‡ | 80.5 [39.5 to 121.6] |
| Free testosterone, calculated (ng/dL) | 0.79 [0.49 to 1.29]‡ | 1.02 [0.40 to 1.64] |
| AUCinsulin (µIU•min•ml−1) | 6448 [3745 to 11102]‡ | 6529 [3369 to 12656]‡ |
| AUCGlucose mg•min•dL−1) | 14806.5 [13326.7 to 16286.4] | 15010.5 [12968.4 to 17052.5] |
| Plasma DCI (nmol/L) | 83.0 [48.4 to 142.2] ‡ | 1022.0 [122.0 to 8559]‡ |
| AUCDCI-IPG (%•min) | 13417 [11009 to 16353]‡ | 14738 [10972 to 19795] ‡ |
| AUCDCI-IPG/AUCinsulin | 2.08 [1.10 to 3.92]‡ | 3.22 [1.52 to 4.92] |
| Si (min−1/mu/L) | 2.42 [0.95 to 6.17]‡ | 3.28 [1.40 to 8.15]‡ |
| AIRg (mu •L−1 •min) | 378.3 [168.1 to 851.4]‡ | 460.7 [222.5 to 699.0] |
| Disposition index (AIRg •Si) | 1153.5 [447.6 to 1859.6 | 1204.9 [477.9 to 3037.6]‡ |
Data are means [95% CI], or geometric means [95% CI] when indicated by ‡
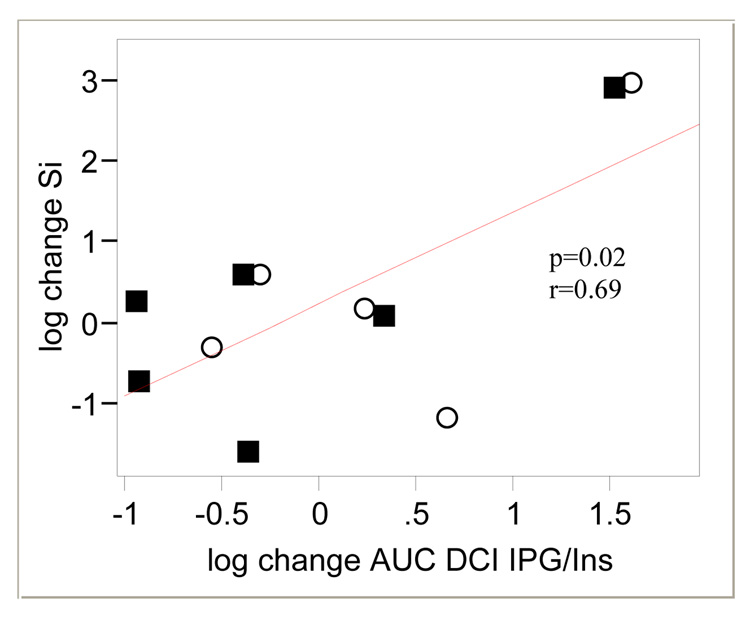
In all subjects, there was a positive correlation between the change in AUCDCI-IPG and the change in Si during the 6 weeks of treatment (r=0.57, p=0.06) which barely missed statistical significance. Since insulin is thought to mediate the release of DCI-IPG after a glucose load in diabetic subjects [12], the ratio of AUCDCI-IPG / AUCinsulin probably more accurately reflects insulin-mediated release of DCI-IPG than AUCDCI-IPG alone. The relationship between AUCDCI-IPG and Si is further strengthened when AUCDCI-IPG is standardized to the amount of insulin released during OGTT, resulting in a significant correlation between the change in AUCDCI-IPG / AUCinsulin ratio and the change in Si during the 6 weeks of treatment (r=0.69, p=0.02) (Figure 1).
Figure 1.
Relationship between change (baseline to 6 weeks) in Si and change in release of the bioactive DCI-IPG messenger standardized per unit of insulin release during OGTT during 6 weeks of study period
■ DCI
○ Placebo
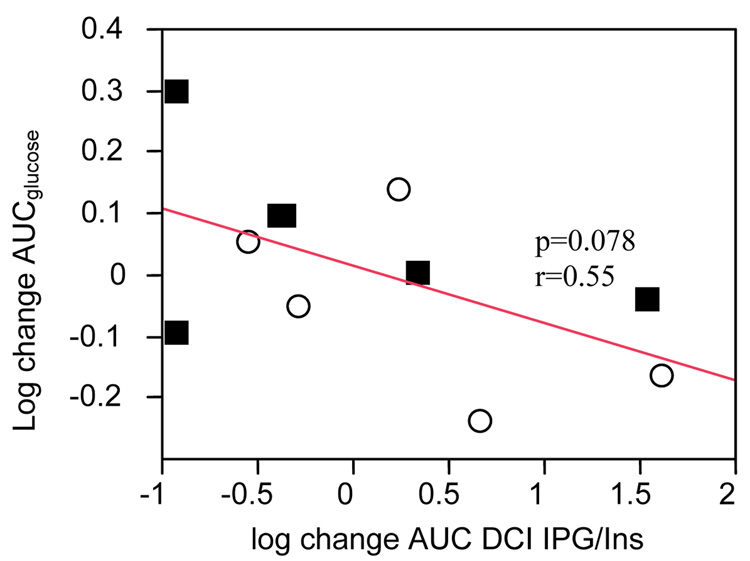
There was no significant relationship between change in the ratio of AUCDCI-IPG / AUCinsulin and change in AIRg. However there was a significant relationship between change in disposition index (AIRg * Si) and change in the AUCDCI-IPG / AUCinsulin ratio (r=0.65, p=0.04). Corollary to the significant relationship between change in AUCDCI-IPG / AUCinsulin ratio and the change in Si,, change in AUCDCI-IPG / AUCinsulin ratio was inversely related to change in AUCglucose during OGTT which barely missed statistical significance (r=0.55, p=0.078) (Figure 2).
Figure 2.
Relationship between change (baseline to 6 weeks) in AUCglucose and change in release of the bioactive DCI-IPG messenger standardized per unit of insulin release during OGTT during 6 weeks of study period
■ DCI
○ Placebo
Association between insulin sensitivity measures and release of bioactive DCI-IPG after adjustment for possible confounders
Because the women in this study presented with a range of BMI at baseline, we evaluated whether baseline BMI influenced the relationship between change in release of bioactive DCI-IPG and change in insulin sensitivity during the study. The significance of the relationship between the change in AUCDCI-IPG / AUCinsulin ratio and the change in Si persisted even after adjustment for baseline BMI (p=0.03).
In addition, treatment assignment and changes in BMI during the study may affect insulin sensitivity independent of the release of bioactive DCI-IPG. We determined whether the relationship between change in Si and the change in AUCDCI-IPG / AUCinsulin ratio would remain significant after adjusting for treatment assignment and change in BMI.
Adjusted for change in BMI during the study, the relationship between change in Si and the change in AUCDCI-IPG / AUCinsulin ratio remained significant (p=0.022). Importantly, change in BMI during the study did not correlate with change in Si independent of the change in AUCDCI-IPG / AUCinsulin ratio (p=0.366). When we further adjusted for treatment allocation in addition to change in BMI, the relationship between change in Si and the change in AUCDCI-IPG / AUCinsulin ratio remained significant (p=0.0261). Notably, in this multivariate model, change in BMI (p=0.320) and treatment assignment (p=0.503) did not predict changes in Si, independent of the change in AUCDCI-IPG / AUCinsulin ratio. There were no significant interactions between independent variables.
Among FSIVGTT indices other than Si, only change in the disposition index (AIRg*Si) during the study was significantly correlated with change in the AUCDCI-IPG / AUCinsulin ratio. We further investigated whether this relationship remains significant in multivariate analyses. After adjustment for change in BMI during the study, the relationship between change in the AUCDCI-IPG / AUCinsulin ratio and change in the disposition index remained statistically significant (p=0.043). After further adjustment for treatment assignment, the relationship barely missed statistical significance (p=0.066).
Discussion
The aim of this study was to test the hypothesis that administration of DCI to women with PCOS would increase insulin sensitivity, and that the increase in insulin sensitivity would be associated with an increase in insulin-stimulated release of the putative DCI-IPG mediator. We conducted a randomized controlled trial in which women with PCOS were assessed at two time-points, at baseline and after 6 weeks of administration of DCI or placebo. Unfortunately, the study was discontinued prematurely because of sudden unavailability of study drug. Nonetheless, in the limited number of women who were studied (6 in the DCI group and 5 in the placebo group), we observed no improvement in insulin sensitivity in the women who had received DCI.
Further inspection of the results yielded a surprising finding. In a number of women who had received DCI, there was no change in DCI-IPG release, suggesting that impaired DCI-IPG release in these women may have represented a functional defect (e.g., intracellular defect in formation or release of the DCI-IPG mediator) rather than a simple nutritional deficiency in the substrate DCI. This idea is supported by the observation that administration of metformin alone increases the insulin-stimulated release of DCI-IPG in women with PCOS [15]. Conversely, for reasons unclear to us but perhaps related to changes in lifestyle (increased activity, altered diet, etc.), DCI-IPG release improved in some women who had received placebo. We also noted that in those women in whom insulin-stimulated DCI-IPG release increased, regardless of group assignment, insulin sensitivity had improved as well. These observations led us to combine the data from both the DCI and placebo groups to test the hypothesis that there would be a positive correlation between change in DCI-IPG release and change in insulin sensitivity.
Indeed, there was a significant positive relationship between release of DCI-IPG mediator and insulin sensitivity. Specifically, an increase in DCI-IPG mediator release during OGTTs, per unit of released insulin (AUCDCI-IPG/AUCinsulin ratio), was significantly and positively correlated with an improvement in Si. A corollary inverse relationship between release of the DCI-IPG mediator (AUCDCI-IPG/AUCinsulin ratio) and AUCglucose during OGTT was also observed.
Furthermore, this relationship between DCI-IPG release and improvement in insulin sensitivity was independent of baseline obesity, changes in BMI during the study, and treatment assignment. This correlation between the change in AUCDCI-IPG/AUCinsulin ratio and change in Si was not merely driven by AUCinsulin (which is expected to be correlated with Si), since there was a borderline significant association between change in Si and change in AUCDCI-IPG (without standardization to AUCinsulin). The significant correlation between increase of AUCDCI-IPG/AUCinsulin ratio and an improvement in Si supports the idea that a relationship exists between the release of the putative DCI-IPG mediator and insulin sensitivity in PCOS.
The findings of this study are congruent with previous reports of the role of DCI-IPG in insulin sensitivity in PCOS. In women with and without PCOS studied at a single time point, we previously documented diminished insulin-stimulated release of the putative DCI-IPG mediator in women with PCOS compared to normal women
Although we observed a significant relationship between release of the DCI-IPG mediator and insulin sensitivity, changes in these parameters over the 6-week study period was not different between the placebo and DCI groups. This contrasts with our previous study with Venezuelan women with PCOS in whom DCI supplementation improved AUCinsulin, ovulation, testosterone levels, lipids and blood pressure, all suggesting an improvement in insulin sensitivity with DCI treatment [16]. In addition, in a randomized, double-blind and placebo-controlled trial of 283 Italian women with PCOS, frequency of ovulation was increased by almost 2-fold in women who received DCI, and serum HDL cholesterol also increased, effects again consistent with improved insulin sensitivity [17]. Although availability of bioactive DCI-IPG release and insulin sensitivity were not directly measured, the findings of these studies suggested that improved availability of DCI through oral supplementation may improve insulin sensitivity, at least in non-American women.
A possible explanation for the disparate findings between our current study and previous reports is that impaired release of the DCI-IPG mediator in studies evaluating non-American women may have been related to a nutritional deficiency of the substrate of DCI-IPG, namely, DCI, which was easily corrected by the administration of DCI. Conversely, impaired release of the DCI-IPG mediator in the women in the present study who did not respond to administration of DCI may have been related to a functional defect of DCI release rather than a nutritional deficiency. One can envision a multitude of abnormalities that would all present as impaired release of DCI-IPG, such as deficient availability of substrate (due either to dietary deficiency or a functional derangement of cellular uptake of DCI), defective intracellular processing of DCI into the DCI-IPG mediator, and defective coupling of DCI-IPG release to insulin release). It would be reasonable to assume that all manners of impaired DCI-IPG release might be represented in the PCOS population-at-large, accounting for why some women with PCOS responded to administration of DCI with improved DCI-IPG release whereas others did not. Depending on the availability of DCI in the diet in a specific country, the prevalence of impaired DCI-IPG release due to dietary DCI deficiency would also be expected to vary widely.
Furthermore, evidence for a functional defect in DCI-IPG release in many women with PCOS in the U.S. is the fact that administration of metformin alone, without DCI supplementation, improves both DCI-IPG release and insulin sensitivity in women with PCOS [15].
Hence, it is possible that multiple defects in DCI metabolism may result in the identical end result. That is, deficient insulin-stimulated release of DCI-IPG may be related to a true deficiency in DCI (as suggested by the studies in Venezuela and Italy) or to an intracellular defect in either processing of DCI to DCI-IPG or in secretion of DCI-IPG. The former defect could be remedied by administration of DCI, whereas the latter would not. Since the liberation of DCI-IPG from the outer cell membrane is mediated by a specific phospholipase C that is activated by insulin [11], interventions that improve insulin action, such as metformin, might increase DCI-IPG secretion via improved insulin-mediated activation of this phospholipase C.
Because the defect in DCI metabolism may be at the tissue level in American women, future studies should also evaluate tissue DCI-IPG availability. Significantly decreased muscle DCI-IPG bioactivity and decreased total DCI content have been noted in needle biopsies [13] and autopsy specimens [14] from type 2 diabetic subjects compared with controls. In addition, DCI-IPG (or P-type IPG) has been shown to be decreased in human term placentas in preeclamptic individuals, who also concomitantly exhibit decreased insulin signaling, when compared to healthy women [28].
In this current study, it is unknown why release of DCI-IPG (and concurrently insulin sensitivity) increased in some women taking placebo; possibilities include undetected changes in diet, exercise or other aspects of lifestyle. Nonetheless, it is noteworthy that when DCI-IPG release increased, insulin sensitivity improved as well. This finding is consonant with the apparent close relationship between DCI-IPG release and insulin sensitivity.
Alternatively, while evidence suggests that DCI-IPG increases insulin sensitivity, it is possible that insulin resistance and/or insulin itself modulates DCI or DCI-IPG metabolism or induces a defect in DCI-IPG release in PCOS. That is, insulin resistance and/or hyperinsulinemia may mediate a defect that leads to deficient release of DCI-IPG, which in turn worsens insulin resistance, resulting in a vicious cycle. Future studies should elucidate the etiologies of impaired DCI-IPG release in women with PCOS.
Our study has its limitations. We were able to study only 11 women because of sudden unavailability of the study medication. Also, the relationship between the release of DCI-IPG per unit of insulin and insulin sensitivity may be an epiphenomenon. However, this is unlikely given the previous evidence in normal and type 2 diabetes subjects, suggesting that a deficiency in the putative DCI-IPG mediator may result in insulin resistance. Additionally, we did not have a control group of women without PCOS in this study. Although the study would be strengthened with a normal cohort, we were primarily interested in changes in the release of DCI-IPG mediator in women with PCOS and its relationship with insulin sensitivity. Furthermore, it would be elucidating to evaluate the relationship between DCI-IPG release and insulin sensitivity separately in obese and lean women with glucose, which our current study was not designed to address. Lastly, given our findings, it may be useful in future studies to evaluate tissue availability of DCI-IPG as the defect in DCI metabolism in American women with PCOS may be evident at the tissue level in terms of DCI uptake or release, and not dietary in nature.
In conclusion, our findings indicate that an increase in the release of DCI-IPG is significantly and positively correlated with an improvement in insulin sensitivity in women with PCOS. Regardless of whether DCI or placebo is administered, if insulin-stimulated release of DCI-IPG is increased, insulin sensitivity increases as well, and vice versa. These findings, combined with those of previous studies, suggest that release of the DCI-IPG mediator may play an important role in insulin sensitivity in women with PCOS.
Acknowledgments
Supported in part by:
National Institutes of Health Grants R01HD35629 (to J.E.N.), K24HD40237 (to J.E.N.), K23HD049454 (to K.I.C) and R01DK58698 (to R.E.O.), the Fond de Recherche en Santé du Québec #2834 (to J.P.B.), and National Institutes of Health Clinical Research Center Grant M01-RR00065, NCRR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement:
K.I.C., P.A.E., T.A., L.I. and R.E.O. have nothing to declare. J.P.B. received lecture fees from GSK and Abbott. J.E.N. is on the speaker bureau for Sanofi-Aventis, Novartis and Pfizer.
Reference List
- 1.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic ovary syndrome. Boston: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Nestler JE. Role of hyperinsulinemia in the pathogenesis of the polycystic ovary syndrome, and its clinical implications. Semin Reprod Endocrinol. 1997;15:111–122. doi: 10.1055/s-2007-1016294. [DOI] [PubMed] [Google Scholar]
- 4.Dunaif A, Segal KR, Futterweit W, et al. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 5.Nestler JE, Jakubowicz DJ. Lean women with polycystic ovary syndrome respond to insulin reduction with decreases in ovarian P450c17 alpha activity and serum androgens. J Clin Endocrinol Metab. 1997;82:4075–4079. doi: 10.1210/jcem.82.12.4431. [DOI] [PubMed] [Google Scholar]
- 6.Cheang KI, Sharma ST, Nestler JE. Is metformin a primary ovulatory agent in patients with polycystic ovary syndrome? Gynecol Endocrinol. 2006;22:595–604. doi: 10.1080/09513590601005847. [DOI] [PubMed] [Google Scholar]
- 7.Apridonidze T, Essah PA, Iuorno MJ, et al. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929–1935. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 8.Sharma ST, Nestler JE. Prevention of diabetes and cardiovascular disease in women with PCOS: treatment with insulin sensitizers. Best Pract Res Clin Endocrinol Metab. 2006;20:245–260. doi: 10.1016/j.beem.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Larner J. Mediators of postreceptor action of insulin. Am J Med. 1983;74:38–51. doi: 10.1016/0002-9343(83)90653-8. [DOI] [PubMed] [Google Scholar]
- 10.Larner J. Insulin-signaling mechanisms. Lessons from the old testament of glycogen metabolism and the new testament of molecular biology. Diabetes. 1988;37:262–275. doi: 10.2337/diab.37.3.262. [DOI] [PubMed] [Google Scholar]
- 11.Saltiel AR. Second messengers of insulin action. Diabetes Care. 1990;13:244–256. doi: 10.2337/diacare.13.3.244. [DOI] [PubMed] [Google Scholar]
- 12.Shashkin PN, Shashkina EF, Fernqvist-Forbes E, et al. Insulin mediators in man: effects of glucose ingestion and insulin resistance. Diabetologia. 1997;40:557–563. doi: 10.1007/s001250050715. [DOI] [PubMed] [Google Scholar]
- 13.Kennington AS, Hill CR, Craig J, et al. Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. N Engl J Med. 1990;323:373–378. doi: 10.1056/NEJM199008093230603. [DOI] [PubMed] [Google Scholar]
- 14.Asplin I, Galasko G, Larner J. Chiro-inositol deficiency and insulin resistance: a comparison of the chiro-inositol- and the myo-inositol-containing insulin mediators isolated from urine, hemodialysate, and muscle of control and type II diabetic subjects. Proc Natl Acad Sci U S A. 1993;90:5924–5928. doi: 10.1073/pnas.90.13.5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baillargeon JP, Iuorno MJ, Jakubowicz DJ, et al. Metformin therapy increases insulin-stimulated release of D-chiro-inositol-containing inositolphosphoglycan mediator in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:242–249. doi: 10.1210/jc.2003-030437. [DOI] [PubMed] [Google Scholar]
- 16.Nestler JE, Jakubowicz DJ, Reamer P, et al. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N Engl J Med. 1999;340:1314–1320. doi: 10.1056/NEJM199904293401703. [DOI] [PubMed] [Google Scholar]
- 17.Gerli S, Mignosa M, Di Renzo GC. Effects of inositol on ovarian function and metabolic factors in women with PCOS: a randomized double blind placebo-controlled trial. Eur Rev Med Pharmacol Sci. 2003;7:151–159. [PubMed] [Google Scholar]
- 18.Gerli S, Papaleo E, Ferrari A, et al. Randomized, double blind placebo-controlled trial: effects of myo-inositol on ovarian function and metabolic factors in women with PCOS. Eur Rev Med Pharmacol Sci. 2007;11:347–354. [PubMed] [Google Scholar]
- 19.Papaleo E, Unfer V, Baillargeon JP, et al. Myo-inositol in patients with polycystic ovary syndrome: a novel method for ovulation induction. Gynecol Endocrinol. 2007;23:700–703. doi: 10.1080/09513590701672405. [DOI] [PubMed] [Google Scholar]
- 20.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang YJ, Youn JH, Bergman RN. Modified protocols improve insulin sensitivity estimation using the minimal model. Am J Physiol. 1987;253:E595–E602. doi: 10.1152/ajpendo.1987.253.6.E595. [DOI] [PubMed] [Google Scholar]
- 22.Bergman RN, Beard JC, Chen M. The minimal modeling method. Asessment of insulin sensitivity and B-cell function in vivo. Meth Diabetes Res. 1986;2:15–34. [Google Scholar]
- 23.Nestler JE, Usiskin KS, Barlascini CO, et al. Suppression of serum dehydroepiandrosterone sulfate levels by insulin: an evaluation of possible mechanisms. J Clin Endocrinol Metab. 1989;69:1040–1046. doi: 10.1210/jcem-69-5-1040. [DOI] [PubMed] [Google Scholar]
- 24.Nestler JE, Powers LP, Matt DW, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83–89. doi: 10.1210/jcem-72-1-83. [DOI] [PubMed] [Google Scholar]
- 25.Nestler JE, Beer NA, Jakubowicz DJ, et al. Effects of a reduction in circulating insulin by metformin on serum dehydroepiandrosterone sulfate in nondiabetic men. J Clin Endocrinol Metab. 1994;78:549–554. doi: 10.1210/jcem.78.3.8126125. [DOI] [PubMed] [Google Scholar]
- 26.Sodergard R, Backstrom T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 27.Ostlund RE, Jr, McGill JB, Herskowitz I, et al. D-chiro-inositol metabolism in diabetes mellitus. Proc Natl Acad Sci U S A. 1993;90:9988–9992. doi: 10.1073/pnas.90.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scioscia M, Gumaa K, Kunjara S, et al. Insulin resistance in human preeclamptic placenta is mediated by serine phosphorylation of insulin receptor substrate-1 and -2. J Clin Endocrinol Metab. 2006;91:709–717. doi: 10.1210/jc.2005-1965. [DOI] [PubMed] [Google Scholar]