Abstract
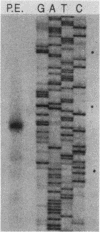
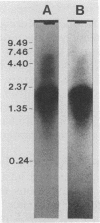
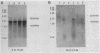
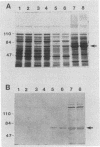
Chancroid is a sexually transmitted genital ulcer disease caused by Haemophilus ducreyi. Previously, we developed diagnostic DNA probes for H. ducreyi (L. M. Parsons, M. Shayegani, A. L. Waring, and L. H. Bopp, J. Clin. Microbiol. 27:1441-1445, 1989). In the present study, DNA sequencing of one of the diagnostic probes revealed two adjacent open reading frames (ORFs). These H. ducreyi ORFs and the encoded proteins show significant homology with the groE genes and GroES and GroEL heat shock proteins from several bacterial pathogens and with conserved eukaryotic 60-kDa heat shock proteins. The first H. ducreyi ORF (groES) is preceded by sequences similar to those of the Escherichia coli consensus heat shock promoters and is 288 nucleotides long and is capable of encoding a protein of 10.3 kDa. The second ORF (groEL) is 1,641 nucleotides long and is capable of encoding a protein of 57.8 kDa. Northern (RNA blot) analysis demonstrated the presence of a high level of groE mRNA in exponential-phase H. ducreyi grown in hemin broth at the organism's optimal growth temperature (33 degrees C), with increased levels seen following heat shock. Heat shock also increased the thermostability of the organisms, since stressed cells were more resistant to the lethal effects of rapid chilling. Electrophoretic analysis and immunoblots demonstrated that the predominant protein produced by exponential-phase H. ducreyi was a heat-inducible, immunoreactive protein of approximately 60 kDa (GroEL). Also, H. ducreyi groE mRNA and GroEL were expressed and inducible by heat in E. coli. This is the first report describing the cloning, sequencing, and expression of H. ducreyi protein-encoding genes.
Full text
PDF



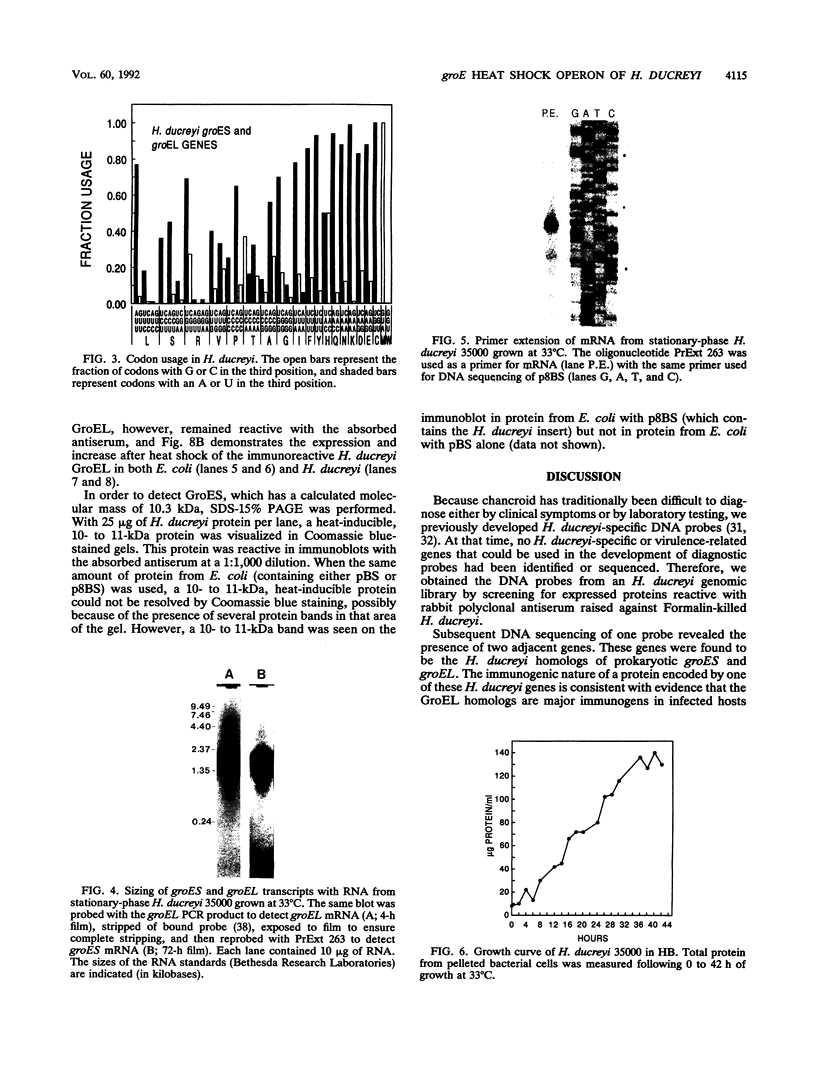
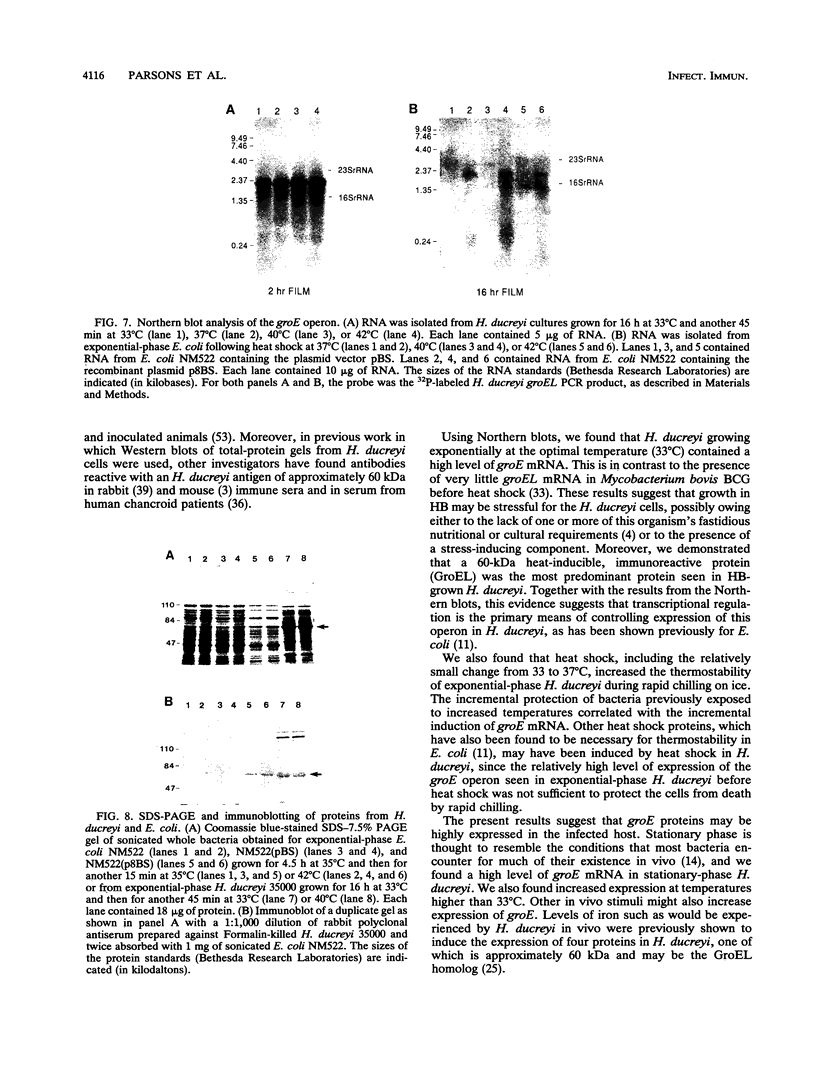


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeck D., Johnson A. P., Hartinger A., Kollmann M., Korting H. C., Ballard R. C., Braun-Falco O. Absence of extracellular enzyme activity and cytotoxicity in cell-free culture filtrates of Haemophilus ducreyi. Microb Pathog. 1991 May;10(5):405–410. doi: 10.1016/0882-4010(91)90085-o. [DOI] [PubMed] [Google Scholar]
- Abeck D., Johnson A. P., Taylor-Robinson D. Antigenic analysis of Haemophilus ducreyi by Western blotting. Epidemiol Infect. 1988 Aug;101(1):151–157. doi: 10.1017/s0950268800029319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton W. L. Biology of Haemophilus ducreyi. Microbiol Rev. 1989 Dec;53(4):377–389. doi: 10.1128/mr.53.4.377-389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham M. E., Carney S., Butler R., Colston M. J. A mycobacterial 65-kD heat shock protein induces antigen-specific suppression of adjuvant arthritis, but is not itself arthritogenic. J Exp Med. 1990 Jan 1;171(1):339–344. doi: 10.1084/jem.171.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Campagnari A. A., Wild L. M., Griffiths G. E., Karalus R. J., Wirth M. A., Spinola S. M. Role of lipooligosaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect Immun. 1991 Aug;59(8):2601–2608. doi: 10.1128/iai.59.8.2601-2608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsdorf J. R., Marrs C. F., McCrea K. W., Forney L. J. Cloning, expression, and sequence analysis of the Haemophilus influenzae type b strain M43p+ pilin gene. Infect Immun. 1990 Apr;58(4):1065–1072. doi: 10.1128/iai.58.4.1065-1072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Lian C. J., Wilt J. C., Ronald A. R. Comparison of specimen collection and laboratory techniques for isolation of Haemophilus ducreyi. J Clin Microbiol. 1978 Jan;7(1):39–43. doi: 10.1128/jcm.7.1.39-43.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R., Klein W., Lange R., Rimmele M., Boos W. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J Bacteriol. 1991 Dec;173(24):7918–7924. doi: 10.1128/jb.173.24.7918-7924.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. S., Houston L., Butler C. A. Legionella pneumophila htpAB heat shock operon: nucleotide sequence and expression of the 60-kilodalton antigen in L. pneumophila-infected HeLa cells. Infect Immun. 1990 Oct;58(10):3380–3387. doi: 10.1128/iai.58.10.3380-3387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. E., Schultz J. E., Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessamine P. G., Plummer F. A., Ndinya Achola J. O., Wainberg M. A., Wamola I., D'Costa L. J., Cameron D. W., Simonsen J. N., Plourde P., Ronald A. R. Human immunodeficiency virus, genital ulcers and the male foreskin: synergism in HIV-1 transmission. Scand J Infect Dis Suppl. 1990;69:181–186. [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Wand-Württenberger A., DeBruyn J., Munk M. E., Schoel B., Kaufmann S. H. T cells against a bacterial heat shock protein recognize stressed macrophages. Science. 1989 Sep 8;245(4922):1112–1115. doi: 10.1126/science.2788923. [DOI] [PubMed] [Google Scholar]
- Lee B. C. Iron sources for Haemophilus ducreyi. J Med Microbiol. 1991 Jun;34(6):317–322. doi: 10.1099/00222615-34-6-317. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992 Jan;174(1):1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Su H., Lyng K., Yuan Y. The Chlamydia trachomatis hyp operon is homologous to the groE stress response operon of Escherichia coli. Infect Immun. 1990 Aug;58(8):2701–2705. doi: 10.1128/iai.58.8.2701-2705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons L. M., Shayegani M., Waring A. L., Bopp L. H. DNA probes for the identification of Haemophilus ducreyi. J Clin Microbiol. 1989 Jul;27(7):1441–1445. doi: 10.1128/jcm.27.7.1441-1445.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B. K., Banerjee D. K., Butcher P. D. Characterization of the heat shock response in Mycobacterium bovis BCG. J Bacteriol. 1991 Dec;173(24):7982–7987. doi: 10.1128/jb.173.24.7982-7987.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell B. K., Richardson J. A., Radolf J. D., Hansen E. J. A temperature-dependent rabbit model for production of dermal lesions by Haemophilus ducreyi. J Infect Dis. 1991 Aug;164(2):359–367. doi: 10.1093/infdis/164.2.359. [DOI] [PubMed] [Google Scholar]
- Purvén M., Lagergård T. Haemophilus ducreyi, a cytotoxin-producing bacterium. Infect Immun. 1992 Mar;60(3):1156–1162. doi: 10.1128/iai.60.3.1156-1162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggen E. L., De Breucker S., van Dyck E., Piot P. Antigenic diversity in Haemophilus ducreyi as shown by western blot (immunoblot) analysis. Infect Immun. 1992 Feb;60(2):590–595. doi: 10.1128/iai.60.2.590-595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossau R., Duhamel M., Jannes G., Decourt J. L., Van Heuverswyn H. The development of specific rRNA-derived oligonucleotide probes for Haemophilus ducreyi, the causative agent of chancroid. J Gen Microbiol. 1991 Feb;137(2):277–285. doi: 10.1099/00221287-137-2-277. [DOI] [PubMed] [Google Scholar]
- Saunders J. M., Folds J. D. Immunoblot analysis of antigens associated with Haemophilus ducreyi using serum from immunised rabbits. Genitourin Med. 1986 Oct;62(5):321–328. doi: 10.1136/sti.62.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanafelt M. C., Hindersson P., Soderberg C., Mensi N., Turck C. W., Webb D., Yssel H., Peltz G. T cell and antibody reactivity with the Borrelia burgdorferi 60-kDa heat shock protein in Lyme arthritis. J Immunol. 1991 Jun 1;146(11):3985–3992. [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegele D. A., Kolter R. Life after log. J Bacteriol. 1992 Jan;174(2):345–348. doi: 10.1128/jb.174.2.345-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos A., Klocke M., Frosch M. Cloning and sequencing of the genes coding for the 10- and 60-kDa heat shock proteins from Pseudomonas aeruginosa and mapping of a species-specific epitope. Infect Immun. 1991 Sep;59(9):3219–3226. doi: 10.1128/iai.59.9.3219-3226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C. K., Marana D. P., Dasch G. A., Oaks E. V. Molecular cloning and sequence analysis of the Sta58 major antigen gene of Rickettsia tsutsugamushi: sequence homology and antigenic comparison of Sta58 to the 60-kilodalton family of stress proteins. Infect Immun. 1990 May;58(5):1360–1368. doi: 10.1128/iai.58.5.1360-1368.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuffrey M., Alexander F., Ballard R. C., Taylor-Robinson D. Characterization of skin lesions in mice following intradermal inoculation of Haemophilus ducreyi. J Exp Pathol (Oxford) 1990 Apr;71(2):233–244. [PMC free article] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C. A heat shock operon in Coxiella burnetti produces a major antigen homologous to a protein in both mycobacteria and Escherichia coli. J Bacteriol. 1988 Mar;170(3):1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wood D. O. Codon usage in selected AT-rich bacteria. Biochimie. 1988 Aug;70(8):977–986. doi: 10.1016/0300-9084(88)90262-3. [DOI] [PubMed] [Google Scholar]
- Yelton D. B., Limberger R. J., Curci K., Malinosky-Rummell F., Slivienski L., Schouls L. M., van Embden J. D., Charon N. W. Treponema phagedenis encodes and expresses homologs of the Treponema pallidum TmpA and TmpB proteins. Infect Immun. 1991 Oct;59(10):3685–3693. doi: 10.1128/iai.59.10.3685-3693.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B. Stress proteins and the immune response. Antonie Van Leeuwenhoek. 1990 Oct;58(3):203–208. doi: 10.1007/BF00548934. [DOI] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]