Abstract
PURPOSE
Squamous metaplasia occurs in ocular surface diseases like Sjögren’s syndrome (SS). It is a phenotypic change whereby epithelial cells initiate synthesis of squamous cell-specific proteins such as small proline-rich protein 1B (SPRR1B) that result in pathologic keratin formation on the ocular surface. The authors hypothesized that inflammation is a key inducer of pathologic keratinization and that SPRR1B represents an analytical biomarker for the study of the molecular mechanisms.
METHODS
Real-time quantitative RT-PCR and immunohistochemistry were used to examine SPRR1B mRNA and protein in two different mouse models of dry eye and patients with SS. Adoptive transfer of mature lymphocytes from mice lacking the autoimmune regulator (aire) gene was performed to examine the role of inflammation as an inducer of squamous metaplasia. SPRR1B expression in response to several cytokines was examined in vitro, whereas the expression of cytokines IL1β and IFNγ was quantified in ocular tissues of aire-deficient mice and patients with SS.
RESULTS
SPRR1B was increased across the ocular surface of mice with both desiccating stress and autoimmune-mediated, aqueous-deficient dry eye and in patients with SS. Adoptive transfer of CD4+ T cells from aire-deficient mice to immunodeficient recipients caused advanced ocular surface keratinization. IL1α, IL1β, IL6, IFNγ, and TNFα induced SPRR1B expression in vitro and the local expression of IL1β and IFNγ was elevated in ocular tissues of patients with SS and aire-deficient mice.
CONCLUSIONS
SPRR1B is a valid biomarker for the study of the molecular mechanisms of squamous metaplasia. There is a definitive link between inflammation and squamous metaplasia in autoimmune-mediated dry eye disease, with IL1β and IFNγ likely acting as key participants.
Severe ocular surface diseases, such as Stevens-Johnson syndrome (SJS), ocular cicatricial pemphigoid (OCP), and Sjögren’s syndrome (SS) present some of the most challenging clinical cases facing eye care providers today.1,2 These patients experience numerous problems, including symblepharon formation, corneal vascularization, and squamous metaplasia. Squamous metaplasia is a serious clinical problem in that it causes pathologic keratinization of the ocular surface in response to disease processes that are autoimmune mediated (e.g., SS, SJS, OCP, rheumatoid arthritis, lupus, and scleroderma), infectious (e.g., trachoma), allergic (e.g., atopic dermatitis), and injury related (e.g., alkali burns). Very little is known about the molecular mechanisms mediating squamous metaplasia and efforts to inhibit it locally are markedly unsuccessful. Of interest, the presence of squamous metaplasia has been extensively correlated with proinflammatory activity of the ocular surface,3–6 yet the possibility that proinflammatory cytokines released from infiltrating cells actually contribute to squamous metaplasia has just recently begun to be examined.7
Squamous metaplasia, by definition, is a phenotypic change whereby epithelial cells initiate synthesis of specialized, squamous cell-specific proteins, including small proline-rich proteins (SPRRs), loricrin, involucrin, envoplakin, elafin, filaggrin, keratins, and the enzyme transglutaminase-1. These proteins form the cornified envelope (keratinization) typical of squamous cells.8–12 An outcome of the synthesis of these proteins is a change in the appearance of the cells, which convert from a nonkeratinized, stratified epithelium to a nonsecretory, keratinized epithelium.13 In the human eye, this process is accompanied by the loss of conjunctival goblet cells, an increase in epithelial stratification, and an enlargement of the superficial epithelial cells.6,14–17 Clinically, these patients experience destabilization of the cornea–tear interface, which can ultimately lead to decreased vision from loss of corneal transparency.
Since squamous metaplasia is a histologic phenomenon, to understand it at a molecular level and to identify potential targets for therapeutic intervention requires the availability of a surrogate biomarker to be used in analytical studies. SPRR proteins are such a biomarker, having been shown to be expressed early in the squamous differentiation process.18 SPRR1B belongs to an SPRR multigene family consisting of two SPRR1 genes (1A and 1B), approximately seven SPRR2 genes, one SPRR3 gene, and one SPRR4 gene.19–22 SPRRs are cross-linked either to themselves and/or to other cornified envelope precursor proteins and thereby participate in barrier formation.11 Although SPRR expression is a normal feature of external squamous tissues (i.e., skin, scalp, footpad, and vaginal epithelia), it is a sign of disease when present in mucosal tissues such as the bladder, lung, or ocular surface.18,23
Recent studies have shown that SPRR1B mRNA is expressed at very low levels in cultured human corneal cells and this expression decreases with confluence and differentiation of the cultures.24 We hypothesized that SPRR1B is increased in response to biological processes that favor pathologic keratinization and thereby serves as an important endpoint for determining which molecular events favor the development of squamous metaplasia in human eyes.
In this study, we examined SPRR1B in two different mouse models of dry eye disease. We defined a specific role for autoreactive T cells as inducers of squamous metaplasia and identified the cytokines IL1β and IFNγ as potential participants in this process. SPRR1B, IL1β, and IFNγ gene expression are increased in ocular surface tissues of mice and patients with autoimmune-mediated dry eye disease, and a direct link between the presence of these cytokines and induction of SPRR1B has been verified in vitro. This work provides an important connection between inflammation and autoimmune-mediated keratinizing ocular surface disease while introducing model systems that will help to define therapeutic approaches to prevent pathologic keratinization and vision loss.
METHODS
Human Subject Recruitment
Patients with Sjögren’s syndrome (SS) were recruited from the University of California, San Francisco (UCSF) SS clinic. Patients who had keratoconjunctivitis sicca (KCS) as well as a focal lymphocytic sialadenitis (FLS) of the labial salivary gland were included for participation in this study. FLS and KCS were scored as outlined by Daniels and Whitcher.25 Briefly, FLS is defined as the presence of greater than 1 inflammatory focus per 4 mm2 of glandular tissue, a focus being an aggregate of 50 or more lymphocytes with few plasma cells. The presence of KCS was assessed by the degree and pattern of epithelial keratitis revealed by fluorescein staining and the degree and pattern of lissamine green staining of the bulbar conjunctiva. The severity of KCS was graded on a scale from 0 to 3, where 0 is no KCS, 0.5 is very mild, 1 is mild, 1.5 is mild to moderate, 2 is moderate, 2.5 is moderate to severe, and 3 is severe. Patients with FLS and a KCS score ≥ 1 in at least one eye underwent impression cytology of the temporal bulbar conjunctiva. Age- and sex-matched control subjects with a normal ocular surface were recruited from the UCSF community and underwent identical procedures. A summary of patient demographics and KCS scores is provided in Table 1.
TABLE 1.
Summary of Control and Patients with SS
| Subjects | Age, y (Mean ± SD) | Sex | Samples (n) | KCS Score (Mean ± SD) |
|---|---|---|---|---|
| SS (n = 21) | 55 ± 12 | Female (n = 20) | 39 | 2 ± 0.5 |
| Male (n = 1) | ||||
| Control (n = 20) | 55 ± 15 | Female (n = 19) | 29 | 0 ± 0.5 |
| Male (n = 1) |
Exclusion criteria included anyone less than 21 years of age, a known diagnosis of hepatitis C, HIV, sarcoidosis, amyloidosis, graft versus host disease, cicatrizing conjunctivitis, preexisting lymphoma in patients with no prior diagnosis of SS, use of anticholinergic medication(s), active tuberculosis, pregnancy, past head and neck radiation, glaucoma with daily eye drops, corneal or eyelid surgery in the past 5 years, contact lens wear for 6 hours or more per day, a physical or mental condition interfering with successful participation, or a known diagnosis of autoimmune connective tissue disease (i.e., rheumatoid arthritis, systemic lupus erythematosus, or mixed connective tissue disease, scleroderma). All procedures were approved by the UCSF Committee for Human Research and adhered to the tenets of the Declaration of Helsinki.
Impression Cytology
Mixed cellulose ester membranes (Millipore, Bedford, MA) were used to collect cells from the conjunctival surface for RNA isolation. A 2 × 20-mm strip was placed against the temporal bulbar conjunctiva and gently pressed using closed forceps tips. The membrane was removed and stored in a cryovial containing stabilization reagent (RNAlater; Ambion, Austin, TX). The membranes were left at 4°C for 24 hours and stored at −80°C until RNA extraction.
Sample Size Planning
Sample size was determined by the power to detect a difference between SPRR1B mRNA expression in impression cytology specimens collected from patients with SS versus age-matched controls. Using preliminary real-time quantitative RT-PCR (qPCR) comparative Ct values, we calculated a threefold increase in SPRR1B expression in patients with SS (mean ± SE, Ct = 0.3 ± 0.1 vs. 0.9 ± 0.3 in normal subjects versus SS, respectively). We estimated that 30 samples in each group would provide 80% power to detect a 0.6 increase in SPRR1B expression in patients with SS (α = 0.05, two-tailed). We examined 39 impression cytology specimens from 21 patients with SS and 29 specimens from 20 age- and sex-matched control subjects. The potential correlation between eyes of the same patient was controlled by using the Huber-White sandwich estimator (i.e., the cluster command in Stata; StataCorp LP, College Station, TX).
Materials
Primary human corneal epithelial cells and medium (EpiLife) supplemented with human corneal growth supplement in the absence of antibiotics and antimycotics, were obtained from Cascade Biologics, Inc. (Portland, OR). Eagle’s minimum essential medium, Ham’s F-12, fetal bovine serum (FBS), human epidermal growth factor (EGF), penicillin-streptomycin-amphotericin, and trypsin/EDTA were from Invitrogen (Carlsbad, CA). Cholera toxin, bovine pituitary extract, insulin, dimethyl sulfoxide (DMSO) and cytokines were from Sigma-Aldrich (St. Louis, MO). Stabilization reagent (RNAlater) and the kit for RNA extraction (Micro Kit; RNeasy) were from Qiagen (Valencia, CA).
Cell Culture and Stimulation
Primary human corneal epithelial cells were seeded onto six-well plates and grown at 1 × 105 cells/well in 2 mL of medium (EpiLife; Cascade Biologics, Inc.). SV40-transformed human corneal epithelial cells (SV40-HCECs)26 were plated similarly and grown in 2 mL of EMEM-Ham’s (1:1 mixture) medium supplemented with 10% FBS, insulin (5 µg/mL), cholera toxin (0.1 µg/mL), EGF (0.01 µg/mL), 0.5% DMSO, and penicillin-streptomycin-amphotericin. At ~70% confluence, cell cultures were treated with DMSO or cytokine (20 ng/mL) for 12 hours before lysis for protein or RNA analysis.
Mice
Experimental dry eye was induced in 129SvEv/CD-1 white mice, as previously described.27,28 The desiccating stress model of dry eye using this strain of mouse has been shown to induce the activation of proinflammatory signaling pathways with increased mRNA expression of IL1β in both the tear fluid and corneal– conjunctival epithelium. All tissues and corneal epithelial cells used in this study were kindly provided by De-Quan Li and Stephen Pflugfelder (Cullen Eye Institute, Baylor College of Medicine, Houston, TX).
Aire (autoimmune regulator)-deficient mice were generated by targeted disruption of the murine aire gene as previously described.29 Aire-deficient mice were backcrossed into the BALB/c and NOD Lt/J backgrounds for more than 10 generations. All mice were housed in a pathogen-free barrier facility at the University of California, San Francisco (UCSF). Offspring mice were genotyped for the aire mutation by PCR of their genomic DNA, as previously described.29 The dry eye phenotype was assessed through topical application of lissamine green dye at 4, 8, and 15 weeks. The mice were euthanatized by carbon dioxide inhalation and thoracotomy, followed by cardiac perfusion with 0.1 M phosphate-buffered saline (PBS, pH 7.4). Their eyes were harvested for RNA isolation and immunohistochemistry. At least three aire-deficient (knockout) and three aire-sufficient (heterozygous) mice were used per experiment, and each experiment was repeated a minimum of three times. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco, and firmly adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Adoptive Transfer
Mature lymphocytes from the regional lymph node and spleen of NOD aire-deficient mice were used for adoptive transfer studies, as previously described.30 CD4+ and CD8+ T cells were depleted by using complement. Lymphocyte populations (5 × 106 cells/mouse) enriched for CD4+ T cells, mixed CD4+/CD8+ T cells or depleted of CD4+ or CD8+ T cells were transferred via intravenous tail vein injection into immunodeficient (SCID) recipients. On days 0, 5, 19, and 33, animals were treated with 0.5 mg/mouse of anti-CD4 (GK1.5, CD4+ depleted) or anti-CD8 (YTS169.4, CD8+ depleted), to remove residual CD4+ or CD8+ T cells. Animals were euthanatized 40 days after transfer and analyzed for ocular surface SPRR1B expression.
Histology, Immunofluorescence, and Immunostaining
The eyeball together with eyelids was dissected, embedded in OCT (for immunostaining) or fixed in 10% phosphate-buffered formalin and embedded in paraffin (for histology). For immunofluorescence, sections were exposed to primary anti-SPRR1B antibody at a 1:2000 dilution to final 3 ng/mL (gift of Reen Wu, University of California, Davis) and visualized with labeled secondary antibody at 333 ng/mL (FluoroLink Cy3; GE Healthcare, Piscataway, NJ). Nuclei were counterstained with DAPI (4′,6′-diamino-2-phenylindole). Immune cell types were visualized by immunostaining as previously described.30 Briefly, anti-CD4 (550280; BD-PharMingen, San Diego, CA) was used at 1:50, to a final concentration of 625 ng/mL, and anti-CD8a (550281; BD-PharMingen) was used at 1:50, to a final concentration of 625 ng/mL. Horseradish peroxidase– conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) was applied for 1 hour at 4 µg/mL. Paraffin-embedded sections were stained with periodic acid-Schiff (PAS), to visualize mucus-secreting goblet cells. The total number of goblet cells in the tarsal conjunctiva and fornix was counted in each section to quantify expression.
Western Blot Analysis
Primary and SV40-transformed human corneal epithelial cells were cultured in six-well plates to near confluence and treated with cytokines for 12 hours. After culture media were aspirated and the cells were washed with PBS, the cells were lysed with ice-cold buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, 2 mM EDTA, 1 mM EGTA, 1% Triton X-100, 0.5% DOC, 1 mM PMSF, and protease inhibitor cocktail (P2714; Sigma-Aldrich). Protein concentrations were determined by the Bradford (Bio-Rad) method. Equal amounts of protein (20 µg) from control or treated cells were resolved on 12% Bis-Tris gels (NuPAGE; Invitrogen). After electrophoresis, the proteins were blotted to nitrocellulose membranes by electrotransferring in transfer buffer (NuPAGE) containing 20% methanol. Blots were washed in TBS-T (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% Tween 20) and blocked in TBS-T containing 5% BSA for 60 minutes at room temperature. The blots were incubated overnight with primary antibody specific to human SPRR1B diluted at 1:5000 in TBS-T containing 5% BSA. After they were washed extensively, the blots were incubated with the appropriate horseradish peroxidase–linked secondary antibody (GE Healthcare, Piscataway, NJ) and exposed to film (Super RX; Fuji, Tokyo, Japan). Blots were then stripped, reprobed with anti-β-tubulin (BD Biosciences, San Jose, CA) and analyzed by densitometry using NIH Image J ver. 1.37 (http://www.ncbi.nlm.nih.gov/Genbank; provided in the public domain by the National Center for Biotechnology Information, Bethesda, MD).
RNA Isolation, Reverse Transcription, and Real-Time PCR
Total RNA from human ocular impressions, human corneal epithelial cells and mouse corneas was extracted (RNeasy Micro Kit; Qiagen). The quality and concentrations of the RNA samples were assessed (model 2100 Bioanalyzer; Agilent Technologies, Böblingen, Germany). First-strand cDNA was synthesized by using a reverse transcription kit (TaqMan; Applied Biosystems, Foster City, CA) as instructed by the manufacturer. Real-time quantitative PCR (q)PCR was performed with the SYBR Green detection method (SuperArray Bioscience Corp., Frederick, MD). PCR primer sets for human and mouse SPRR1B, IL1β, IFNγ, cyclophilin, and GAPDH were validated by SuperArray Biosciences. Cycle thresholds (Ct) from each sample were normalized with GAPDH and cyclophilin and expressed as the comparative Ct, as described in user bulletin 2 (Applied Biosystems).
Statistics
Linear regression was performed to assess the correlation between each group and qPCR results (Stata 9.0; Stata LP). The potential correlation between two eyes in the same patient was controlled by using the Huber-White sandwich estimator (cluster command in Stata 9.0; StataCorp LP). We looked at the expression of SPRR1B, IL1β, and IFNγ in patients with SS and aire-deficient mice. For studies in human subjects, we excluded impression cytology specimens that resulted in an endogenous reference (GAPDH) Ct greater than 30, because these samples did not substantiate a clinically relevant sample for accurate analysis due to low RNA yield. The comparative Ct was used to assess the statistical significance of qPCR results. Linear regression was also used to compare goblet cell expression in aire-deficient and aire-sufficient mice. One eye of each mouse was used for this analysis with a total of 11 mice in each group.
RESULTS
In Vivo Analysis of SPRR1B Expression in Mouse Models of Dry Eye Disease
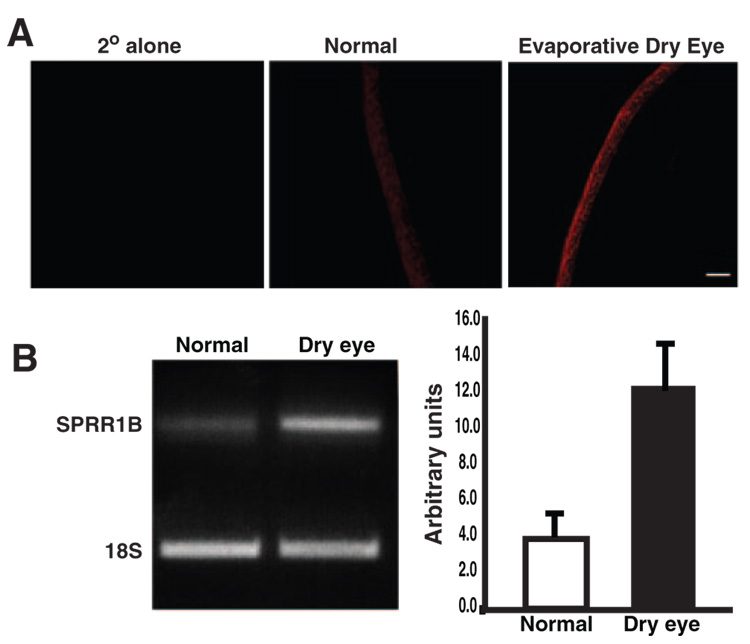
To establish the importance of SPRR1B as a biomarker for squamous metaplasia and the feasibility of using it as an endpoint for monitoring squamous metaplasia in our studies, we examined SPRR1B protein and mRNA expression in two different mouse models of dry eye. The first was a combined aqueous-deficient– evaporative mouse model induced by using anti-cholinergic agents to block lacrimal gland muscarinic receptors. Cholinergic blockade was combined with environmental stress consisting of exposure to continuous air flow for 10 to 18 h/d in an environmentally controlled room (humidity 50%, 18°C).28 This well-established model experimentally induces dry eye with features that mimic human disease (including inflammation).28 As shown in Figure 1A, SPRR1B protein is localized to the superficial layers of the epithelium and is increased in dry eye mice after 8 days of cholinergic blockade and air current desiccation (Fig. 1A, right). SPRR1B mRNA expression was also increased in corneal epithelial cells scraped from the ocular surface after 7 days of scopolamine treatment versus cells obtained from normal control mice (Fig. 1B).
FIGURE 1.
Corneal epithelial expression of SPRR1B protein and mRNA in an aqueous-deficient– evaporative mouse model of dry eye. (A) Anti-SPRR1B antibody detected with anti-rabbit Cy3 (red) showed increased expression of SPRR1B in a dry-eye mouse (right) compared with a normal one (middle). Secondary antibody alone was negative (left), as was the rabbit IgG isotype control (not shown). SPRR1B-positive immunostaining is representative of that observed in three sections obtained from three different dry eye and control mice. Scale bar, 50 µm. (B) SPRR1B mRNA expression is increased in the desiccating stress mouse model of dry eye. Corneal epithelial cells were scraped from the ocular surface and lysed for RNA extraction after 7 days of cholinergic blockade and air current desiccation. Results were compared to cells obtained from normal control mice (scraped cells were pooled from multiple dry eye and control eyes to generate 1 µg of RNA for each group) and were normalized using primers for 18S RNA. Right: densitometric analysis.
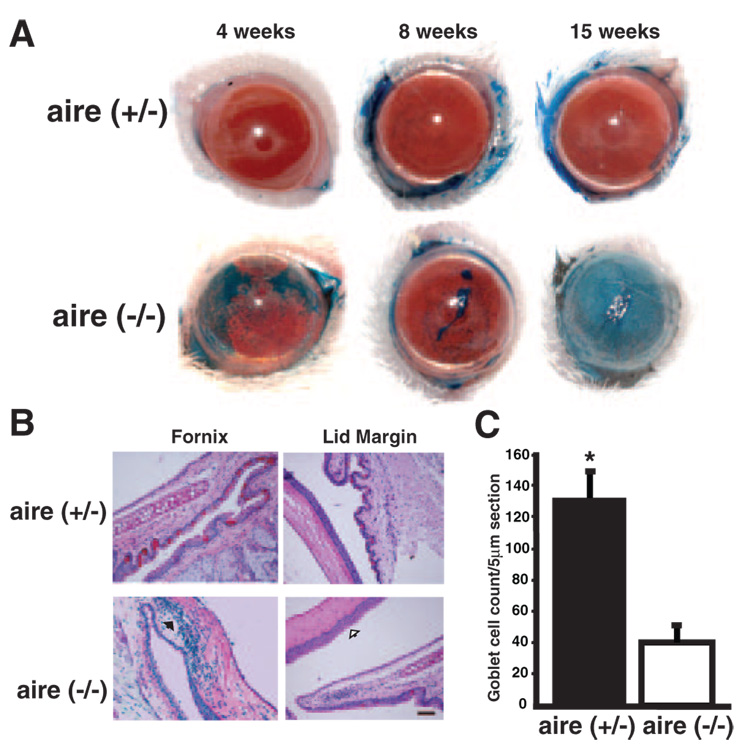
Next, we examined an autoimmune-mediated, aqueous-deficient dry eye mouse model of aire deficiency that has recently emerged as a model in the study of the immunopathogenesis of SS (Chan MF, et al. IOVS 2007;48:ARVO E-Abstract 2833).31 The aire gene controls the expression of ectopic antigens and plays an important role in central tolerance.29,32 In aire-deficient mice, and presumably in humans lacking the aire gene with autoimmune polyendocrine-candidiasis ectodermal dystrophy (APECED),29,31 the lack of aire expression leads to the loss of self-antigen presentation in the thymus, survival of self-reactive thymocytes, and the development of a population of mature, autoreactive T cells capable of mediating autoimmune disease. Our studies show signs of aqueous tear deficiency including a strikingly irregular ocular surface with areas of intense staining with lissamine green present in all aire-deficient mice by 15 weeks (Fig. 2A). Changes in the ocular surface were verified through histologic analysis of the ocular surface and quantification of PAS-stained conjunctival goblet cells. Corneal epithelial stratification was increased, goblet cell density was decreased, and there was significant cellular infiltration of the ocular surface tissues in aire-deficient eyes compared with aire-sufficient littermates (Figs. 2B, 2C).
FIGURE 2.
Dry eye phenotype in aire-deficient mice. (A) Punctate corneal epithelial erosions were apparent with lissamine green staining as early as 4 weeks and appeared to progress to a filamentary keratitis by 8 weeks of age. Moderate to severe disruption of ocular surface integrity was present in all aire−/− mice by 15 weeks. (B) Hallmark histologic signs of dry eye in aire-deficient mice were accompanied by increased epithelial stratification (open black arrow), cellular infiltration of the tarsal conjunctiva and cornea (solid black arrow), and loss of goblet cells. Scale bar, 50 µm; magnification, ×200. (C) Goblet cells were quantified in aire-deficient vs. aire-sufficient mice at 15 weeks by PAS staining. Counts are expressed as mean ± SE per 5-µm section (aire+/− vs. aire−/−, 133 ± 16.0 vs. 45 ± 10.4, respectively; *P < 0.0001).
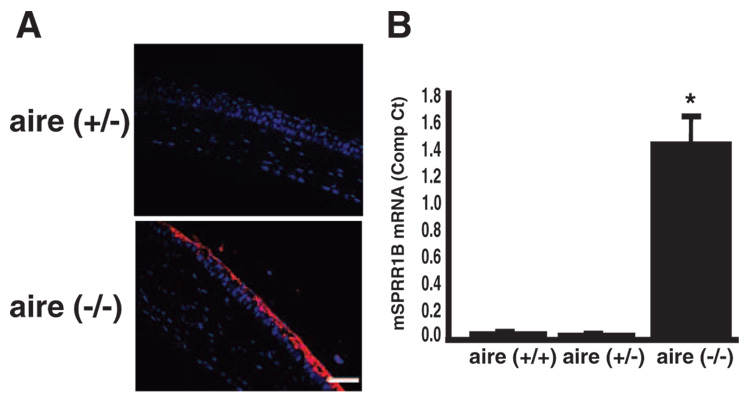
These hallmark signs of squamous metaplasia in aqueous-deficient dry eye disease were accompanied by superficial overexpression of SPRR1B across the corneal surface (Fig. 3A) and upregulation of SPRR1B endogenous gene expression in the corneal epithelium (Fig. 3B) of aire-deficient mice. Together, these results show transformation of a normal, mucus-secreting ocular surface to one that is pathologically keratinized in the setting of aire deficiency and confirm that this transformation is accompanied by increased protein and mRNA expression of the squamous cell biomarker, SPRR1B.
FIGURE 3.
The squamous cell biomarker, SPRR1B, was increased across the ocular surface of aire-deficient mice. (A) Representative example of SPRR1B protein expression on the corneal surface of aire-deficient mice at 15 weeks. Anti-SPRR1B antibody detected with Cy3 secondary (red) was associated with the superficial layers of the corneal epithelium in aire−/− vs. littermate control (aire+/−). Magnification, ×400. Blue: nuclei stained with DAPI. Scale bar, 50 µm. (B) Comparison of SPRR1B mRNA expression in the corneas of aire+/+ (wild-type), aire+/− (heterozygous), and aire−/− (knockout) mice using RT-qPCR. A minimum of three eyes from three different animals were combined for each experiment. Experiments were repeated a minimum of three times, and results are expressed as the mean ± SE (wild-type undetected; heterozygous, undetected; knockout = 1.43 ± 0.224; *P < 0.01).
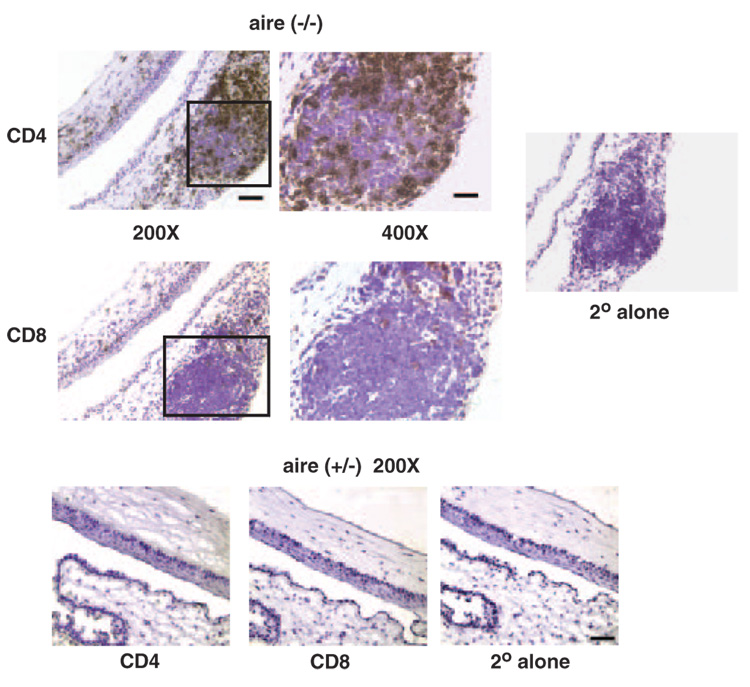
Previous examinations of conjunctival tissues in patients with SS suggest that they are a target for inflammation.4,33–35 Lymphocytic infiltration of the substantia propria and expression of notable amounts of various cytokines have been reported.36,37 Immunohistologic examination of aire-deficient animals verified accompanying lymphocytic infiltration of corneal and lid tissues with CD4+ and, to a lesser extent, CD8+ T cells (Fig. 4). Whether the presence of these cells or specific mediators released from them (or other cells) are the principal inducers of the histopathology is of interest.
FIGURE 4.
The ocular surface and lids (box, ×400) of aire-deficient mice are infiltrated with CD4+ and CD8+ T cells. OCT embedded sections of aire−/− BALB/c mice were stained with antibodies directed against CD4+ (top) and CD8+ (middle) T cells, as described.30 Staining of heterozygous littermates (bottom) was negative for both cell populations. Secondary antibody–alone control staining was negative in all tissues. Specimens are representative of CD4+ and CD8+ immunostaining observed in tissue sections obtained from 8 knockout and 12 heterozygous mice. Scale bar for ×400, 30 µm; for ×200, 50 µm.
CD4+ T Cells and Ocular Surface Squamous Transformation
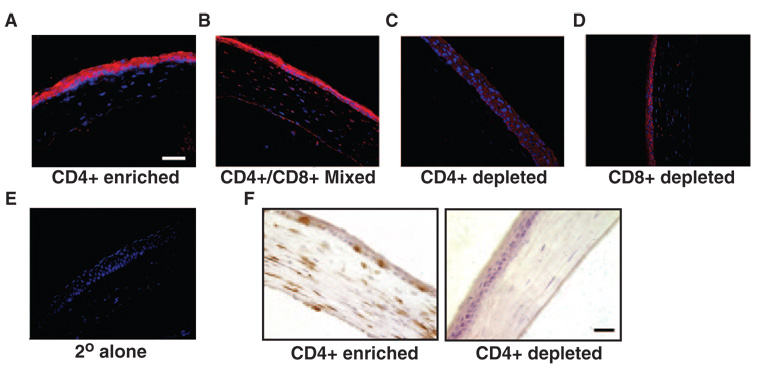
To establish a link between squamous metaplasia and inflammation in the aire-deficient mouse, we examined the effects of transferring lymphocytes obtained from NOD aire-deficient donors to wild type, immunodeficient (SCID) recipients. Of interest, we were able to transfer the squamous phenotype to wild-type mice by using lymphocytes obtained from aire-deficient donors (Fig. 5A–E). Pooled lymphocytes enriched for CD4+ T cells and CD4+/CD8+ mixed T cell populations caused advanced keratinization of the cornea in immunodeficient recipients that was not observed in populations that were depleted of CD4+ cells. Pathologic changes in the corneal surface appeared to progress from a thickened, hyperplastic epithelium with disorganization of the basal cell layer to a keratinizing squamous epithelium that eventually underwent considerable cornification and atrophy. Immunostaining confirmed lymphocytic infiltration of the ocular surface with CD4+ T cells in CD4+ enriched (Fig. 5F), CD8+ depleted and the mixed CD4/CD8 phenotype (not shown). The severity of pathologic keratinization in these groups of adoptive transfer mice compared to the CD4+-depleted group suggests that squamous metaplasia is predominantly mediated by CD4+ T cells. Whether the presence of these cells and/or specific mediators released from them (and other cells) are the principle inducers of the histopathology is unclear; however, the significance of autoreactive T cells in driving pathologic keratinization in autoimmune-mediated dry eye is apparent.
FIGURE 5.
The squamous phenotype can be induced by adoptive transfer of lymphocytes from the regional lymph node and spleen of aire-deficient mice. Pooled lymphocyte populations were (A) enriched for CD4+ T cell, (B) enriched for CD4+ and CD8+ T cells, (C) depleted of CD4+ T cells, or (D) depleted of CD8+ T cells. Cells were transferred via intravenous tail vein injection into immunodeficient (SCID) recipients at 1 × 107 cells per mouse (n = 5 per group). Immunofluorescence with anti-SPRR1B antibody and Cy3 secondary (red) shows localization throughout the corneal epithelium that is absent in eyes of (E) secondary alone control animals and those (C) depleted of CD4+ T cells. (F) Immunohistochemical analysis of the same tissues demonstrated extensive infiltration of the ocular surface with CD4+ T cells (labeled brown with DAB) in CD4+ enriched animals (left) that was absent in CD4+ depleted animals (right). Blue: nuclei stained with DAPI. Magnification, ×400; scale bar, 50 µm.
In Vitro Analysis of SPRR1B Expression and Regulation
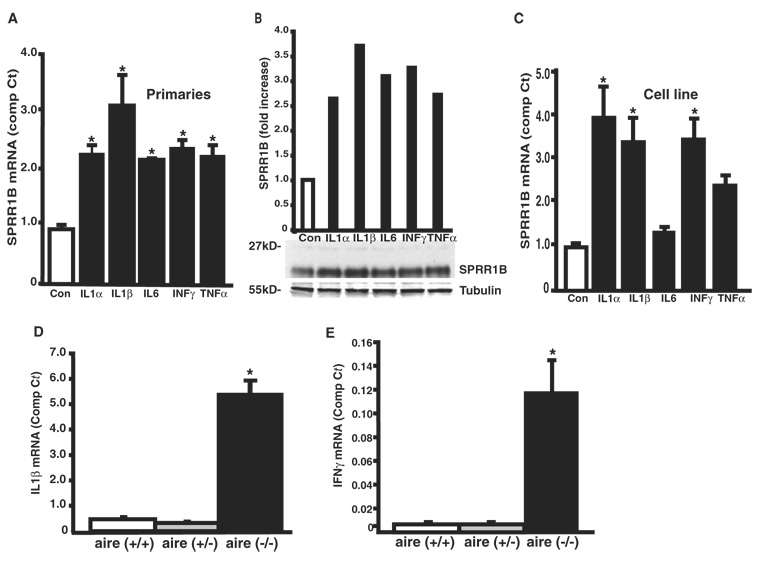
Having confirmed that SPRR1B is upregulated in two distinct mouse models of dry eye, we hypothesized that this induction might be the direct result of a local inflammatory response initiated by the influx of activated T cells. In humans, several cytokines released by activated lymphocytes, other immune cells, and/or epithelial cells have been reported to be increased in dry eye tissues, including: TNFα, IL1α, IL1β, MIP-2, IL6, and IL8, monokine induced by γ-interferon (MIG), IL4, IFNγ, and others.36–39 We examined the role of cytokines as direct inducers of SPRR1B gene expression in vitro and their potential role as inducers of squamous metaplasia in vivo by exposing human corneal epithelial cells to the cytokines IL1α, IL1β, IL6, IFNγ, and TNFα. Consistent with the idea that squamous metaplasia is mediated by a local inflammatory response, SPRR1B was induced by all these cytokines in primary human corneal epithelial cells (Fig. 6A), with the response to IL1β being the most robust. This induction of SPRR1B was also noted at the protein level by immunoblot analysis (Fig. 6B). An identical analysis performed in the SV40-transformed cell line suggests that they responded in a similar fashion (Fig. 6C). Further study confirmed that at least two of these cytokines, IL1β and IFNγ, were also significantly elevated in the corneas of aire-deficient mice (Figs. 6D, 6E) and there was a strong correlation between the expression of these cytokines and SPRR1B in the corneas of these animals (r = 0.92, P < 0.001 for IL1β; r = 0.81, P < 0.01 for IFNγ).
FIGURE 6.
Upregulation of SPRR1B (A) mRNA and (B) protein in primary human corneal epithelial cells after a 12-hour exposure to the cytokines IL1α, IL1β, IL6, IFNγ, and TNFα. Real-time PCR results are expressed as the mean ± SE comparative Ct calculated using GAPDH as an endogenous reference (control = 0.92 ± 0.082, IL1α 2.18 ± 0.147, IL1β = 3.00 ± 0.504, IL6 = 2.08 ± 0.026, IFNγ = 2.28 ± 0.132, and TNFα = 2.15 ± 0.183). (C) Similar levels of induction were observed in SV40-HCE cells (control = 0.98 ± 0.095, IL1α = 3.92 ± 0.714, IL1β = 3.37 ± 0.561, IL6 = 1.34 ± 0.114, IFNγ = 3.44 ± 0.475, and TNFα = 2.39 ± 0.237, respectively). (D) IL1β and (E) IFNγ mRNA were significantly increased in aire-deficient mice compared to aire-sufficient littermates. The mean ± SE comparative Ct for IL1β and IFNγ was compared in corneal tissues of wild-type, heterozygous, and knockout mice by RT-qPCR (IL1β = 0.424 ± 0.161, 0.284 ± 0.096, and 5.37 ± 0.878, respectively; IFNγ = undetected, undetected, and 0.120 ± 0.023, respectively) *P < 0.05. Experiments were repeated a minimum of three times. Both IL1β and IFNγ expression correlated highly with SPRR1B expression in aire-deficient mice (r = 0.92, P < 0.001 for IL1β and r = 0.81, P < 0.01 for IFNγ).
SPRR1B Expression in Patients with SS
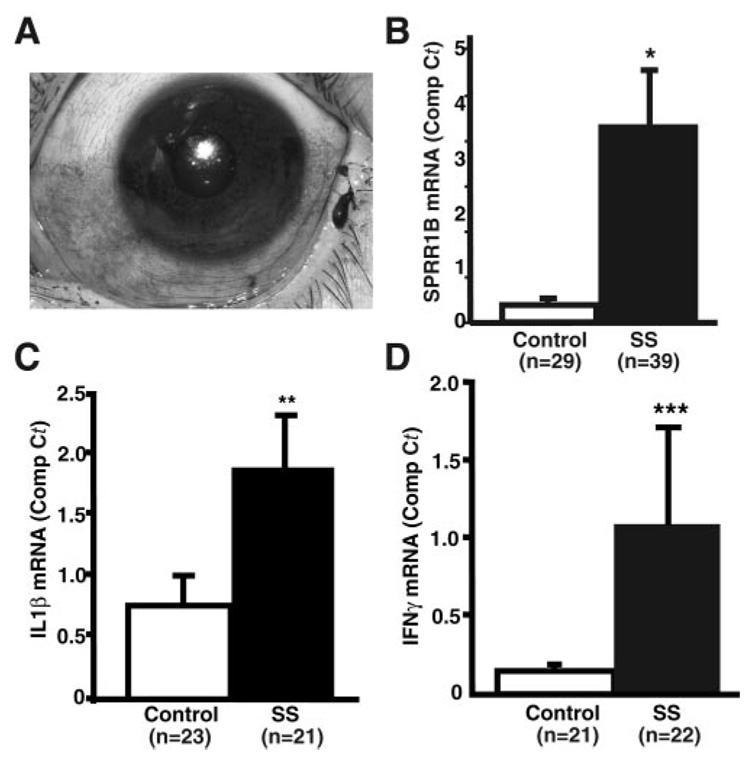
Given the significant increase in SPRR1B expression in two different mouse models of dry eye disease as well as the direct induction of SPRR1B by autoreactive CD4+ T cells in vivo and by several proinflammatory cytokines in vitro, we sought to validate the use of SPRR1B as an analytical biomarker of keratinizing ocular surface disease in human eyes. Using ocular surface conjunctival cells obtained from human subjects by impression cytology, we examined SPRR1B. Impressions were collected from lissamine green–stained areas of conjunctiva in patients with SS (Fig. 7A) and compared with age-matched control subjects without lissamine green staining. A significant increase in SPRR1B mRNA expression was noted in patients with SS by RT-qPCR (Fig. 7B), and this increase was significantly correlated with the severity of ocular surface disease (r = 0.34, P < 0.005 for SPRR1B and KCS score). Increased SPRR1B was also accompanied by elevated expression of both IL1β and IFNγ in a subgroup of the same cohort (Figs. 7C, 7D).
FIGURE 7.
SPRR1B is upregulated in patients with SS. (A) Impression cytology specimens were collected from lissamine green–stained areas of the temporal bulbar conjunctiva in patients with SS and compared to age-matched control subjects without staining. RT-qPCR was used to determine the comparative Ct for (B) SPRR1B, (C) IL1β, and (D) IFNγ. Expressed as the mean ± SE in control versus patients with SS: SPRR1B = 0.31 ± 0.087 vs. 3.37 ± 1.00, *P < 0.001; IL1β = 0.766 ± 0.198 vs. 1.88 ± 0.633, **P = 0.085; and IFNγ = 0.149 ± 0.034 vs. 1.097 ± 0.593, ***P = 0.11.
DISCUSSION
Over the past several years a growing body of evidence has suggested that inflammation provides a driving force for the initiation and maintenance of ocular surface disease associated with dry eye. In this study, we examined the hypothesis that a dysregulated immune response (e.g., SS-associated dry eye) or a stress response to chronic deficiencies in the quantity and/or quality of the tear film (e.g., non-SS dry eye or evaporative dry eye) provides the underlying driving force for pathologic keratinization of the ocular surface. With either scenario, inflammation sets off a defense response that leads to increased expression of the cornified envelope proteins present in keratin and loss of mucus-secreting goblet cells. This innate defense is most likely an attempt to “cover up” and protect the mucosal surface from further insult. Unfortunately, it ultimately leads to significant disease that can be sight-threatening when there is corneal involvement (e.g., OCP, SJS).
Given the inherent complexity of deciphering the specific inflammatory events that elicit pathologic expression of keratinizing proteins, we examined the possibility of using SPRR1B as a biomarker for squamous metaplasia in several model systems. SPRR1B provides a convenient endpoint for quantifying pathologic keratinization because it is expressed at negligible levels in nonkeratinized mucosal tissues and quickly becomes overexpressed in the setting of stress-induced and/or immunemediated disease.18,23 Our studies show that SPRR1B is increased across the ocular surface in two different animal models of dry eye disease and in conjunctival samples obtained from the eyes of patients with SS. Adoptive transfer studies using mature lymphocytes from the aire-deficient mouse model confirmed that autoreactive T cells can cause squamous transformation of the ocular surface epithelium in immunodeficient recipients, providing a definitive link between ocular surface inflammation and squamous metaplasia.
To explore inflammation further as an inducer of squamous cell marker expression, we focused on examining the effects of several cytokines reported to be increased in the conjunctival epithelium and/or tear fluid of patients and animals with keratinizing ocular surface disease.5,37,40–43 Some of the mediators are well known to effect differentiation and gene expression in a variety of cell types. For example, in human epidermal keratinocytes, IL1α has been shown to upregulate SPRR1B.44 Also in keratinocytes, the inflammatory cytokine IFNγ causes irreversible growth arrest and a 7- to 15-fold increase in the expression of both transglutaminase type 1 and SPRR1B, which precedes expression of the squamous phenotype.45 More recent work has shown a role for IFNγ as an inducer of transglutaminase type 146 and SPRR27 in conjunctival epithelial cells, whereas IL1α has no effect.46 Unexpectedly, involucrin gene expression was repressed by IFNγ suggesting that the etiology of squamous metaplasia is complicated and may involve multiple participants.
In our own studies, we found direct induction of SPRR1B gene expression and protein in response to IL1α, IL1β, IL6, IFNγ, and TNFα, with IL1β proving to be the most potent inducer. We found increased IL1β and IFNγ expression in the ocular surface tissues of aire-deficient mice and in bulbar conjunctival cells from patients with SS. Direct induction of SPRR1B in response to these cytokines suggests that they may function to trigger downstream events that mediate the pathogenesis of dry eye disease, including squamous metaplasia. This possibility is in agreement with a recent study showing a role for IFNγ as an inducer of squamous metaplasia in mice in the desiccating stress model of dry eye.7
In addition to its role as a precursor protein for squamous metaplasia, recent work suggests that the regulation of SPRR genes may not be limited to the induction of epithelial cell differentiation. For example, SPRR1A is involved in axonal outgrowth and is highly expressed at the growth cones of axotomized neurons.47 SPRR1A also confers cardiomyocyte protection in response to IL6 family cytokines after biomechanical –ischemic stress and may play a critical role in the dynamic regulation of actin cytoskeleton assembly.47,48 Of significance to our work is the hypothesis that SPRR genes may play a central role in the induction of an innate defense response after injury or in the setting of primary inflammatory conditions. This notion is supported by studies showing the induction of SPRR2A in mucosal tissue infected by Helicobacter pylori in models of gastric injury.49 Even the Th2 cytokine, IL13, has been shown to induce SPRR2 in subsets of airway epithelial cells and mononuclear cells associated with inflammation in the asthmatic lung.50 These studies support our hypothesis that SPRR genes are induced in response to stress injury and thereby contribute to cell protection and/or tissue remodeling. They also extend the potential role of SPRR proteins as mediators of genetic or environmental stress where induction may offer protection, such as cardioprotection in the setting of ischemic heart disease, or cause disease, as we find in the setting of keratinizing ocular surface disease.
In summary, we report that SPRR1B is a stress-induced transcript on the ocular surface that is upregulated in both evaporative and immune-mediated, aqueous-deficient dry eye disease. SPRR1B expression is increased in the eyes of patients with mild to moderate KCS and this increase correlates with disease severity. Our results also provide a definitive link between ocular surface inflammation and squamous metaplasia and set the stage for further studies to define the molecular sequence of events that mediates the induction of cornified envelope proteins. Whether a single inflammatory mediator drives the process of squamous metaplasia in autoimmune-mediated keratinizing ocular surface disease is not clear. It is also possible that stimuli such as androgens or tear film hypertonicity could initiate the process of squamous metaplasia. Moreover, studies of SPRR protein and gene expression in the setting of innate immunity may point to additional biological functions for these structural proteins that move beyond their role of forming the cornified envelope. A complete understanding of each role is necessary to preferentially modulate the expression of SPRRs in favor of the host. Further in vitro studies of the gene regulatory mechanisms governing squamous metaplasia in response to cytokines are necessary as well as in vivo studies to identify other key players in the immunopathogenesis of squamous metaplasia. This combination of approaches will guide our studies of therapeutic targets to control this process in sight-threatening ocular surface disease.
Acknowledgments
The authors thank Trinka Vijmasi for assistance with tissue preparation, Kathryn Ray and Travis Porco for statistical advice, and Marianne Gallup for helping to prepare the manuscript.
Supported by National Eye Institute Grants R01EY 016203 (NAM), R01EY 016408 (MSA, JD), and EY014419; Research to Prevent Blindness; James S. Adams Scholarship (ECS); and the Giannini Foundation (JD).
Footnotes
Disclosure: S. Li, None; K. Nikulina, None; J. DeVoss, None; A.J. Wu, None; E.C. Strauss, None; M.S. Anderson, None; N.A. McNamara, None
References
- 1.Nakamura T, Nishida K, Dota A, Kinoshita S. Changes in conjunctival clusterin expression in severe ocular surface disease. Invest Ophthalmol Vis Sci. 2002;43:1702–1707. [PubMed] [Google Scholar]
- 2.Tsubota K, Satake Y, Kaido M, et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340:1697–1703. doi: 10.1056/NEJM199906033402201. [DOI] [PubMed] [Google Scholar]
- 3.Sacks EH, Jakobiec FA, Wieczorek R, Donnenfeld E, Perry H, Knowles DM., Jr Immunophenotypic analysis of the inflammatory infiltrate in ocular cicatricial pemphigoid: further evidence for a T cell-mediated disease. Ophthalmology. 1989;96:236–243. doi: 10.1016/s0161-6420(89)32922-8. [DOI] [PubMed] [Google Scholar]
- 4.Raphael M, Bellefqih S, Piette JC, Le Hoang P, Debre P, Chomette G. Conjunctival biopsy in Sjögren’s syndrome: correlations between histological and immunohistochemical features. Histopathology. 1988;13:191–202. doi: 10.1111/j.1365-2559.1988.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 5.Pflugfelder SC, Huang AJ, Feuer W, Chuchovski PT, Pereira IC, Tseng SC. Conjunctival cytologic features of primary Sjögren’s syndrome. Ophthalmology. 1990;97:985–991. doi: 10.1016/s0161-6420(90)32478-8. [DOI] [PubMed] [Google Scholar]
- 6.Nishida K, Yamanishi K, Yamada K, et al. Epithelial hyperproliferation and transglutaminase 1 gene expression in Stevens-Johnson syndrome conjunctiva. Am J Pathol. 1999;154:331–336. doi: 10.1016/S0002-9440(10)65279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-γ. Invest Ophthalmol Vis Sci. 2007;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 8.Eckert RL, Yaffe MB, Crish JF, Murthy S, Rorke EA, Welter JF. Involucrin: structure and role in envelope assembly. J Invest Dermatol. 1993;100:613–617. doi: 10.1111/1523-1747.ep12472288. [DOI] [PubMed] [Google Scholar]
- 9.Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995;270:17702–17711. doi: 10.1074/jbc.270.30.17702. [DOI] [PubMed] [Google Scholar]
- 10.Steinert PM, Marekov LN. Direct evidence that involucrin is a major early isopeptide cross-linked component of the keratinocyte cornified cell envelope. J Biol Chem. 1997;272:2021–2030. doi: 10.1074/jbc.272.3.2021. [DOI] [PubMed] [Google Scholar]
- 11.Steinert PM, Candi E, Kartasova T, Marekov L. Small proline-rich proteins are cross-bridging proteins in the cornified cell envelopes of stratified squamous epithelia. J Struct Biol. 1998;122:76–85. doi: 10.1006/jsbi.1998.3957. [DOI] [PubMed] [Google Scholar]
- 12.Steinert PM, Marekov LN. Initiation of assembly of the cell envelope barrier structure of stratified squamous epithelia. Mol Biol Cell. 1999;10:4247–4261. doi: 10.1091/mbc.10.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jetten AM. Multistep process of squamous differentiation in tracheobronchial epithelial cells in vitro: analogy with epidermal differentiation. Environ Health Perspect. 1989;80:149–160. doi: 10.1289/ehp.8980149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ralph RA. Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest Ophthalmol. 1975;14:299–302. [PubMed] [Google Scholar]
- 15.Friend J, Kiorpes T, Thoft RA. Conjunctival goblet cell frequency after alkali injury is not accurately reflected by aqueous tear mucin content. Invest Ophthalmol Vis Sci. 1983;24:612–618. [PubMed] [Google Scholar]
- 16.Kinoshita S, Kiorpes TC, Friend J, Thoft RA. Goblet cell density in ocular surface disease: a better indicator than tear mucin. Arch Ophthalmol. 1983;101:1284–1287. doi: 10.1001/archopht.1983.01040020286025. [DOI] [PubMed] [Google Scholar]
- 17.Tseng SC, Hirst LW, Maumenee AE, Kenyon KR, Sun TT, Green WR. Possible mechanisms for the loss of goblet cells in mucin-deficient disorders. Ophthalmology. 1984;91:545–552. doi: 10.1016/s0161-6420(84)34251-8. [DOI] [PubMed] [Google Scholar]
- 18.Tesfaigzi J, Carlson DM. Expression, regulation, and function of the SPR family of proteins: a review. Cell Biochem Biophys. 1999;30:243–265. doi: 10.1007/BF02738069. [DOI] [PubMed] [Google Scholar]
- 19.Kartasova T, van de Putte P. Isolation, characterization, and UV-stimulated expression of two families of genes encoding polypeptides of related structure in human epidermal keratinocytes. Mol Cell Biol. 1988;8:2195–2203. doi: 10.1128/mcb.8.5.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kartasova T, van Muijen GN, van Pelt-Heerschap H, van de Putte P. Novel protein in human epidermal keratinocytes: regulation of expression during differentiation. Mol Cell Biol. 1988;8:2204–2210. doi: 10.1128/mcb.8.5.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbs S, Fijneman R, Wiegant J, van Kessel AG, van De Putte P, Backendorf C. Molecular characterization and evolution of the SPRR family of keratinocyte differentiation markers encoding small proline-rich proteins. Genomics. 1993;16:630–637. doi: 10.1006/geno.1993.1240. [DOI] [PubMed] [Google Scholar]
- 22.Cabral A, Sayin A, de Winter S, Fischer DF, Pavel S, Backendorf C. SPRR4, a novel cornified envelope precursor: UV-dependent epidermal expression and selective incorporation into fragile envelopes. J Cell Sci. 2001;114:3837–3843. doi: 10.1242/jcs.114.21.3837. [DOI] [PubMed] [Google Scholar]
- 23.Carrasco D, Bravo R. Tissue-specific expression of the fos-related transcription factor fra-2 during mouse development. Oncogene. 1995;10:1069–1079. [PubMed] [Google Scholar]
- 24.Tong L, Corrales RM, Chen Z, et al. Expression and regulation of cornified envelope proteins in human corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:1938–1946. doi: 10.1167/iovs.05-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniels TE, Whitcher JP. Association of patterns of labial salivary gland inflammation with keratoconjunctivitis sicca: analysis of 618 patients with suspected Sjögren’s syndrome. Arthritis Rheum. 1994;37:869–877. doi: 10.1002/art.1780370615. [DOI] [PubMed] [Google Scholar]
- 26.Araki-Sasaki K, Ohashi Y, Sasabe T, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 27.Luo L, Li D, Doshi A, Farley W, Corrales RW, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 28.Dursun D, Wang M, Monroy D, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43:632–638. [PubMed] [Google Scholar]
- 29.Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the Aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 30.DeVoss J, Hou Y, Johannes K, et al. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuroda N, Mitani T, Takeda N, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174:1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 32.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Pflugfelder SC, Tseng SC, Yoshino K, Monroy D, Felix C, Reis BL. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology. 1997;104:223–235. doi: 10.1016/s0161-6420(97)30330-3. [DOI] [PubMed] [Google Scholar]
- 34.Rivas L, Oroza MA, Perez-Esteban A, Murube-del-Castillo J. Morphological changes in ocular surface in dry eyes and other disorders by impression cytology. Graefes Arch Clin Exp Ophthalmol. 1992;230:329–334. doi: 10.1007/BF00165940. [DOI] [PubMed] [Google Scholar]
- 35.Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjögren’s and non-Sjögren’s patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–2614. [PubMed] [Google Scholar]
- 36.Jones DT, Monroy D, Ji Z, Atherton SS, Pflugfelder SC. Sjögren’s syndrome: cytokine and Epstein-Barr viral gene expression within the conjunctival epithelium. Invest Ophthalmol Vis Sci. 1994;35:3493–3504. [PubMed] [Google Scholar]
- 37.Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 38.Kawasaki S, Kawamoto S, Yokoi N, et al. Up-regulated gene expression in the conjunctival epithelium of patients with Sjögren’s syndrome. Exp Eye Res. 2003;77:17–26. doi: 10.1016/s0014-4835(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 39.Stern ME, Siemasko KF, Gao J, Calonge M, Niederkorn JY, Pflugfelder SC. Evaluation of ocular surface inflammation in the presence of dry eye and allergic conjunctival disease. Ocul Surf. 2005;3:S161–S164. doi: 10.1016/s1542-0124(12)70246-x. [DOI] [PubMed] [Google Scholar]
- 40.Afonso AA, Sobrin L, Monroy DC, Selzer M, Lokeshwar B, Pflugfelder SC. Tear fluid gelatinase B activity correlates with IL-1α concentration and fluorescein clearance in ocular rosacea. Invest Ophthalmol Vis Sci. 1999;40:2506–2512. [PubMed] [Google Scholar]
- 41.Tishler M, Yaron I, Geyer O, Shirazi I, Naftaliev E, Yaron M. Elevated tear interleukin-6 levels in patients with Sjögren syndrome. Ophthalmology. 1998;105:2327–2329. doi: 10.1016/S0161-6420(98)91236-2. [DOI] [PubMed] [Google Scholar]
- 42.Jones DT, Monroy D, Ji Z, Pflugfelder SC. Alterations of ocular surface gene expression in Sjögren’s syndrome. Adv Exp Med Biol. 1998;438:533–536. doi: 10.1007/978-1-4615-5359-5_75. [DOI] [PubMed] [Google Scholar]
- 43.Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 44.Eller MS, Yaar M, Ostrom K, Harkness DD, Gilchrest BA. A role for interleukin-1 in epidermal differentiation: regulation by expression of functional versus decoy receptors. J Cell Sci. 1995;108:2741–2746. doi: 10.1242/jcs.108.8.2741. [DOI] [PubMed] [Google Scholar]
- 45.Saunders NA, Jetten AM. Control of growth regulatory and differentiation-specific genes in human epidermal keratinocytes by interferon gamma: antagonism by retinoic acid and transforming growth factor beta 1. J Biol Chem. 1994;269:2016–2022. [PubMed] [Google Scholar]
- 46.Hirai N, Kawasaki S, Tanioka H, et al. Pathological keratinisation in the conjunctival epithelium of Sjögren’s syndrome. Exp Eye Res. 2006;82:371–378. doi: 10.1016/j.exer.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pradervand S, Yasukawa H, Muller OG, et al. Small proline-rich protein 1A is a gp130 pathway- and stress-inducible cardioprotective protein. EMBO J. 2004;23:4517–4525. doi: 10.1038/sj.emboj.7600454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mueller A, O’Rourke J, Grimm J, et al. Distinct gene expression profiles characterize the histopathological stages of disease in Helicobacter-induced mucosa-associated lymphoid tissue lymphoma. Proc Natl Acad Sci USA. 2003;100:1292–1297. doi: 10.1073/pnas.242741699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmermann N, Doepker MP, Witte DP, et al. Expression and regulation of small proline-rich protein 2 in allergic inflammation. Am J Respir Cell Mol Biol. 2005;32:428–435. doi: 10.1165/rcmb.2004-0269OC. [DOI] [PubMed] [Google Scholar]