Abstract
The UV hypersensitive CHO cell mutant UV41 is the archetypal XPF mammalian cell mutant, and was essential for cloning the human nucleotide excision repair (NER) gene XPF by DNA transfection and rescue. The ERCC1 and XPF genes encode proteins that form the heterodimer responsible for making incisions required in NER and the processing of certain types of recombination intermediates. In this study, we cloned and sequenced the CHO cell XPF cDNA, determining that the XPF mutation in UV41 is a +1 insertion in exon 8 generating a premature stop codon at amino acid position 499; however, the second allele of XPF is apparently unaltered in UV41, resulting in XPF heterozygosity. XPF expression was found to be several-fold lower in UV41 compared to its parental cell line, AA8. Using approaches we previously developed to study intrachromosomal recombination in CHO cells, we modified UV41 and its parental cell line AA8 to allow site-specific gene targeting at a Flp Recombination Target (FRT) in intron 3 of the endogenous adenine phosphoribosyltransferase (APRT) locus. Using FLP/FRT targeting, we integrated a plasmid containing an I-SceI endonuclease sequence into this site in the paired cell lines to generate a heteroallelic APRT duplication. Frequencies of intrachromosomal recombination between APRT heteroalleles and the structures of resulting recombinants were analyzed after I-SceI induction of site-specific double-strand breaks (DSBs) in a non-homologous insertion contained within APRT homology. Our results show that I-SceI induced a small proportion of aberrant recombinants reflecting DSB-induced deletions/rearrangements in parental, repair-proficient AA8 cells. However, in XPF mutant UV41, XPF heterozygosity is responsible for a similar, but much more pronounced genomic instability phenotype, manifested independently of DSB induction. In addition, gene conversions were suppressed in UV41 cells compared to wild-type cells. These observations suggest that UV41 exhibits a genomic instability phenotype of aberrant recombinational repair, confirming a critical role for XPF in mammalian cell recombination.
Keywords: XPF, UV41, recombinational repair, genomic instability
1. Introduction
The ERCC1-XPF heterodimer is a structure-specific endonuclease involved in several aspects of DNA metabolism, including nucleotide excision repair (NER), the processing of specific types of intermediates in strand-invasion and single-strand annealing (SSA) recombination pathways, and telomere maintenance [1–7]. The structure specific incision activity of ERCC1-XPF is critical to these functions, generating the incision on the 5’ side of the NER bubble [1], and precisely removing 3’-OH end-blocking single-stranded heterologous DNA from homologous recombination intermediates [2–5]. In telomere metabolism, ERCC1-XPF removes the 3’ overhang from uncapped telomeres, allowing chromosome end fusions, and is postulated to suppress recombination between telomeres and interstitial telomere related sequences [6]. Much of our knowledge concerning the roles of the ERCC1 and XPF genes in these critical processes has been gained by exploiting CHO cell mutants, including the molecular cloning of the human ERCC1 and XPF genes [8–10], and the implication of ERCC1 in targeted homologous recombination [2,3].
Both ERCC1 and XPF mutants derived from a common parental CHO cell line, AA8, have been used in numerous studies of DNA repair and mutagenesis [11–14]. The ERCC1 gene maps to a monosome (chromosome 9) in CHO cells [15], and is therefore hemizygous; this characteristic has facilitated ERCC1 mutational analyses and somatic cell gene knock-out strategies [16,17] Based on the paucity of CHO XPF mutants obtained in mutant hunts, Busch et al. speculated that XPF is present as two alleles in CHO cells [12]. One purpose of this study was to determine the nature of the XPF mutation in UV41, which is the archetypal CHO XPF mutant, having been the most extensively characterized and widely utilized rodent XPF mutant cell line in cytotoxicity, repair and mutagenesis studies [13].
Experiments using ERCC1 deficient rodent cell lines have shown that the ERCC1-XPF endonuclease is required for removing lengthy nonhomologous DNA sequences which block homologous DNA ends during gene targeting [2], and for efficient gene disruption by “ends-out” configuration targeting vectors [3]. We have also shown that ERCC1 is required for the generation of recombinants with crossover structures resulting from the precise removal of nonhomologous DNA sequences interrupting homology during intrachromosomal recombination between direct repeat substrates integrated at the endogenous CHO APRT locus. In this study, lack of incision by ERCC1-XPF at sites of overhanging 3′homologous DNA sequence was implicated as the primary source of recombination-dependent rearrangements in ERCC1 deficient CHO cells [4]. Our previously reported results from stimulating homologous recombination between direct repeats with I-SceI endonuclease also suggested that ERCC1 might be required to generate rearrangements arising after induced DSBs [5]. A recent study similarly investigated recombinational repair of I-SceI-induced DSBs in heteroallelic direct repeats in ERCC1-deficient UV4 cells and showed that generation of both SSA and gene conversion recombinants required ERCC1 [18].
The majority of studies investigating the role of ERCC1-XPF in mitotic recombination have used ERCC1-deficient cell lines. The endonuclease activity of the ERCC1-XPF heterodimer resides in the XPF subunit [19]. However, a recent study has shown that nuclease-inactive XPF is functional in TRF2-mediated telomere shortening [7]; furthermore, a number of recent reports have implicated ERCC1 in IGF1 endocrine signaling pathways with a role in development and metabolism distinct from its role in NER (briefly reviewed in [20]). Therefore, in the present study, we characterized the XPF mutation in UV41 cells and performed experiments to directly examine the role of XPF deficiency on mitotic recombination. We determined that UV41 is a XPF heterozygote and that XPF expression was significantly reduced in UV41 cells compared to its parent, AA8. We genetically modified UV41 and parental AA8 cell lines to allow analysis of DSB-induced intrachromosomal recombination, and conducted a series of experiments to determine the influence of XPF heterozygosity in UV41 cells on its recombination phenotype. Our results show that I-SceI-induced DSBs significantly affected the recombination phenotypes of both AA8 and UV41 cell lines. As expected, DSBs dramatically increased the overall frequencies of recombination in both cell lines and stimulated SSA in wild-type cells. In addition, a small number of recombinants characterized by deletion/rearrangement structures was induced by DSBs in AA8 cells. Surprisingly, this aberrant recombination phenotype was exhibited to a relatively high degree in non-induced UV41 cells, but was not further enhanced by DSB induction. Our results also show that UV41 cells are somewhat deficient in generating gene conversions, supporting results of a recent study of CHO ERCC1 mutant UV4 cells demonstrating that DSB-induced gene conversions require ERCC1 [18]. Taken together, the results of our study show that XPF deficiency leads directly to a persistent, DSB induction-independent derangement of pathways regulating recombination between directly repeated sequences in mammalian cells.
2. Materials and methods
2.1. Plasmids and cell line constructions
Plasmids pGS100 and pAK45 were constructed and used in previous studies of intrachromosomal recombination in CHO cells [4,5,21]. In the present study, we used both plasmids to modify cell lines derived from CHO AA8 (wild-type) and UV41 (XPF mutant) cells in a two-step gene modification procedure [21]. Briefly, after isolating APRT− hemizygotes of AA8 and UV41 using selection in 8-azaadenine (8-AA) [22], we used targeted gene correction [2] to integrate plasmid pGS100 at the endogenous APRT locus in each cell line. After verifying the integrated structures by Southern analysis, we selected for “pop-out” recombinants using 8-AA and gancyclovir (GANC), resulting in AA8- and UV41-derived cell lines which each contained one copy (the same allele) of APRT, which was inactive due to a pre-existing EcoRV site-loss transversion mutation in exon 2. The APRT allele in each cell line retained a Flp recombination target (FRT) sequence in intron 3, donated from pGS100. Each of these “founder” cell lines was further modified to contain a direct repeat heteroallelic APRT intrachromosomal recombination substrate by FLP/FRT targeting with pAK45. These procedures are described in detail in references [5] and [21], and resulted in wild-type, AA8-derived cell line AA8-AK45 and XPF mutant, UV41-derived cell line U41-AK45. The end result was a pair of cell lines, derived respectively from XPF mutant UV41 and its parent, AA8, which each contained exactly the same recombination substrate (integrated pAK45) fixed at the endogenous, hemizygous APRT locus.
Other plasmids used in this study were the I-SceI expression vector, pCMV-I-SceI (kindly provided by Maria Jasin, Memorial Sloan-Kettering, New York NY), the luciferase reporter plasmid pRL-CMV (Promega, Madison WI), and pCMV-Empty (pCMV-E), an empty vector containing the CMV promoter that was constructed by removing the luciferase gene from pRL-CMV. In addition, two XPF expression vectors were constructed in this study from plasmid pCDN3.1 (−) Hygromycin (Invitrogen, Carlsbad CA); pLDCXPF42 contained the wild-type XPF cDNA and pLDCXPF43 contained a XPF cDNA cloned from UV41 with the exon 8 mutation we identified in this study (see 2.3 below).
2.2. Cell culture and selection conditions
All cell lines were cultured in alpha-modified Minimal Essential Medium (α-MEM) containing 10% fetal calf serum (FCS) in a humidified atmosphere of 5%CO2/95% air at 37 °C, unless otherwise stated. For selection of APRT− clones, a-MEM containing 10% dialyzed FCS (DFCS) and 8-azaadenine (8-AA) at a concentration of 0.4 mM was used; for counterselection against the HSV-TK marker contained in plasmids and intrachromosomal recombination substrates, gancyclovir (GANC) at a concentration of 0.4 mM was added to a-MEM containing 10% DFCS. In some recombination assays, co-selection with both 8-AA and GANC was used to isolate APRT−/HSV-TK− clones (see 2.5 below).
2.3. Characterization of the UV41 XPF mutation and XPF expression levels in UV41 and AA8 cell lines
To perform DNA sequence analysis in CHO cells, it was necessary to clone and sequence the CHO cell XPF full-length cDNA. A lambda FIXII CHO genomic library purchased from Stratagene (La Jolla, CA) was screened using a 910 bp fragment of the human ERCC4 cDNA fragment (generously provided by Dr. L. Thompson, Lawrence Livermore National Laboratory, Livermore CA) and sequencing of DNA from positive plaques revealed CHO cell XPF sequences on several lambda clones; from this information, oligonucleotide primers were designed which were used to amplify the full-length CHO cell XPF cDNA sequence - Forward: 5′-ATGGACCGGGGCATATCG-3′ and Reverse: 5′-TCATTTCTCACTCTGCCTCCG-3′.
To characterize the XPF mutation in UV41 cells, we adopted a strategy of first sequencing cDNA clones generated from RNA and then confirming any putative mutations by genomic sequencing; we previously used this strategy to identify the mutations in several CHO cell ERCC1 mutants [17]. Total RNA was isolated from cell pellets (5 × 106) using TRIzol Reagent (Invitrogen) according to the supplier's directions. After purification and suspension in nuclease-free water, RNA was treated with DNase I for 15 min at ambient temperature and cDNA synthesis was performed using the SuperScript II First Strand synthesis kit, followed by PCR amplification using random hexamers. Amplimers generated using XPF-specific primers that amplified the entire cDNA sequence (see above) were subcloned into the pCR-Blunt II TOPO or pCR 2.1-TOPO cloning vectors (Invitrogen) and sequenced using the BigDye Terminator cycle sequencing kit on an ABI Prism 377 DNA Sequencer (Applied Biosystems, Foster City CA). Genomic sequencing of XPF exon 8 was performed to verify results of the sequencing analysis of cDNA clones, using exon 8 specific sequencing primers Forward: 5′-TGAGAAGGACAGCAAAGCCCG-3′ and Reverse 5′-CAGAACCGCCAAGCACAAAC-3".
Real-time PCR (RT-PCR) analysis was used to measure XPF expression levels in cell lines. Total RNA was isolated from AA8 and UV41-derived cell lines using RNA-Stat60 according to the manufacturer’s recommendations (Tel-Test, Friendswood TX), and quantified using the Nanodrop ND-1000 spectrophotometer. A total of 1 µg RNA was reverse transcribed using the Superscript II First Strand synthesis kit (Invitrogen). From this reaction, 1 µl of 20 µl total was used for RT-PCR analysis. Two-step RT-PCR was performed using the Taqman Universal PCR Master Mix (Applied Biosystems) on the ABI Prism 7700 Sequence Detection System. Stage 1 of the reaction was carried out at 50 °C for 2 minutes to allow for uracil-N-glycosylase degradation of possible PCR product carryover contamination, followed by stage 2 denaturation at 95 °C for 10 minutes. Stage 3 consisted of reaction at 95 °C for 15 seconds followed by annealing/extension at 60 °C for 1 minute for a total of 40 cycles. Real time PCR primer and probe sequences for XPF (XPFForward: 5′-GAGTGATGAGGAACCTTTTTGTGA-3′; XPFReverse: 5′-TGTTGTTCCAAGAACGAGTTGACT-3′; XPFProbe: 5′/56-FAM/AAGCTCTACCTGTGGCCAAGGTTCCATGTA/36-TAMTph/-3′) and 18S RNA (18SS1: 5′-TGCCGGAGTCTCGTTCGT-3′; 18SAS1: 5′-GGTGCATGGCCGTTCTTAGT-3′; 18SProbe: 5′-/56-FAM/TCGGCCTTAACCAGACAAATCGCTCCAC/36-TAMTph/-3′) were designed using PrimerExpress software (Applied Biosystems). Both probes were designed with 6-carboxyfluorescein (FAM) as the reporter molecule and 6-carboxytetramethyl-rhodamin (TAMRA) as the quencher. The relative comparison ΔΔCt method for RT-PCR [23] was used to evaluate normalized XPF expression levels (normalized to 18S RNA) in AA8- and UV41- derived cell lines.
Northern blotting was used as a semi-quantitative measurement to confirm differences in the levels of XPF transcripts between the two cell lines. Northern blotting was performed as previously described [17] on 2 µg samples of polyA RNA isolated from cell lines using the polyA Pure Kit (Ambion, Austin TX) and probed with the XPF cDNA probe; blots were stripped and re-probed with GAPDH probe as a loading control.
2.4. Complementation of cells with mutant XPF cDNA
CHO XPF cDNA expression vectors were constructed by amplifying cDNAs from AA8 and UV41 cell lines by PCR, then ligating with expression plasmid pCDN3.1 (−)Hygro (Invitrogen) using ApaI and KpnI restriction endonuclease sites, resulting in wild-type XPF expression vector pLDCXPF42 and mutant XPF expression vector pLDCXPF43, which recapitulates the exon 8 mutation in the UV41 mutant allele; both vectors contain the hygromycin cassette to enable selection for stable transfectants.
UV41 or AA8 cells were electroporated with pLDCXPF42 or pLDCXPF43 and independent hygromycin-resistant clones were picked, expanded and tested for sensitivity to mitomycin C (MMC). Cells were plated into 6-well dishes and refed 24 hours later with media containing 0, 7.5, 18.75, 37.5, 75 or 187.5 nM MMC, then stained and examined under the microscope after 72 hours. Control plates with AA8 and UV41 cells were also MMC treated.
2.5. Determination of DSB- induced and non-induced intrachromosomal recombination frequencies
Independent populations of each cell line (AA8-AK45 and U41-AK45) were initiated at 50 cells/well in 12-well cell culture dishes. After 5–6 days, populations were trypsinized, counted and divided into 3 subpopulations by replating into triplicate 12-well dishes. The first subpopulation (designated L) was Fugene-treated with a luciferase expression plasmid. The second subpopulation (designated N) was treated with pCMV-E, an empty vector containing the CMV promoter. The third subpopulation (designated I) was treated with the I-SceI expression vector pCMV-I-SceI to site-specifically induce a double-strand break in the HPRT-derived sequence contained as a non-homologous insertion in the downstream APRT heteroallele. Fugene treatments were initiated 24 hours after division of the cell cultures into subpopulations, using (per well) 2.4 µl Fugene and 0.8 µg plasmid DNA dissolved in 80 µl SFM. N and I subpopulations were expanded to 107 cells each in T-75 flasks and the N populations were plated into selection media containing 10% dialyzed FCS: 0.4 mM 8-azaadenine was added for APRT− selections (5 plates/flask) and 0.4 mM 8-AA + 0.4 µM GANC was added for APRT−/HSV-TK− selections (10 plates/flask); I populations were plated at 1/20 the plating densities used for N populations. One independent clone from each culture and selection condition was chosen for analysis by Southern blotting, and PCR analysis. Corrected induced recombination frequencies were determined by subtracting the N recombination frequency from the I recombination frequency for each independent population. Recombination frequencies for each selection condition were determined by dividing the total number of recombinant clones by total number of viable cells plated. The corrected recombination frequency is represented as the average ± standard error of the mean (S.E.M.) with significance (p) determined by an analysis of variance (ANOVA) test.
To assess any differences in transfection efficiencies between AA8-AK45 and U41-AK45 cell lines, subpopulations (L) were transfected with the pRL-CMV vector (Promega, Madison, WI) and assayed for luciferase activity 24 hours post-transfection according to manufacturer’s instructions. An arbitrary unit of relative light units/cell number was calculated for each independent subpopulation and compared between the AA8-AK45 and U41-AK45 cell lines. Significance was analyzed through an ANOVA test.
2.6. Analysis of recombinant structures
Southern analysis was performed as previously described [5] on DNA samples isolated from cell pellets (approx. 2 × 107 cells) using GenElute spin columns (Sigma, St. Louis MO), according to the manufacturer’s instructions. Analysis of restriction fragment patterns enables the deduction of recombinant structures, facilitated by the facts that APRT is hemizygous in AA8-AK45 and U41-AK45 cell lines and the integrated pAK45 recombination substrate is fixed at the endogenous APRT locus. For determination of APRT− gene conversions in which the EcoRV transversion mutation in exon 2 is transferred from the downstream to the upstream APRT heteroallele, PCR was used to discriminate between recombinants and background mutations: PCR analysis of the APRT exon 1 – exon 2 region shows that neither amplimer is digested by EcoRV in conversion recombinants, and reveals a 1:1 distribution of products digested with EcoRV in APRT− mutants. Any population determined in this way to be an APRT− mutant was discarded from all calculations and statistical analyses. Statistical analysis of the proportional distribution of recombinants in non-induced and induced condtions was performed using Fisher’s exact test.
3. Results
3.1. Results of XPF mutation analysis in UV41 cells
We first generated cDNA clones from RNA isolated from wild-type CHO cell line AA8 and XPF mutant UV41 and sequenced the cDNA inserts to identify any putative mutations in UV41. Sequencing of 10 independently cloned full-length cDNA amplimers generated from CHO AA8 cells (wild-type) revealed no deviation from the wild-type CHO XPF cDNA sequence (GenBank Accession Number 1064271). However, sequencing of 12 independently cloned full-length cDNA amplimers from UV41 cells showed that 5 clones were wild-type, and that 7 clones had a +1 G insertion in a run of 4 Gs in exon 8. This insertion mutation was confirmed by genomic sequencing of DNA isolated from AA8 and UV41 cell lines. Results shown in Fig. 1 indicate the location of the UV41 mutation in exon 8 of the CHO XPF gene. Of the two XPF alleles in UV41, the mutated allele has a +1 G insertion in a GGGG run that begins at nucleotide 1509 of the CHO XPF cDNA; this causes a frameshift that results in a premature stop codon as shown in Fig. 1. These results indicate that UV41 is heterozygous for XPF, with one wild-type and one mutant allele, both of which are transcribed.
Figure 1. Identity and location of the nonsense mutation in one XPF allele of UV41 cells.
DNA sequencing of expressed XPF cDNA, confirmed by genomic sequencing, reveals a “G” insertion after position 1513 in exon 8 of the wild-type CHO cell XPF gene. This insertion causes a frameshift in one XPF allele of UV41 which results in a premature stop codon (X) being generated at amino acid position 499. Asterisks indicate amino acid substitutions in the predicted mutant UV41 XPF allele.
3.2. Results of XPF expression analysis in AA8 and UV41 cell lines
Since our results (section 3.1) indicated that UV41 is a XPF heterozygote, apparently expressing both wild-type and mutant alleles, it was not obvious why it exhibits its DNA repair deficient phenotype. One possible explanation was that XPF expression might be suppressed in UV41 cells, since in a XPF knock-in mouse model with an exon 8 mutation, such was found to be the case [24]. Therefore, to determine the relative expression levels of XPF transcripts in AA8 and UV41 cell lines, we used real-time PCR methods as described above (section 2.3). The relative comparison ΔΔCt method for RT-PCR [23] was used to compare XPF expression levels (normalized to 18S RNA) in AA8- and UV41- derived cell lines. These results showed that XPF is expressed at ~ 6-fold higher levels in AA8 compared to UV41 (ΔΔCt values for XPF relative to 18S RNA were calculated as 5.95 for AA8 and 1.0 for UV41 samples). Results of northern blotting (Fig. 2) confirmed that XPF transcript is expressed in both cell lines, at noticeably higher levels in AA8 compared to UV41 cells, supporting the results of RT-PCR analysis.
Figure 2. Northern blot analysis of XPF expression in AA8 and UV41 cell lines.

Lane 1: RNA from AA8 cells; Lane 2: RNA from UV41 cells. The top panel was probed with hamster XPF sequence, as described in Materials and methods; the bottom panel is the same nylon membrane stripped and re-probed with GAPDH sequence.
3.3. Results of MMC sensitivity tests of complemented UV41 and AA8 cell lines
Another possible explanation for the DNA repair deficient phenotype of UV41 was that the XPF mutant allele in UV41 confers a dominant negative phenotype. To test this possibility, we constructed two expression vectors, pLCDXPF42 ( containing wild-type XPF cDNA) and pLCDXPF43 (containing UV41 exon 8 mutant XPF cDNA) as described in section 2.1; these expression vectors carry the hygromycin resistance marker and drive cDNA expression from a CMV promoter. To demonstrate complementation of the extreme cytotoxicity exhibited by UV41 to mitomycin C (MMC) [14], we electroporated each expression vector into UV41 cells and isolated independent hygromycin-resistant clones; these clones were then individually tested in multiwell dishes for survival (colony formation) in MMC as described in section 2.4. The results were as follows: Of 20 independent, hygromycin-resistant UV41 clones stably transformed with plasmid pLDCXPF42 (wild-type XPF cDNA), 14 exhibited MMC sensitivity comparable to wild-type AA8 cells; Of 20 independent, hygromycin-resistant UV41 clones stably transformed with plasmid pLDCXPF43 (exon 8 mutant XPF cDNA), none exhibited MMC sensitivity comparable to wild-type AA8 cells, but instead resembled untransfected UV41 cell control wells (i.e., no colony formation was observed). To determine if the exon 8 mutated XPF allele could confer a dominant negative phenotype, we electroporated AA8 cells with pLCDXPF43, selected independent hygromycin-resistant clones and tested these for MMC sensitivity as described in section 2.4. When AA8 cells were transformed with pLDCXPF43, none of the 20 independent, hygromycin-resistant clones tested showed any effect on MMC sensitivity (i.e., all clones exhibited wild-type sensitivity), indicating that expression of the exon 8 mutant XPF allele in UV41 cells does not induce MMC sensitivity in wild-type AA8 cells. Thus, results with the two XPF expression vectors showed (i) that wild-type XPF cDNA complemented the MMC-sensitivity phenotype of UV41, whereas the UV41 exon 8 mutant XPF cDNA did not, and (ii) that the exon 8 mutant XPF allele recovered from UV41 did not induce a dominant negative phenotype in wild-type AA8 cells.
3.4. Results of DSB-induced intrachromosomal recombination in AA8- and UV41-derived cell lines
After determining the nature of the XPF deficiency in UV41 cells, we performed recombination assays designed to interrogate specific recombination pathways in which ERCC1-XPF would be predicted to function. The structure of the direct repeat heteroallelic APRT construct in AA8-AK45 and U41-AK45 cell lines in shown in Fig. 3A (top). In this intrachromosomal recombination substrate, the upstream APRT copy harbors a transversion mutation in exon 2 (vertical line) and is truncated at its 3’ end (black bar), whereas the downstream APRT copy is functional, but has a large ~900 bp insertion of heterologous DNA (derived from human HGPRT) in intron 3 [5]; the 18-bp I-SceI recognition site (vertical arrow) is contained within the HGPRT-derived heterologous sequence. The two APRT heteroalleles are separated by ~ 9.9 kb of sequence containing the HSV-TK and GPT markers. Expression of I-SceI endonuclease in cells causes induction of site-specific DSBs within the HGPRT-derived heterology embedded in intron 3 of the downstream APRT copy in AA8-AK45 and U41-AK45 cell lines. After induction of DSBs and exonucleolytic processing of the ends, lengthy single-stranded non-homologous “tails” are generated that block the ends of APRT homologous sequence in the downstream APRT copy [5]. ERCC1-XPF endonuclease is predicted to be required for incision and removal of these non-homologous tails to allow the resolution of recombination intermediates into the ‘legitimate’ products shown in Fig. 3A, a and b, as crossover and conversion structures, respectively; structure c shown in Fig. 3A represents a variety of inappropriate resolutions which are characterized by variable sized deletions and rearrangements.
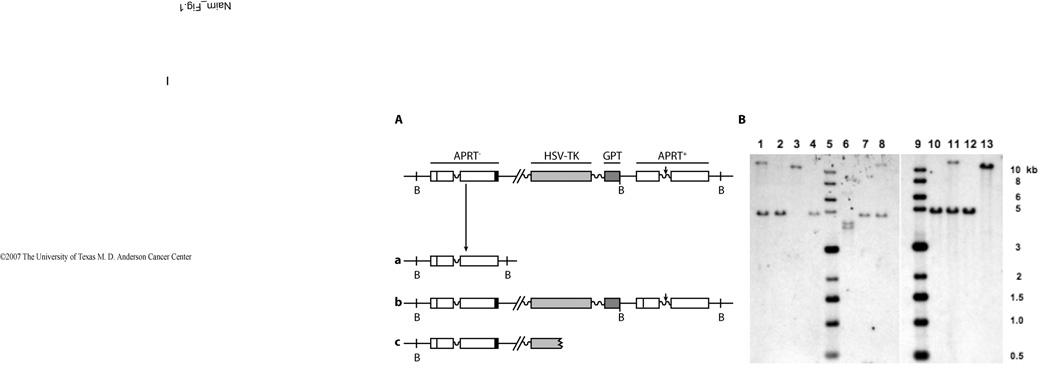
Figure 3. Recombinant APRT structures and representative results of Southern blot analyses.
A. Structure of the pAK45 recombination reporter substrate integrated at the endogenous APRT locus in CHO cell lines AA8-AK45 and U41-AK45. The upstream APRT heteroallele is inactive, due to a transversion mutation in exon 2 (vertical line) which results in loss of an EcoRV site, and a deletion at its 3’ end resulting in loss of exon 5 (black bar). The downstream APRT heteroallele is functional, and contains an I-SceI site (vertical arrow) embedded in heterologous DNA in APRT intron 3 (wavy line). Two markers, HSV-TK and GPT are contained in the intervening DNA sequence donated by plasmid pAK45. Different types of recombinant structures can be generated from the heteroallelic, direct repeat APRT recombination substrate contained in AA8-AK45 and U41-AK45 cell lines after selection against APRT function (recombinant products a, b and c). Structure “a” shows the result of the precise deletion of all plasmid-donated sequences generated from the integration of pAK45 into the endogenous APRT locus (crossover product); this recombination event regenerates the original APRT allele in AA8- or U41-derived cell lines. Structure “b” (conversion product) retains the heteroallelic duplication, but the downstream APRT copy picks up the inactivating exon 2 transversion mutation (vertical line) from the upstream copy. Structure “c” is characterized by random breakage downstream from the inactive APRT allele. B. Examples of Southern blots analyzing DNA from induced (I) and non-induced (N) recombinant clones; DNA was digested with BamHI and probed with radiolabelled APRT sequence as described in Materials and methods. Lanes 1: AA8-AK45 DNA control; 2: non-induced U41-AK45 recombinant (a); 3: non-induced U41-AK45 recombinant (c); 4: non-induced U41-AK45 recombinant (a); 5: molecular size ladder; 6: induced AA8-AK45 recombinant (c); 7: non-induced AA8-AK45 recombinant (a); 8: induced AA8-AK45 recombinant (a); 9: molecular size ladder; 10: non-induced U41-AK45 recombinant (a); 11: induced U41-AK45 recombinant (b); 12: induced U41-AK45 recombinant (a); 13: non-induced U41-AK45 recombinant (c).
Two selection regimes were used. In selecting against both APRT and HSV-TK function by using both 8-AA and GANC in selection media, crossover recombinants, which have a structure reflecting the precise deletion of the pAK45 plasmid sequences from the genomic integration site (Fig. 3A, a), would be predicted to predominate in wild-type cells, because of selective pressure against retaining the HSV-TK marker. On the other hand, selection against APRT function alone by using only 8-AA reports both conversion and crossover recombinants. Since single-strand annealing (SSA) is accepted as the most likely mechanism to generate crossovers from direct repeat substrates in mammalian cells [25−27], the 8-AA +GANC selection “window” primarily interrogates the SSA pathway, whereas 8-AA selection alone provides a wider selection window and allows examination of the role of ERCC1-XPF in other recombinant resolution pathways in addition to SSA.
Table 1 presents corrected DSB-induced recombination frequencies for each selection condition (i.e., selection for the APRT−/HSV-TK− phenotype in 8-AA + GANC, and selection for only the APRT− phenotype in 8-AA alone). Corrected DSB-induced frequencies were calculated by subtracting the recombination frequency for a population treated with empty vector (N, or non-induced) from the frequency of the same population treated with the pCMV-I-SceI vector (I, or induced) as described in “Materials and methods.” These corrected frequencies indicate a significant decrease of 2.4-fold (p<0.001, ANOVA) in U41-AK45 induced frequencies for selection of APRT−/TK− recombinants compared to wild-type AA8-AK45 cells; there was no significant difference observed between the corrected DSB-induced frequencies for selection of APRT− recombinants in independent populations of the two cell lines. Results of luciferase assays in replicate samples, performed 24 hours after pRL-CMV transfection, showed no statistically significant differences in transfection efficiencies between AA8-pAK45 and U41-pAK45 populations.
TABLE 1.
Double-strand break-induced recombination frequencies in AA8-AK45 (XPF+) and U41-AK45 (XPF−) cell lines.
| APRT−/TK− | APRT− | |
|---|---|---|
| Induced Frequency* | Induced Frequency* | |
| Cell Line |
(Average ± SEM) × 10−3 |
(Average ± SEM) × 10−3 |
| AA8-AK45 | 1.48 ± 0.12 | 2.91 ± 0.26 |
| (n = 35) | (n = 35) | |
| U41-AK45 | 0.61 ± 0.06 | 2.75 ± 0.27 |
| (n = 40) | (n = 39) |
Induced frequencies (I) corrected for background (N); (see text)
Our statistical treatment of the induced frequency data, as presented in Table 1, was based on the experimental design of seeding replicate, parallel subpopulations and subtracting frequencies observed in a N subpopulation from its corresponding I subpopulation (see section 2.5). However, additional comparisons are available by considering the uncorrected average frequency data, which are presented in Table 2 (row 3). For example, to examine the effects of DSB induction on the generation of recombinants, the averages of uncorrected frequencies from N and I populations can be separately compared. These comparisons reflect a 71.9-fold increase in DSB-induced recombination in the wild-type AA8-AK45 cell line (i.e., 20.9 to 1500 × 10−6), compared to 18.7-fold (34.4 to 631 × 10−6) in U41-AK45 cells for induction of APRT−/HSV-TK− recombinants. Likewise, DSB-induced recombination generating APRT− recombinants resulted in an induction of 29-fold in AA8-AK45 cells and 14.5-fold in U41-AK45 cells. Although N values for U41-AK45 are higher than N values for AA8-AK45 in both selection conditions, these differences were not statistically significant within each selection regime.
TABLE 2.
Distribution of APRT recombinant products in uninduced (N) and I-SceI-induced (I) AA8- and UV41- derived cell lines.
| Phenotype | APRT−/HSV-TK− | APRT− | ||||||
|---|---|---|---|---|---|---|---|---|
| Cell Line | AA8-AK45 | AA8-AK45 | U41-AK45 | U41-AK45 | AA8-AK45 | AA8-AK45 | U41-AK45 | U41-AK45 |
| Condition | N | I | N | I | N | I | N | I |
| Overall Frequency of Recombination Events ( × 106) | 20.9 ± 6.9 (n = 31) | 1500 ± 130 (n = 29) | 34.4 ± 8.9 (n = 30) | 641 ± 61 (n = 29) | 104 ± 13 (n = 25) | 3020 ± 260 (n = 29) | 204 ± 61 (n = 22) | 2960 ± 260 (n = 27) |
| Precise Deletion Events (Crossover -type structures) | 31/31 (100%) | 27/29 (93%) | 21/30 (70%) | 19/29 (66%) | 5/25 (20%) | 15/29 (52%) | 7/22 (32%) | 10/27 (37%) |
| Gene Conversion Events | 0/31 (0%) | 0/29 (0%) | 0/30 (0%) | 0/29 (0%) | 20/25 (80%) | 12/29 (41%) | 7/22 (32%) | 6/27 (22%) |
| Deletion/Rearrangement Events | 0/31 (0%) | 2/29 (7%) | 9/30 (30%) | 10/29 (33%) | 0/25 (0%) | 2/29 (7%) | 8/22 (36%) | 11/27 (41%) |
3.5. Distribution of recombinant products depending on induction conditions and XPF status
To evaluate how XPF heterozygosity and deficiency in UV41 cells affected DSB-induced intrachromosomal recombination, Southern analysis was performed on independent recombinants isolated from N and I experimental regimes. Only one clone was analyzed from each population to ensure independence of events. Representative results of Southern analyses showing structures of different recombinant classes are presented in Fig. 3B. Results presented in Table 2 show the distributions of recombinant products for AA8-AK45 and U41-AK45 in induced (I) and non-induced (N) conditions. Selection in 8-AA + GANC (for APRT−/HSV-TK−recombinants) would be expected to result predominantly in precise deletion of the integrated pAK45 sequence and generate recombinants with crossover structures in repair proficient cells [5]. This expected result was observed in non-induced parental AA8-AK45 cells; however, I-SceI induction of DSBs in the downstream APRT heteroallele (see Fig. 3A) caused not only a dramatic increase in the overall frequency of recombination (~ 72-fold), but also generated recombinants reflecting deletion/rearrangement structures (see Fig. 3A, c), which were not observed in AA8 cells in non-induced conditions. By contrast, in U41-AK45 cells a substantial proportion (30%, p<0.001) of APRT−/HSV-TK− recombinants showing deletion/rearrangement was generated in even in non-induced conditions, and I-SceI induction of DSBs, while markedly increasing the overall recombination frequency (~ 18-fold) did not appreciably increase the proportion of recombinants in this category (33%). When selection was applied only for the APRT− phenotype, allowing recovery of conversion recombinants in addition to crossovers, the several-fold suppression in the UV41 cell background of induced recombination frequency relative to AA8 was apparently relieved; the corrected, induced recombination frequencies for both cell lines were thus approximately the same (see Table 1). However, DSB induction in AA8-derived cells increased the proportion of crossover-type recombinants from 20% to 52% (p=0.024, Fisher’s exact test), and halved the gene conversion fraction (from 80% to 41%, p=0.006, Fisher’s exact test); as in the case of APRT−/HSV-TK−selections, deletion/rearrangement recombinants were only generated in the case of DSB induction in AA8-AK45 cells. In U41-AK45 cells, as in selections for APRT−/HSV-TK− recombinants, selection solely for APRT− recombinants after I-SceI induction revealed an unaltered distribution of recombinant classes, even though there was a substantial increase in induced recombinants overall.
Collectively, the results of these analyses show that the XPF mutation in UV41 cells which results in its hypomorphic phenotype causes a persistent instability of the integrated pAK45 recombination reporter substrate. This instability is reflected in significant increases compared to wild-type cells in deletion/rearrangement recombinants generated from both non-induced (p<0.001) and DSB-induced (p=0.021) experiments when selecting for the APRT−/HSV-TK− phenotype, which interrogates primarily the SSA pathway. Similar statistically significant differential increases in the proportions of deletion/rearrangement recombinants between wild-type and UV41 cells in non-induced (p<0.001) and induced (p=0.004) conditions were observed in APRT− selections, which allows recovery of gene conversion as well as SSA recombinants. Furthermore, results of Table 1 and Table 2 reveal that DSB-induced generation of recombinants by SSA is significantly suppressed in UV41 compared to wild-type AA8 cells (Table 1), while selection for the APRT− phenotype alone shows that gene conversions per se were also suppressed in UV41 relative to AA8 in both non-induced and DSB-induced conditions (Table 2). Induction of DSBs by I-SceI in all cases dramatically increased the generation of recombinants, but surprisingly had no apparent effect on the proportion of deletion/rearrangement recombinants generated in U41-AK45 cells, suggesting that the underlying genomic instability in XPF hypomorphic UV41 cells is a determinative mechanistic factor in the pathway leading to these events.
4. Discussion
One of the goals of this study was to determine the nature of the XPF mutation in the UV41 cell line. UV41 is one of only a few CHO cell XPF mutants isolated in the series of large screens of mutagenized AA8 cells performed by Larry Thompson, David Busch and their colleagues in the early 1980s [11,12]. UV41 cells exhibit hypersensitivity to UV radiation and other DNA damaging treatments that induce adducts requiring NER for repair. In addition, UV41 cells show extreme hypersensitivity to agents causing DNA interstrand crosslinks (ICLs), sharing this phenotype only with ERCC1 defective cells among mammalian NER mutants [13,14]. Our results demonstrate unambiguously that UV41 is a XPF heterozygote, expressing both wild-type and mutant alleles. Although our results also showed that XPF expression was significantly lower in UV41 than AA8 cells, it was surprising that this condition results in such pronounced cytotoxicity phenotypes, especially regarding ICL sensitivity. Since transfection of wild-type AA8 cells with a XPF cDNA expression vector containing the exon 8 mutation (pLCDXPF43) failed to induce a ICL hypersensitive phenotype (see section 3.3), we conclude that the truncated XPF protein predicted by the position of the exon 8 mutation is not conferring a dominant negative phenotype. It remains possible, however, that the XPF mutation in UV41 could exert a weak dominant negative effect in conditions of low XPF expression, since XPF expression is much lower (~6-fold) in UV41 than in AA8 cells. However, suppression of XPF expression in UV41 also may be due to nonsense-mediated mRNA decay [28]. It is notable that a nonsense mutation in XPF exon 8 at glycine 445, also generating a stop codon, was introduced by knock-in into mice to construct a mutant XPF mouse, and the phenotype reflected a significant suppression (> 10-fold) of XPF transcription as measured by RT-PCR [24]. Considering these observations, it is likely that UV41 is hypomorphic for XPF and that this condition underlies its cellular phenotypes.
The other goal of this study was to investigate the effect of XPF deficiency specifically on mitotic recombination. ERCC1 deficient CHO cell mutants have been used by a number of investigators, including ourselves, to address a variety of questions concerning the role of ERCC1-XPF in different pathways of homologous recombination. Gene targeting studies have shown that the ERCC1-XPF structure-specific endonuclease is required for removal of non-homologous sequences blocking strand invasion and allowing efficient targeted gene correction [2] and gene disruption [3]. We previously showed that ERCC1 is required to suppress rearrangements and deletions generated when nonhomologous DNA sequences block the resolution of intermediates formed during spontaneous intrachromosomal homologous recombination between directly repeated sequences [4]. A recent study by Al-Minawi et al. [18] used ERCC1 mutant UV4 cells to show that ERCC1-XPF was required both for efficient SSA and gene conversion. Their study employed an intrachromosomal direct-repeat recombination substrate in which DSBs introduced by I-SceI in an upstream NEO-based heteroallele initiated recombination. This recombination reporter assay differs from the one used in our present study in several ways: (i) In their assay, both NEO heteroalleles are inactive, and the endpoint selection requires restoration of NEO function, whereas our assay reports inactivation of the one active APRT duplication heteroallele in pAK45; (ii) The I-SceI site in their assay is contained within NEO homology, whereas in pAK45, the I-SceI site is within a large heterologous insertion in the (active) downstream APRT heteroallele; (iii) The NEO recombination reporter is randomly integrated in their assay, whereas the integrated pAK45 substrate is fixed at the endogenous CHO APRT locus. Despite these differences, our results using XPF mutant UV41-derived cells are in general agreement with, and support, the conclusions by these workers using ERCC1 mutant UV4-derived cells. Both UV4 and UV41 were isolated from the same CHO parental cell line, AA8 [11–14].
We previously used the integrated pAK45 recombination reporter substrate in experiments with ERCC1 knock-out cells [5], but did not measure conversions. In this previous study, we showed that induced DSBs in ERCC1 knock-out cells dramatically enhanced recombination, to a similar degree as we observed in the present study in XPF deficient cells [5]. However, in ERCC1 knock-out cells, there was ~ 30-fold suppression of APRT−/HSV-TK− recombinants with crossover structures, arising from SSA, compared to 2.4-fold suppression of SSA generated crossover recombinants in UV41 cells observed in the present study. There may be several reasons for this discrepancy. One obvious possibility is that, since UV41 is hypomorphic for XPF and not completely deficient, there could be residual XPF function that results in more DSB-induced stimulation of SSA compared to ERCC1 knock-out cells. We also note that the I-SceI stimulation results for ERCC1 knock-out cells from our previous study were based on only 2 experiments, and showed significant experimental variation. In any case, our results here confirm the necessity for fully functional ERCC1-XPF to realize the maximum stimulation of DSB-induced recombination which results in generation of crossover-type recombinants arising from SSA.
In the present study, we also subjected the I-SceI-induced cell populations to selection in 8-AA alone, which reports both conversion and crossover recombinants. In wild-type AA8-AK45 cells, induced DSBs increased the recombination frequency, and also shifted the proportions of conversions and crossovers from a ratio of 4:1 (conversions:crossovers) in non-induced cells to approximately 1:1 in I-SceI-induced cells (Table 2). The 4:1 ratio in non-induced cells is comparable to the preference for conversions observed in similar experiments performed in other wild-type mammalian cell lines [29]. The shift in this ratio after DSB induction is consistent with the channeling of recombination intermediates from conversion to crossover resolutions that would be predicted from DSB stimulation of SSA, which requires ERCC1-XPF [5,18]. In UV41-AK45 cells, in spite of robust stimulation of recombination by I-SceI, the spectrum of APRT− recombinants was not significantly altered in induced and non-induced conditions (Table 2, columns 7 and 8). However, conversions were significantly reduced in non-induced UV41-AK45 cells compared to non-induced AA8-AK45 cells (p < 0.001) and imprecise deletion/rearrangement recombinants were significantly increased in UV41-AK45 cells compared to AA8-AK45 cells in both non-induced (p < 0.001) and induced (p < 0.004) conditions. The significant elevation of these "illegitimate" recombination events in UV41-derived cells supports a requirement for ERCC1-XPF to precisely remove nonhomologous sequences that would otherwise interfere with the resolution of SSA recombination intermediates. Furthermore, the defective crossover recombination phenotype of UV41-AK45 cells, as reflected by the large proportion of deletion/rearrangement clones from APRT−/TK− selection experiments, is mirrored in the APRT− selection results. Interestingly, however, in both induced and non-induced conditions, the mechanism for resolving recombination intermediates leading to conversions is apparently suppressed in XPF mutant UV41 compared to wild-type cells, suggesting a role for ERCC1-XPF in the pathway leading to gene conversions; our observation in this regard thus supports the conclusions of Al-Minawi et al. [18] that ERCC1-XPF is necessary for both SSA and gene conversion pathways. In Saccharomyces cerevisiae, RAD1 mutants exhibit reduced conversion tract lengths [30], and it is possible that a similar defect in UV41 cells contributes to this recombination phenotype.
Finally, our results show that UV41 exhibits a recombination phenotype of instability at direct repeat sequences, manifested as significantly elevated frequencies of deletion/rearrangement recombinants compared to wild-type cells (up to 41% of recombinants; see Table 2). Surprisingly, this instability is independent of DSB stimulation of the SSA pathway, which would be expected to lead to increased crossover-type recombinants. Since, as discussed above, conversions are apparently suppressed in UV41- relative to AA8-derived cells, this suggests that the hypomorphic XPF insufficiency in UV41 results in a persistent alteration of pathway choice for resolving direct repeat recombination intermediates, which is independent of whether or not induced DSBs contribute more SSA intermediates. Many progeroid syndromes exhibit various types of genomic instability [20,31,32], and this XPF phenotype in particular may be important to the recent implication of ERCC1 and XPF genetic defects in aging and aging pathologies. For example, two patients exhibiting premature aging were recently described, one affected in XPF and the other in ERCC1 [33,34]. The complexity of ERCC1 and XPF recombination phenotypes, as described in this and previous studies [2–5,18], may underlie to some extent the newly described progeroid syndrome XFE [33,35]. In this condition, some XPF and ERCC1 mutations in humans, as well as a hypomorphic ERCC1 mouse model [36], display premature aging and metabolic derangement. Further studies of aberrant recombination, as well as other ERCC1 and XPF deficiency phenotypes, may lead to greater insight into the role of ERCC1-XPF in maintaining genomic stability and mitigating both premature aging and cancer susceptibility.
Acknowledgements
We thank Christine Brown for artwork and Becky Brooks for assistance in manuscript preparation. This work was supported by grant CA097175 from the National Cancer Institute and NIEHS Center of Excellence award ES007784.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bessho T, Sancar A, Thompson LH, Thelen MP. Reconstitution of human excision nuclease with recombinant XPF-ERCC1 complex. J. Biol. Chem. 1997;272:3833–3837. doi: 10.1074/jbc.272.6.3833. [DOI] [PubMed] [Google Scholar]
- 2.Adair GM, Rolig RL, Moore-Faver D, Zabelshansky M, Wilson JH, Nairn RS. Role of ERCC1 in removal of long non-homologous tails during targeted homologous recombination. EMBO J. 2000;19:5552–5561. doi: 10.1093/emboj/19.20.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niedernhofer LJ, Essers J, Weeda G, Beverloo B, de Wit J, Muijtjens M, Odijk H, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease Ercc1-Xpf is required for targeted gene replacement in embryonic stem cells. EMBO J. 2001;20:6540–6549. doi: 10.1093/emboj/20.22.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sargent RG, Rolig RL, Kilburn AE, Adair GM, Wilson JH, Nairn RS. Recombination-dependent deletion formation in mammalian cells deficient in the nucleotide excision repair gene ERCC1. Proc Natl. Acad. Sci. USA. 1997;94:13122–13127. doi: 10.1073/pnas.94.24.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sargent RG, Meservy JL, Perkins BD, Kilburn AE, Intody Z, Adair GM, Nairn RS, Wilson JH. Role of the nucleotide excision repair gene ERCC1 in formation of recombination-dependent rearrangements in mammalian cells. Nucl. Acids Res. 2000;28:3771–3778. doi: 10.1093/nar/28.19.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu XD, Niedernhofer L, Kuster B, Mann M, Hoeijmakers JH, de Lange T. ERCC1/XPF removes the 3' overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Mol. Cell. 2003;12:1489–1498. doi: 10.1016/s1097-2765(03)00478-7. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Zacal NJ, Rainbow AJ, Zhu XD. XPF with mutations in its conserved nuclease domain is defective in DNA repair but functions in TRF2-mediated telomere shortening. DNA Repair. 2007;6:157–166. doi: 10.1016/j.dnarep.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Westerveld A, Hoeijmakers JH, van Duin M, de Wit J, Odijk H, Pastink A, Wood RD, Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984;310:425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- 9.Brookman KW, Lamerdin JE, Thelen MP, Hwang M, Reardon JT, Sancar A, Zhou ZQ, Walter CA, Parris CN, Thompson LH. ERCC4 (XPF) encodes a human nucleotide excision repair protein with eukaryotic recombination homologs. Mol. Cell. Biol. 1996;16:6553–6562. doi: 10.1128/mcb.16.11.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sijbers AM, de Laat WL, Ariza RR, Biggerstaff M, Wei YF, Moggs JG, Carter KC, Shell BK, Evans E, de Jong MC, Rademakers S, de Rooij J, Jaspers NG, Hoeijmakers JH, Wood RD. Xeroderma pigmentosum group F caused by a defect in a structure-specific DNA repair endonuclease. Cell. 1996;86:811–822. doi: 10.1016/s0092-8674(00)80155-5. [DOI] [PubMed] [Google Scholar]
- 11.Thompson LH, Busch DB, Brookman K, Mooney CL, Glaser DA. Genetic diversity of UV-sensitive DNA repair mutants of Chinese hamster ovary cells. Proc. Natl. Acad. Sci. USA. 1981;78:3734–3737. doi: 10.1073/pnas.78.6.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busch D, Greiner C, Lewis K, Ford R, Adair G, Thompson L. Summary of complementation groups of UV-sensitive CHO cell mutants isolated by large-scale screening. Mutagenesis. 1989;4:349–354. doi: 10.1093/mutage/4.5.349. [DOI] [PubMed] [Google Scholar]
- 13.Collins AR. Mutant rodent cell lines sensitive to ultraviolet light, ionizing radiation and cross-linking agents: a comprehensive survey of genetic and biochemical characteristics. Mutat. Res. 1993;293:99–118. doi: 10.1016/0921-8777(93)90062-l. [DOI] [PubMed] [Google Scholar]
- 14.Busch DB, van Vuuren H, de Wit J, Collins A, Zdzienicka MZ, Mitchell DL, Brookman KW, Stefanini M, Riboni R, Thompson LH, Albert RB, van Gool AJ, Hoeijmakers J. Phenotypic heterogeneity in nucleotide excision repair mutants of rodent complementation groups 1 and 4. Mutat. Res. 1997;383:91–106. doi: 10.1016/s0921-8777(96)00048-1. [DOI] [PubMed] [Google Scholar]
- 15.Thompson LH, Bachinski LL, Stallings RL, Dolf G, Weber CA, Westerveld A, Siciliano MJ. Complementation of repair gene mutations on the hemizygous chromosome 9 in CHO: a third repair gene on human chromosome 19. Genomics. 1989;5:670–679. doi: 10.1016/0888-7543(89)90107-9. [DOI] [PubMed] [Google Scholar]
- 16.Rolig RL, Layher SK, Santi B, Adair GM, Gu F, Rainbow AJ, Nairn RS. Survival, mutagenesis, and host cell reactivation in a Chinese hamster ovary cell ERCC1 knock-out mutant. Mutagenesis. 1997;12:277–283. doi: 10.1093/mutage/12.4.277. [DOI] [PubMed] [Google Scholar]
- 17.Rolig RL, Lowery MP, Adair GM, Nairn RS. Characterization and analysis of Chinese hamster ovary cell ERCC1 mutant alleles. Mutagenesis. 1998;13:357–365. doi: 10.1093/mutage/13.4.357. [DOI] [PubMed] [Google Scholar]
- 18.Al-Minawi AZ, Saleh-Gohari N, Helleday T. The ERCC1/XPF endonuclease is required for efficient single-strand annealing and gene conversion in mammalian cells. Nucl. Acids Res. 2008;36:1–9. doi: 10.1093/nar/gkm888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enzlin JH, Scharer OD. The active site of the DNA repair endonuclease XPF-ERCC1 forms a highly conserved nuclease motif. EMBO J. 2002;21:2045–2053. doi: 10.1093/emboj/21.8.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monnat RJ. From broken to old: DNA damage, IGF1 endocrine suppression and aging. DNA Repair. 2007;6:1386–1390. doi: 10.1016/j.dnarep.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merrihew RV, Sargent RG, Wilson JH. Efficient modification of the APRT gene by FLP/FRT site-specific targeting. Somat. Cell Mol. Genet. 1995;21:299–307. doi: 10.1007/BF02257465. [DOI] [PubMed] [Google Scholar]
- 22.Adair GM, Stallings RL, Siciliano MJ. Chromosomal rearrangements and gene expression in CHO cells: mapping of alleles for eight enzyme loci on CHO chromosomes Z3, Z4, Z5, and Z7. Somat. Cell Mol. Genet. 1984;10:283–295. doi: 10.1007/BF01535250. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Tian M, Shinkura R, Shinkura N, Alt FW. Growth retardation, early death, and DNA repair defects in mice deficient for the nucleotide excision repair enzyme XPF. Mol. Cell. Biol. 2004;24:1200–1205. doi: 10.1128/MCB.24.3.1200-1205.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson C, Jasin M. Frequent chromosomal translocations induced by DNA double-strand breaks. Nature. 2000;405:697–700. doi: 10.1038/35015097. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RD, Jasin M. Sister chromatid gene conversion is a prominent double-strand break repair pathway in mammalian cells. EMBO J. 2000;19:3398–3407. doi: 10.1093/emboj/19.13.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tutt A, Bertwistle D, Valentine J, Gabriel A, Swift S, Ross G, Griffin C, Thacker J, Ashworth A. Mutation in Brca2 stimulates error-prone homology-directed repair of DNA double-strand breaks occuring between repeated sequences. EMBO J. 2001;20:4704–4716. doi: 10.1093/emboj/20.17.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maquat LE. Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nat. Rev. Mol. Cell. Biol. 2004;5:89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- 29.Bollag RJ, Liskay RM. Direct-repeat analysis of chromatid interactions during intrachromosomal recombination in mouse cells. Mol. Cell. Biol. 1991;11:4839–4845. doi: 10.1128/mcb.11.10.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguilera A, Klein HL. Yeast intrachromosomal recombination: long gene conversion tracts are preferentially associated with reciprocal exchange and require the RAD1 and RAD3 gene products. Genetics. 1989;123:683–694. doi: 10.1093/genetics/123.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince PR, Emond MJ, Monnat RJ., Jr Loss of Werner syndrome protein function promotes aberrant mitotic recombination. Genes Dev. 2001;15:933–938. doi: 10.1101/gad.877001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Opresko PL, Cheng W-H, von Kobbe C, Harrigan JA, Bohr Vilhelm. Werner syndrome and the function of the Werner protein; what they can teach us about the molecular aging process. Carcinogenesis. 2003;24:791–802. doi: 10.1093/carcin/bgg034. [DOI] [PubMed] [Google Scholar]
- 33.Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 34.Jaspers NG, Raams A, Silengo MC, Wijgers N, Niedernhofer LJ, Robinson AR, Giglia-Mari G, Hoogstraten D, Kleijer WJ, Hoeijmakers JH, Vermeulen W. First reported patient with human ERCC1 deficiency has cerebro-oculo-facio-skeletal syndrome with a mild defect in nucleotide excision repair and severe developmental failure. Am. J. Hum. Genet. 2007;80:457–466. doi: 10.1086/512486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goymer P. New syndrome reconciles theories of ageing. Nature Reviews Genet. 2007;8:90. [Google Scholar]
- 36.Weeda G, Donker I, de Wit J, Morreau H, Janssens R, Vissers CJ, Nigg A, van Steeg H, Bootsma D, Hoeijmakers JH. Disruption of mouse ERCC1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr. Biol. 1997;7:427–439. doi: 10.1016/s0960-9822(06)00190-4. [DOI] [PubMed] [Google Scholar]